Abstract
Background:
Leptin, an adipose-derived hormone, has been implicated in emotional regulation. We have previously shown that systemic administration of leptin produces anxiolytic-like effects and deletion of the leptin receptor, LepRb, in midbrain dopamine neurons leads to an anxiogenic phenotype. This study investigated whether activation or deletion of LepRb in the ventral tegmental area of adult mice is capable of inducing anxiolytic and anxiogenic effects, respectively.
Methods:
Mice were cannulated in the ventral tegmental area and received bilateral intra-ventral tegmental area infusions of leptin or the JAK2/STAT3 inhibitor AG490. Anxiety-like behaviors were assessed using the elevated plus-maze, light-dark box, and novelty suppressed feeding tests. Deletion of LepRb in the ventral tegmental area was achieved by bilateral injection of AAV-Cre into the ventral tegmental area of adult Leprflox/flox mice. Anxiety-related behaviors were evaluated 3 weeks after viral injection.
Results:
Intra-ventral tegmental area infusions of leptin reduced anxiety-like behaviors, as indicated by increased percent open-arm time and open-arm entries in the elevated plus-maze test, increased time spent in the light side and decreased latency to enter the light side of the light-dark box, and decreased latency to feed in the novelty suppressed feeding test. Blockade of JAK2/STAT3 signaling in the ventral tegmental area by AG490 attenuated the anxiolytic effect produced by systemic administration of leptin. Leprflox/flox mice injected with AAV-Cre into the ventral tegmental area showed decreased leptin-induced STAT3 phosphorylation and enhanced anxiety-like behaviors in the elevated plus-maze test and the novelty suppressed feeding test.
Conclusions:
These findings suggest that leptin-LepRb signaling in the ventral tegmental area plays an important role in the regulation of anxiety-related behaviors.
Keywords: Leptin receptor, JAK2/STAT3, elevated plus-maