Abstract
Background:
In major depressive disorder (MDD), electrophysiological and imaging studies suggest reduced neural activity in the parietal and dorsolateral prefrontal cortex regions. In the present study, neural correlates of emotional processing in MDD were analyzed for the first time in a pre-/post-treatment design by means of magnetoencephalography (MEG), allowing for detecting temporal dynamics of brain activation.
Methods:
Twenty-five medication-free Caucasian in-patients with MDD and 25 matched controls underwent a baseline MEG session with passive viewing of pleasant, unpleasant, and neutral pictures. Fifteen patients were followed-up with a second MEG session after 4 weeks of antidepressant monopharmacotherapy with mirtazapine. The corresponding controls received no intervention between the measurements. The clinical course of depression was assessed using the Hamilton Depression scale.
Results:
Prior to treatment, an overall neocortical hypoactivation during emotional processing, particularly at the parietal regions and areas at the right temporoparietal junction, as well as abnormal valence-specific reactions at the right parietal and bilateral dorsolateral prefrontal cortex (dlPFC) regions were observed in patients compared to controls. These effects occurred <150ms, suggesting dysfunctional processing of emotional stimuli at a preconscious level. Successful antidepressant treatment resulted in a normalization of the hypoactivation at the right parietal and right temporoparietal regions. Accordingly, both dlPFC regions revealed an increase of activity after therapy.
Conclusions:
The present study provides neurophysiological evidence for dysfunctional emotional processing in a fronto-parieto-temporal network, possibly contributing to the pathogenesis of MDD. These activation patterns might have the potential to serve as biomarkers of treatment success.
Keywords: Dorsolateral prefrontal cortex, IAPS, MDD, EEG, MEG, parietal hypoactivation, temporoparietal junction
Introduction
In major depressive disorder (MDD), attention and memory biases in favor of negative as compared to positive or neutral information have been observed, which is reversible by antidepressant treatment (Everaert et al., 2012; Harmer and Cowen, 2013). Although such “negative bias effects” dominate the depression literature, researchers also found “positive attenuation effects” in depression, with either reduced sensitivity to positive relative to other stimuli or an absence of a “positivity bias” (Everaert et al., 2012).
As a possible mechanism of a negative bias in depression, the frontal/prefrontal cortex has been suggested to provide insufficient top-down inhibitory control on limbic systems. Indeed, functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies reveal a generally decreased activation in the dorsolateral and dorsomedial prefrontal cortex (PFC) in combination with increased activation of the ventrolateral and orbitofrontal cortical areas in depression (Davidson et al., 2000).
Event-related-potential (ERP) studies consistently showed P300, N400, and slow-wave amplitudes to be elevated in response to negative and reduced in response to positive stimuli in depressive patients and subjects at risk of depression during various behavioral decision tasks (e.g. Shestyuk et al., 2005; Krompinger and Simons, 2009; Bistricky et al., 2014). These findings support both enhanced attention towards negative as well as reduced attention towards positive emotional stimuli in depression.
ERP studies on the processing of emotional scenes without additional task sets and without patients making judgments about emotional stimuli are sparse. In one study (Kayser et al., 2000), depressive patients revealed a reduced emotional reaction towards negative pictures at right parietal sensors, peaking around 460ms. Right parietal sensors also showed a reduced reaction of MDD patients, specifically towards faces with positive expression, though at an earlier N200 component (Deldin et al., 2000). A reduced reaction towards emotionally arousing pictures at right temporo-parietal regions has also been shown by a magnetoencephalography (MEG) study (Moratti et al., 2008). The time course of this effect, however, remained unknown due to the specific steady-state stimulation protocol used in this study.
Electrophysiological resting-state activity in depression consistently showed abnormal regional hemispheric asymmetries of alpha activity, with a relative increase of alpha-band activity in the left versus right frontal electrodes and a relative increase of alpha power in the right verus left electrodes at posterior sites (Henriques and Davidson, 1991; Bruder et al., 1997; Thibodeau et al., 2006; Stewart et al., 2011). Since the posterior alpha is typically inversely correlated with cortical activity (Feige et al., 2005), an enhanced right parietal alpha is interpreted as indicative of a neural hypoactivation, especially at right parietal regions. An enhanced alpha asymmetry (right > left) has also been found in high-risk children and grandchildren of MDD patients (Bruder et al., 2007), supporting the hypothesis of right parietal hypoactivity as an endophenotypic marker of major depression. Posterior, and especially right hemispheric, alpha has also been suggested as a predictor of antidepressant responses, since depressive patients with low posterior alpha seem to respond less to serotonin reuptake inhibitor treatment (Tenke et al., 2011).
Reduced parietal, and especially right parietal, activity in depression, as revealed by electrophysiological event-related and resting state alpha studies, is corroborated by fMRI findings showing decreased parietal metabolism in depression and sadness (Mayberg, 1997; Liotti et al., 2000).
In the present study, we aimed to investigate baseline as well as post-treatment neural correlates of passive emotional picture processing without additional tasks in patients with MDD by means of whole-head MEG. The measure of event-related magnetic fields, in combination with inverse source modeling, allows for detecting even rapid, transient, and preconscious cortical activations, which may not necessarily result in measurable hemodynamic changes and may become object to hemodynamic masking due to compensatory attempts of attenuating the expression or experience of aversive emotions (Drevets, 2007). Our predictions were that medication-free patients with depression as compared to healthy controls would show an overall decreased sensitivity towards arousing pictures, in correlation with a particularly right-sided temporo-parietal hypoactivation and a dysfunctional affective modulation at prefrontal cortex regions. These magnetoencephalographic correlates of disturbed affective processing were expected to normalize along with a clinical response to a standardized antidepressant pharmacological therapy.
Patients and Methods
Subjects
For the present case-control study, 25 right-handed Caucasian in-patients (16 females) with MDD as ascertained by a standardized clinical interview (SCID-I) were recruited at the local Department of Psychiatry (Table 1). Patients with bipolar or psychotic disorders, comorbid substance abuse or anxiety disorders, mental retardation, neurological or neurodegenerative disorders, or other clinically unstable medical illnesses impairing psychiatric evaluation, as well as pregnant patients, were not included in this study. All patients were medication-free at the time of inclusion, either being drug naive at the time of admission or having being tapered off antidepressant medication according to the respective half-life, which was ascertained by drug plasma levels. Patients having received neuroleptic or any other psychotropic medication during a 6-month period before the study were excluded. Benzodiazepines were not used apart from lorazepam (max. 3mg/d) only until 24h before the MEG session.
Table 1.
Demographic and Clinical Data of Patients with MDD and Healthy Controls
| Pre-treatment sample | Patients | Controls | p-value |
|---|---|---|---|
| Males/females a | 9/16 | 9/16 | 1.000 |
| Age (mean ± SD) b | 44.3±14.7 | 44.0±14.7 | 0.939 |
| Age of onset (mean ± SD) | 33.7±14.9 | - | - |
| CGI score (mean ± SD) c | 4.8±0.6 | - | - |
| GAF score (mean ± SD) c | 48.1±6.8 | - | - |
| HAMD score (mean ± SD) b, c | 22.2±5.3 | 1.1±1.6 | <0.001 |
| HAMA score (mean ± SD) b, c | 26.5±9.1 | 3.0±3.0 | <0.001 |
| PANAS positive (mean ± SD) b, c | 19.3±5.1 | 31.6±5.4 | <0.001 |
| PANAS negative (mean ± SD) b, c | 26.8±7.4 | 11.2±1.8 | <0.001 |
| Pre-post-treatment sample | Patients | ||
| Males/females | 5/10 | ||
| Age (mean ± SD) | 45.3+15.8 | ||
| CGI score (mean ± SD) c | 4.8±0.4 | ||
| GAF score (mean ± SD) c | 48.1±5.7 | ||
| HAMD score pre (mean ± SD) c | 22.3±6.0 | ||
| HAMD score post (mean ± SD) d, e | 7.9±8.4 | <0.001 |
a Chi-square test; b two-sample t-test; c scores taken at admission within 2 days before time of inclusion; d scores taken after 4 weeks of treatment with mirtazapine; e one-sample t-test (pre vs. post therapy).
CGI, Clinical Global Impression; GAF, Global Assessment of Functioning; HAMA, Hamilton anxiety scale; HAMD, Hamilton depression scale; MDD, major depressive disorder; PANAS, Positive and Negative Affect Schedule; SD, standard deviation.
For the follow-up study, a subgroup of 15 patients (Table 1) subsequently underwent a 4-week standardized antidepressant monopharmacotherapy with mirtazapine (45mg/d) in a naturalistic clinical in-patient setting. As co-medication, only lorazepam was allowed (max. 3mg/d) up until 24h before the second MEG session. The clinical course of depression was assessed on a weekly basis by experienced psychiatrists using the Hamilton Depression scale (HAMD) and showed significant treatment response (Table 1). Ten patients were not included in the follow-up study, because they either required a change in medication according to clinical judgment (n = 4) or refused participation in a second MEG session (n = 6). These patients (age, 42.8±13.6; Clinical Global Impression, 4.7±0.8; Global Assessment of Functioning, 48.1±8.5; HAMD, 22.1±4.2) did not differ from the ones included in the follow-up study regarding age or clinical characteristics at admission (Table 1; all p > 0.69).
As a control group, 25 healthy participants matched for gender, age, years of education, and handedness were recruited. Inclusion criteria were no history of mental illness as ascertained by SCID-I interview.
All participants had normal or corrected-to-normal visual acuity. The study was approved by the local ethical committee and informed consent was obtained from each participant.
Design, Procedure, and Stimulus Material
All 25 patients and corresponding controls underwent a pre-treatment baseline MEG session. A subgroup of 15 patients treated with a standardized antidepressant pharmacotherapy and corresponding 15 matched controls were followed up with 4 weeks later. MEG volume conductor modeling was based on anatomical T1-MRI images. For later spatial coregistration of anatomy and function, gadolinium landmarks (MRI) and landmark coils (MEG) were attached to the auditory canals and the nasion during the respective scanning.
Based on the normative ratings of emotional valence and arousal, 500 pictures from the International Affective Picture System (Lang et al., 2005) were divided evenly into five picture categories: high arousing positive (erotica, sports, etc.), low arousing positive (family, landscapes, etc.), neutral (people, road-traffic, etc.), low arousing negative (illness, pollution, etc.), and high arousing negative (attack, mutilation, etc.). Statistical tests revealed that critical physical picture parameters did not differ across picture categories. In the MEG session, all pictures were randomly presented for 660ms each as Rapid Serial Visual Presentation (RSVP) without perceivable interstimulus intervals (Junghöfer et al., 2001). Participants were asked to passively view the stream of stimuli while keeping their eyes focused on an overlaid fixation cross.
After RSVP, participants rated hedonic valence and emotional arousal of 36 representative pictures using a computerized version of the Self-Assessment-Manikin (SAM). Hamilton Anxiety Scale (HAMA), State-Trait Anxiety Inventory (STAI), and Positive Affect Negative Affect Schedule (PANAS) scores were taken immediately before and after the respective MEG sessions.
Apparatus and Data Analysis
Visual evoked magnetic fields were acquired using a 275 MEG whole-head sensor system (VSM Medtech Ltd.), sampled down offline to 300 Hz and filtered between 0.01 and 148 Hz. Data were aligned to the stimulus onset, with an averaging epoch ranging from 200ms before to 600ms after the stimulus onset and baseline-adjusted using a 150ms pre-stimulus interval. Single trials were edited and artifacts were corrected following the method for statistical control of artifacts in high-density EEG/MEG data as proposed by Junghöfer and coworkers (2000). In this method, channels contaminated by artifacts are interpolated by weighted spherical splines and trials are removed when too many channels are contaminated. After artifact rejection and averaging, cortical sources of the event-related magnetic fields were estimated using the L2-Minimum-Norm-Estimates method (L2-MNE; Hämäläinen and Ilmoniemi, 1994). The L2-MNE is an inverse modeling technique applied to reconstruct the topography of the primary current underlying the magnetic field distribution. It allows the estimation of distributed neural network activity without a priori assumptions regarding the location and number of current sources. A spherical shell with a radius roughly corresponding to the individual grey matter depth was used as a source model. Topographies of source-direction-independent neural activities were calculated for each participant, condition, and time point. For visualization purposes, source reconstruction results were projected onto a quasi-realistic brain model.
Statistical Data Analysis
Regarding the hypothesis of parietal hypoactivation in depression, t-tests comparing picture-category-independent sustained activity of patients and controls were calculated for the time interval from 0 to 500ms. Guarding against false positive correlations, nonparametric cluster level statistics were applied as suggested by Maris and Oostenveld (2007), with p < 0.05 on both sensor and cluster levels. As this analysis revealed widely distributed effects covering parietal but also right temporal areas, symmetric left and right hemispheric regions of interest were identified based on the dominant difference activations between patients and controls. A repeated-measures ANOVA with the factors of group and hemisphere were performed based on this time interval and—to allow an identification of the temporal onset of sustained hypoactivation—also on moving averaged time intervals of 20ms length. Repeated-measures ANOVAs with the factors of group, affective category, hemisphere, and gender were then calculated for the revealed time interval of sustained hypoactivation (120–500ms).
Testing group–specific differential processing of positive or negative pictures independent of picture arousal, estimated neural activities evoked by low and high arousing positive pictures, as well as low and high arousing negative pictures were averaged for each subject. Subsequently, ANOVAs with the limited factors of valence (positive, negative), group, hemisphere, and gender were applied.
Linear correlations with the Hamilton Depression Scale scores were estimated for the overall cortical activity within the group of 25 patients, as well as for the positive minus negative valence difference activity. Again, cluster-level statistics with significance levels of p < 0.05 on both the sensor and cluster levels were applied. MEG data analysis was conducted with the Matlab-based EMEGS software (Peyk et al., 2011).
Results
Psychological Assessment
Patients and controls differed significantly with respect to their pre- and post-experiment STAI state as well as PANAS scores (all p < 0.001). However, there was no significant difference in STAI state or PANAS scores pre-/post-MEG session within the patient or control group (data not shown).
Behavior
SAM ratings of valence and arousal at baseline were analyzed using repeated-measures ANOVAs, including the factors of group and affective category. Affirming the stimulus selection, participants distinguished between stimulus categories, as indicated by differential valence (F4,192 = 67.5, p < 0.001) and arousal ratings (F4,192 = 66.4, p < 0.001). However, patients and controls did not differ in their valence or arousal ratings (Fs < 1).
MEG Data Baseline Activation Patterns
Parietal Hypoactivation
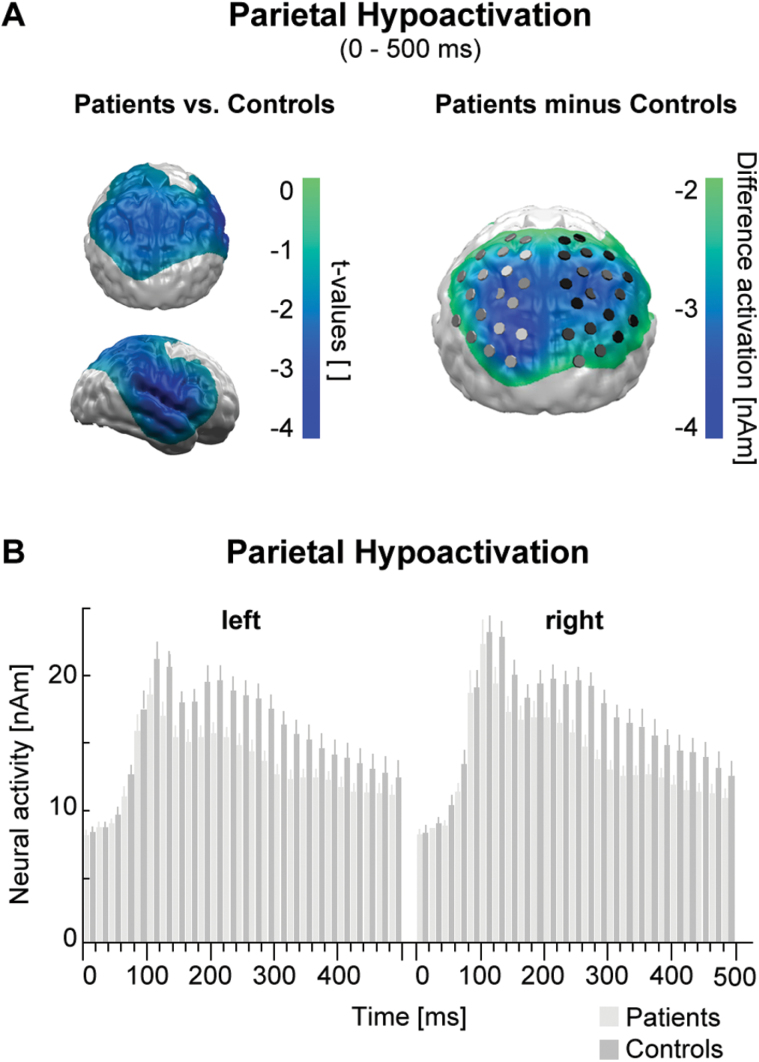
The t-test comparing the sustained (0–500ms) event-related neural activity (Figure 1A, left) and the overall cortical difference activation (Figure 1A, right) between groups revealed a profound diminished neural activity over bilateral parietal areas in patients compared to controls. This was confirmed by a significant main effect of group (F1,48 = 7.9; p < 0.01) and a missing interaction with hemisphere in a group x hemisphere ANOVA. The moving average ANOVA (Figure 1B) revealed a first significant main effect of group within the time interval of 50–70ms (F1,48 = 4.3; p < 0.05), but the following sustained hypoactivation did not get significant before 110–130ms (F1,48 = 4.5; p < 0.05). The group x hemisphere x affective category x gender ANOVA covering the sustained hypoactivation (120–500ms) revealed a significant main effect of hemisphere (F1,46 = 4.6; p < 0.05) with overall stronger right hemispheric activity and a significant main effect of group (F1,46 = 5.7; p < 0.05), but no interaction of both factors and no interactions with affective category or gender.
Figure 1.
(A) Left: topography of t-tests comparing overall picture category independent activity of patients and controls. Areas with significant sustained (0–500ms) hypoactivations in the patient group are color coded (masked at random permutation cluster level p < 0.05). Right: topography of overall difference activations between patients and controls. Markers indicate inverse test sources at right and left parietal regions of interest.
(B) Time course of left and right parietal neural activation for patients and controls. A first significant hypoactivation appeared between 50 and 70ms and was sustained after 120ms.
Differential Positive and Negative Picture Processing at Right Parietal Regions
In the 250–300ms time interval of strongest group effects, a significant valence x group x hemisphere interaction occurred (F1,46 = 11.5; p < 0.001). Hemisphere-specific analyses revealed that preferences of patients and controls differed in the right parietal region only (F1,46 = 7.2; p < 0.01), with controls reacting stronger to positive images (p < 0.07), but patients stronger to negative pictures (p < 0.05). Although this right parietal group x valence interaction was strongest in the 250–300ms interval, qualitatively identical and significant effects also appeared at 150–200ms and 350–400ms intervals, and thus in a 10 Hz (alpha) rhythm. None of the investigated parietal effects correlated with patients’ depression or anxiety scores (data not shown).
Temporoparietal Junction Hypoactivation Correlating with Depression Severity
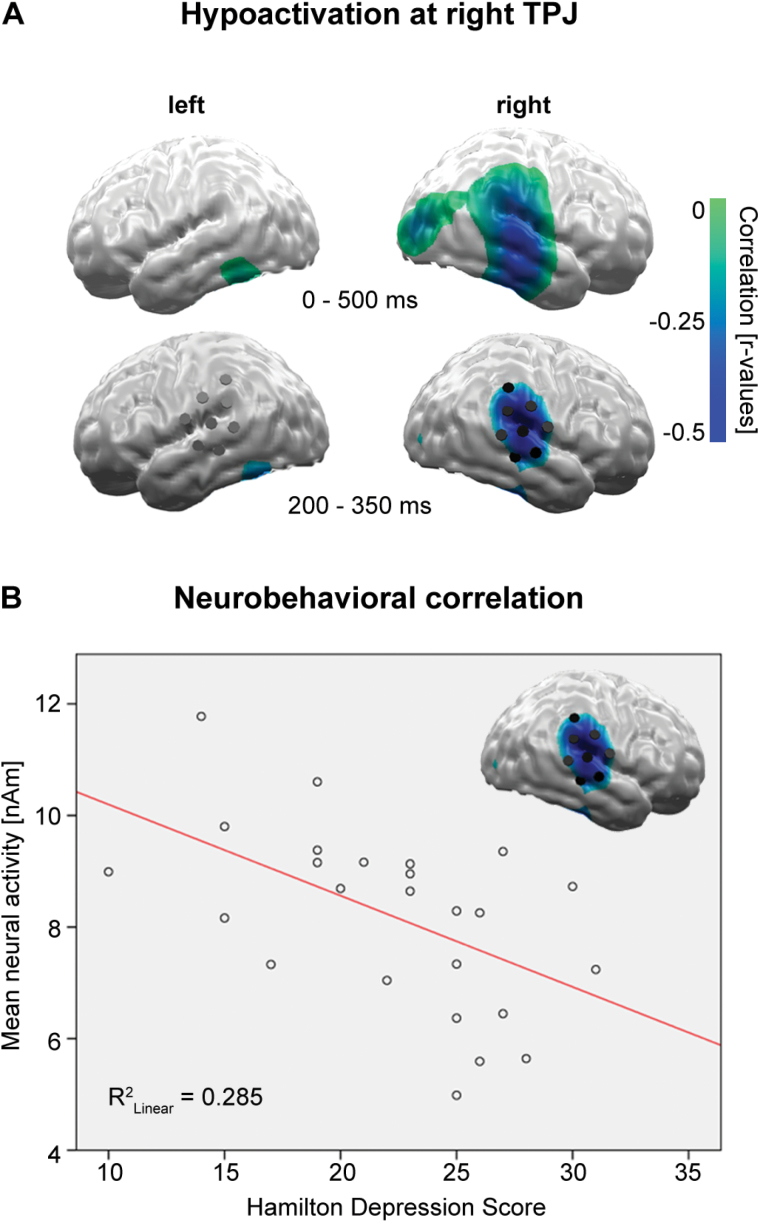
Linear correlations of mean cortical activity with the patients’ HAMD score for the 0–500ms time interval identified an area of significant negative correlations around the right temporo-parietal junction (TPJ; Figure 2A, top), with the strongest effects between 200 and 350ms (Figure 2A, bottom). The group x affective category x hemisphere x gender ANOVA for this interval and bihemispheric regions of interest again revealed an overall right hemispheric dominance (hemisphere, F1,46 = 15.4; p < 0.001) and confirmed an overall TPJ hypoactivation for patients compared to controls (group, F1,46 = 11.0; p < 0.005), predominately at the right hemisphere (group x hemisphere, F1,46 = 4.1; p < 0.05). The strongest negative correlations appeared for high arousing positive (r = -0.55; p < 0.005) and low arousing negative pictures (r = -0.54; p < 0.005). However, responses towards all picture categories showed at least trends for negative correlations, explaining the overall strong correlation of HAMD and right TPJ activity (r = -0.53; p < 0.006; Figure 2B). Right TPJ activity did not correlate with anxiety (HAMA; r = 0.04).
Figure 2.
(A) Top: linear correlations of neural activity with depression severity (Hamilton depression scale) identified an area of significant negative correlation at the right occipito-temporo-parietal regions. Bottom: the strongest linear correlations were found in a 200–350ms time interval at the right temporo-parietal junction (TPJ; p < 0.05 at cluster level).
(B) Linear correlation of Hamilton depression scale score and right TPJ activity.
Differential Appetitive and Aversive Picture Processing at dlPFC Correlating with Depression Severity
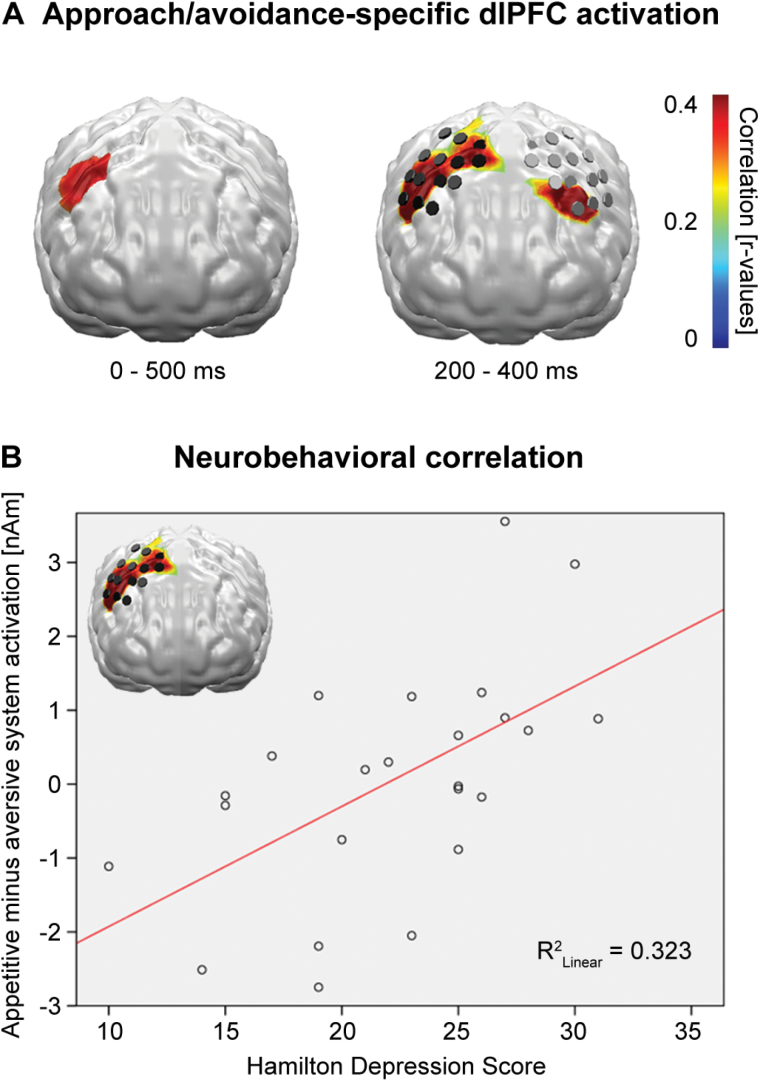
Linear correlations of differential valence reactions (positive minus negative) with the patients’ HAMD score for the 0–500ms time interval identified an area of significant positive correlations at the right dorsolateral prefrontal cortex (dlPFC; Figure 3A, left), which showed the strongest effects between 200 and 400ms. Within this limited interval, a corresponding left hemispheric dlPFC region also revealed significant correlations (Figure 3A, right).
Figure 3.
(A) Left: linear correlations of neural activity evoked by positive minus activity evoked by negative pictures with depression severity (Hamilton depression scale) identified a dorsolateral PFC (dlPFC) area with decreasing activity for aversive and increasing activity for appetitive picture processing with rising severity. Right: strongest linear correlations were found in a time interval between 200 and 400ms, now at the bilateral dlPFC regions (p < 0.05 at cluster level).
(B) Linear correlation of Hamilton depression scale score and right dlPFC difference activity.
Inspection of the correlation coefficients identified one outlying patient, and extraction of this outlier increased the correlation from r = 0.35 (p < 0.05) to r = 0.57 (p < 0.005; Figure 3B).
A parametric linear regression analysis of HAMD scores and right dlPFC activity revealed both negative (T23 = -3.2; p < 0.005) and positive (T23 = +2.7; p < 0.05) interrelations, indicating decreased neural activity to negative pictures and increased neural activity to positive pictures with rising depression severity, respectively (overall regression, F2,23 = 5.7, p = 0.01, R-Square = 0.35).
The corresponding left hemispheric dlPFC area revealed a converging significant regression, with qualitatively identical direction of effects (F2,23 = 3.9, p < 0.05, R-Square = 0.26; negative interrelation, T23 = -2.6; p < 0.05; positive interrelation, T23 = +1.9; p < 0.07).
Again, corresponding regression analyses with anxiety scores (HAMA) did not show any effects (all Fs < 1).
MEG Data Antidepressant Treatment Effects
Parietal Hypoactivation
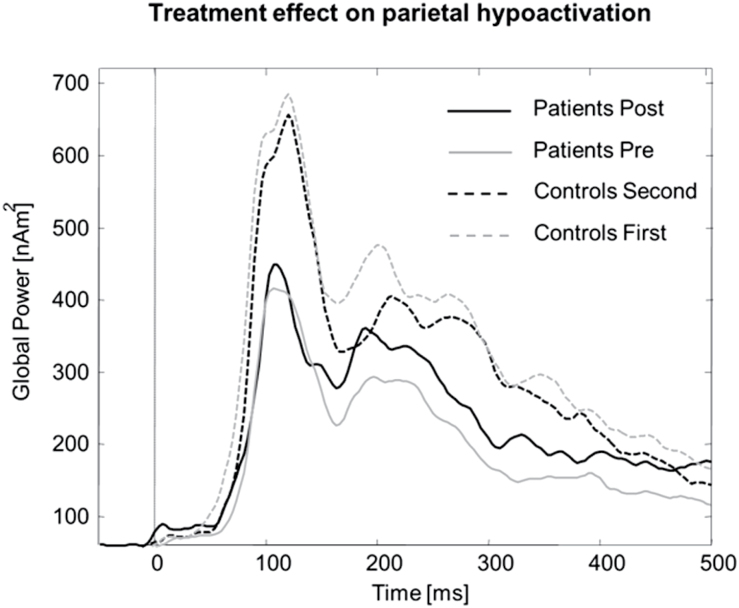
The time course in Figure 4 illustrates a decreasing parietal activation in the control group from the first to second session, but a relative increase in patients after therapy, confirmed by a session x hemisphere x group interaction (F1,26 = 4.6; p < 0.05, 0–500ms). Post hoc analyses revealed a significant interaction of session x group in the right (F1,28 = 5.8; p < 0.05), but not left hemispheric region (F < 1). T-tests affirmed the significant hypoactivation for patients compared to controls before therapy, even in this smaller group of patients and controls (t28 = -2.6; p < 0.02), while after therapy the activity of patients and controls did not differ any more (t < 0.05). Analysis for the limited time interval of strongest pre-therapy hypoactivation (200–300ms) resulted in an even stronger session x group x hemisphere interaction [F(1,26) = 5.9; p < 0.02] and stronger but qualitatively identical post hoc effects. However, Figure 4 indicates residual differences in the MEG waveforms between patients after treatment and controls in the second session, particularly in an early time interval before 150ms after stimulus onset.
Figure 4.
Time course of parietal cortex activity for 15 major depressive disorder patients who participated in both the pre- and post-therapy magnetoencephalography (MEG) sessions, and their matched healthy controls in their first and second MEG sessions. While parietal activation, most probably due to habituation and reduced vigilance, decreased in the control group, patients showed increased parietal activity after therapy in direction normalization.
Differential Appetitive and Aversive Picture Processing at Right Parietal Regions
The system x group x hemisphere interaction in the 250–300ms time interval did not change after therapy (F < 1).
Temporoparietal Junction Hypoactivation
Convergent to the parietal areas, a significant session x group x hemisphere interaction was discerned (F1,26 = 5.9; p < 0.02) for the above defined bilateral TPJ areas (200–350ms). Again, the right (F1,28 = 6.0; p < 0.02) but not the left (F < 1) TPJ area revealed a significant session x group interaction. Post hoc t-tests showed a significantly reduced activation for patients before therapy in the right hemisphere (t28 = -3.3; p < 0.005), which disappeared after therapy (t < 1).
Differential Appetitive and Aversive Pictures Processing at dlPFC
The above defined bihemispheric dlPFC areas (200–400ms) revealed a significant session x group (F1,26 = 4.9; p < 0.05) and a significant session x group x hemisphere interaction (F1,26 = 5.3; p < 0.05). Separate analyses revealed a trend effect for session in the left (F1,28 = 4.0; p < 0.06) and a significant interaction of session x group in the right hemisphere (F1,28 = 6.7; p < 0.02). While left dlPFC activity increased across sessions for both groups, right dlPFC activity increased for patients, but decreased for controls.
Discussion
To the best of our knowledge, this is the first study investigating electrophysiological correlates of emotional processing in depression in a pre-/post-treatment design, and the first study revealing alterations of the spatio-temporal dynamics of passive emotional picture processing in this patient group. The present pre-treatment data provide evidence for an overall neocortical hypoactivation during emotional visual processing in medication-free patients with depression, particularly at parietal regions and areas at the right TPJ, as well as abnormal valence-specific reactions at the bilateral dlPFC and right parietal regions. Four weeks of antidepressant monopharmacotherapy with mirtazapine resulted in a normalization of the patients’ hypoactivation at the right parietal and right temporoparietal regions. Accordingly, both dlPFC regions revealed an increase of activity in the patient group after therapy.
The parietal cortex, and particularly the inferior parietal lobe, is strongly connected to the limbic and frontal system (Morecraft et al., 1993) and has repeatedly been implied in the regulation of emotional arousal, emotional awareness, and attention to emotional contents (for review see Adolphs, 2002). Consistently, weaker activation of the inferior parietal lobes has been observed in patients with depression in response to fearful face presentation (Surguladze et al., 2010) and also during a task on cognitive control over emotional cues (Beevers et al., 2010), while in healthy probands suppression of fearful faces was associated with increased activity in inferior parietal cortices (Amting et al., 2010). Reciprocally, activity of the parietal cortex has been suggested as a potential substrate for successful pharmacological or non-pharmacological treatment of depression (e.g. Mayberg et al., 2000; Sperling et al., 2000; Schutter and van Honk, 2005). Both bilaterally and in the right hemisphere, dominant parietal hypoactivation of the presently event-related neural activity is also in good accordance with relative increased resting state alpha power in right versus left electrodes at posterior sites (e.g. Bruder et al., 1997; Stewart et al., 2011), which is interpreted as hypoactivation in depression, especially at the right parietal regions. However, while increased resting state alpha over right parietal sites does not change with therapy and appears as an endophenotypic marker of major depression (Tenke et al., 2011), the event-related parietal activation measured here increased in direction to normalization with successful treatment.
In emotion-theoretic research, the TPJ has been suggested as a key component of stimulus-driven attentional orienting (Corbetta et al., 2000; Corbetta and Shulman, 2002; Behrmann et al., 2004; Geng and Mangun, 2011). Stimulus-driven shifts of spatial attention in both visual fields (Shulman et al., 2010) and evaluation of the behavioral relevance of salient events outside the current focus of attention (Gruber et al., 2010) have been shown to evoke right-hemisphere dominant temporoparietal activity. The TPJ has thus been suggested to issue a control signal acting as a “circuit breaker” of ongoing cognitive activity when a behaviorally relevant stimulus is perceived (Corbetta and Shulman, 2002). The observed negative correlation of a significantly diminished right TPJ activation with increasing depression severity might thus reflect a dysfunctional capture of the attention mechanism by emotional stimuli in depression.
In addition to the valence independent parietal hypoactivation, activity at the right parietal regions was modulated by picture valence. While controls revealed a typical U-shape emotional arousal–driven reaction with preferential processing of both appetitive and aversive pictures in this region, patients showed a negativity bias, with stronger activations for negative compared to positive pictures. The observation of a left parietal and right temporoparietal general hypoactivation, as well as a valence-specific hypoactivation in the right parietal and temporoparietal regions, is in accordance with an MEG study by Moratti and coworkers (2008). However, while Moratti and colleagues revealed a flattened response towards emotionally arousing pictures in patients compared to controls at the right temporoparietal regions, patients in the study at hand even showed a negativity bias with a disproportionate reduction of appetitive picture processing. The parietal hypoactivation already revealed the first phasic significance around 60ms and showed sustained effects after 120ms, and even the right parietal negativity bias started already around 150ms. Although these early onsets clearly precede the assumed limit of conscious perception of around 300ms (Dehaene and Changeux, 2011) and argue for disturbances of automatic, preconscious processing, issues of automaticity or consciousness have not been specifically tested in this study.
In addition to the right TPJ, activity at the dorsolateral PFC regions strongly correlated with depression severity. The dlPFC has been implied as the main source of top-down control signals to the visual cortex (Desimone and Duncan, 1995; Miller and Cohen, 2001) and serves the purpose of cognitive-emotional control (Ochsner and Gross, 2008). In the present study, bilateral dlPFC regions showed valence-specific effects, with positive correlations for positive but negative correlations for negative picture processing. The first is in line with findings of a positive correlation of bilateral dlPFC activation with global depression severity during appetitive picture processing (Keedwell et al., 2005), with right frontal (though ventral) regions over-regulating positive affect in depression, decreasing along with the therapeutic effect of antidepressants (Light et al., 2011), as well as increasing left dlPFC activation with increasing positivity judgment of pleasant pictures in MDD (Grimm et al., 2008). Decreased dlPFC activity, as presently observed during negative picture processing, has been associated with attentional deficits and cognitive distortion in depression, diminished emotion regulation in depression, and is in line with decreased activity of the dlPFC in functional imaging studies of depression (see Drevets, 1998, and Mayberg, 2003, for comprehensive reviews). The presently observed increase of activity at bilateral dlPFC sites in the patient group after antidepressant treatment might thus reflect a recuperation of cognitive control (inhibition) over particularly negative emotion processing and therefore a normalization of the top-down control of the cortical on the limbic system as previously suggested by fMRI, PET, and MEG studies (e.g. Buchsbaum et al., 1997; Sperling et al., 2000; Kennedy et al., 2001; Mayberg, 2003). However, this interpretation is quite hypothetical and should in the future be tested by relating an increase of dlPFC activity with the efficacy of negative affect self-regulation.
The present finding of both right TPJ and frontal abnormality in depressed subjects is also in line with early evidence for altered activation in these brain regions in depressed patients and normalization with antidepressant pharmacological treatment, respectively. For instance, a classical study by Kronfol et al. (1978) observed poor performance on a variety of right hemispheric neuropsychological tests in depressed patients. Of note with respect to the present results, right hemispheric performance normalized after successful electroconvulsive treatment (Kronfol et al., 1978). In a study of hypnotic induction of depressed mood in healthy probands, Tucker et al. (1981) observed that the depressed mood involved activation (alpha suppression) over the right hemisphere specifically.
Limitations
Since all patients included in the pre-/post-therapy arm responded well to antidepressant pharmacotherapy, the design of the present study does not allow for differentiating brain activation patterns between responders and non-responders. Although the spherical shell conductivity model used here is a quite good approximation of the human head, especially at the fronto-parietal regions of interest, and circumvents the problem of necessary regularization of quasi-radial sources in realistic MEG head modeling (Steinsträter et al., 2010), identification of disorder-related activities at regions with less good spherical fits might benefit from realistic head modeling. Due to the limited depth resolution of MEG and the specific emphasis of the L2-MNE on cortical sources, we here disregarded the potential involvement of subcortical structures.
Summary and Outlook
The present study provides magnetoencephalographic evidence for dysfunctional parietal, temporoparietal, and dorsolateral prefrontal cortex activity to underlie distorted early emotional processing, potentially contributing to the pathogenesis and retention of major depression. In addition to a general flattening of responses, especially in the right hemispheric parietal and temporoparietal regions, simultaneous negative bias and positive attenuation effects were observed in the right parietal and especially the bilateral dlPFC areas. Antidepressant pharmacotherapy normalized the general hypoactivations, but did not affect the valence-specific biases of emotional processing.
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest related to the present manuscript.
Dr Arolt declares that over the last 3 years he has received compensations for his contributions as member of the advisory boards and for presentations from the following companies: Astra-Zeneca, Eli Lily, Janssen-Organon, Lundbeck, Otsuka, Servier, and Trommsdorff. These cooperations have no relevance to the work that is covered in the manuscript.
Dr Kugel declares that over the last 3 years he has received consultation fees from MR:comp GmbH, Testing Services for MR Safety. This cooperation has no relevance to the work that is covered in the manuscript.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, SFB-TRR58-C01).
References
- Adolphs R (2002) Neural systems for recognizing emotion. Curr Opin Neurobiol 12:169–177. [DOI] [PubMed] [Google Scholar]
- Amting JM, Greening SG, Mitchell DG. (2010) Multiple mechanisms of consciousness: the neural correlates of emotional awareness. J Neurosci 30:10039–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Clasen P, Stice E, Schnyer D. (2010) Depression symptoms and cognitive control of emotion cues: a functional magnetic resonance imaging study. Neuroscience 167:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. (2004) Parietal cortex and attention. Curr Opin Neurobiol 14:212–217. [DOI] [PubMed] [Google Scholar]
- Bistricky SL, Atchley RA, Ingram R, O’Hare A. (2014) Biased processing of sad faces: an ERP marker candidate for depression susceptibility. Cogn Emot 28:470–492. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, McGrath PJ, Quitkin FM. (1997) Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol Psychiatry 41:939–948. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Warner V, Weissman MM. (2007) Grandchildren at high and low risk for depression differ in EEG measures of regional brain asymmetry. Biol Psychiatry 62:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C. (1997) Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 41:15–22. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. (2000) Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3:292–297. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. (2000) Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull 126:890–909. [DOI] [PubMed] [Google Scholar]
- Deldin PJ, Keller J, Gergen JA, Miller GA. (2000) Right-posterior face processing anomaly in depression. J Abnorm Psychol 109 (1):116–121. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. (2011) Experimental and theoretical approaches to conscious processing. Neuron 70:200–227. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. (1995) Neural mechanisms of selective visual attention. Annu Rev Neurosci 18:193–222. [DOI] [PubMed] [Google Scholar]
- Drevets WC. (1998) Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med 49:341–361. [DOI] [PubMed] [Google Scholar]
- Drevets WC. (2007) Orbitofrontal cortex function and structure in depression. Ann NY Acad Sci 1121:499–527. [DOI] [PubMed] [Google Scholar]
- Everaert J, Koster EH, Derakshan N. (2012) The combined cognitive bias hypothesis in depression. Clin Psychol Rev 32 (5):413–424. [DOI] [PubMed] [Google Scholar]
- Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. (2005) Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol 93 (5):2864–2872. [DOI] [PubMed] [Google Scholar]
- Geng JJ, Mangun GR. (2011) Right temporoparietal junction activation by a salient contextual cue facilitates target discrimination. Neuroimage 54:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. (2008) Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry 63:369–376. [DOI] [PubMed] [Google Scholar]
- Gruber O, Diekhof EK, Kirchenbauer L, Goschke T. (2010) A neural system for evaluating the behavioural relevance of salient events outside the current focus of attention. Brain Res 1351:212–221. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. (1994) Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput 32:35–42. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Cowen PJ. (2013) ‘It’s the way that you look at it’--a cognitive neuropsychological account of SSRI action in depression. Phil Trans R Soc B 368 (1615):20120407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. (1991) Left frontal hypoactivation in depression. J Abnorm Psychol 100:535–545. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Rockstroh B. (2000) Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology 37:523–532. [PubMed] [Google Scholar]
- Junghöfer M, Bradley MM, Elbert TR, Lang PJ. (2001) Fleeting images: a new look at early emotion discrimination. Psychophysiology 38:175–178. [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JE, Quitkin FM. (2000) Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: differences between depressed patients and healthy adults in P3 amplitude and asymmetry. Int J Psychophysiol 36 (3):211–236. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. (2005) The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 58:843–853. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Krüger S, Mayberg HS, Meyer JH, McCann S, et al. (2001) Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psych 158:899–905. [DOI] [PubMed] [Google Scholar]
- Krompinger JW, Simons RF. (2009) Electrophysiological indicators of emotion processing biases in depressed undergraduates. Biol Psychology 81:153–163. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Hamsher KD, Digre K, Waziri R. (1978) Depression and hemispheric functions: changes associated with unilateral ECT. Br J Psychiatry 132:560–567. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. (2005) International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical report A-6. Gainesville, FL: University of Florida. [Google Scholar]
- Light SN, Heller AS, Johnstone T, Kolden GG, Peterson MJ, Kalin NH, Davidson RJ. (2011) Reduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepressant treatment in major depressive disorder. Biol Psychiatry 70:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. (2000) Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry 48:30–42. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. (2007) Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. (1997) Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9:471–481. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. (2003) Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S. (2000) Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48:830–843. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Moratti S, Rubio G, Campo P, Keil A, Ortiz T. (2008) Hypofunction of right temporoparietal cortex during emotional arousal in depression. Arch Gen Psychiatry 65:532–541. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. (1993) Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention. Arch Neurol 50:279–284. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. (2008) Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci 17:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyk P, De Cesarei A, Junghöfer M. (2011) ElectroMagnetoEncephalography software: overview and integration with other EEG/MEG toolboxes. Comput Intell Neurosci 2011:861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. (2005) A framework for targeting alternative brain regions with repetitive transcranial magnetic stimulation in the treatment of depression. J Psychiatry Neurosci 30:91–97. [PMC free article] [PubMed] [Google Scholar]
- Shestyuk AY, Deldin PJ, Brand JE, Deveney CM. (2005) Reduced sustained brain activity during processing of positive emotional stimuli in major depression. Biol Psychiatry 57:1089–1096. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. (2010) Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30:3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W, Martus P, Alschbach M. (2000) Evaluation of neuronal effects of electroconvulsive therapy by magnetoencephalography (MEG). Prog Neuropsycho-pharmacol Biol Psychiatry 24:1339–1354. [DOI] [PubMed] [Google Scholar]
- Steinsträter O, Sillekens S, Junghoefer M, Burger M, Wolters CH. (2010) Sensitivity of beamformer source analysis to deficiencies in forward modeling. Hum Brain Mapp 31 (12):1907–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Towers DN, Coan JA, Allen JJB. (2011) The oft-neglected role of parietal EEG asymmetry. Psychophysiology 48:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, El-Hage W, Dalgleish T, Radua J, Gohier B, Phillips ML. (2010) Depression is associated with increased sensitivity to signals of disgust: a functional magnetic resonance imaging study. J Psychiatr Res 44:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Manna CG, Fekri S, Kroppmann CJ, Schaller JD, Alschuler DM, Stewart JW, McGrath PJ, Bruder GE. (2011) Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biol Psychiatry 70:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. (2006) Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J Abnorm Psychol 115:715–729. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Stenslie CE, Roth RS, Shearer SL. (1981) Right frontal lobe activation and right hemisphere performance. Decrement during a depressed mood. Arch Gen Psychiatry 38:169–174. [DOI] [PubMed] [Google Scholar]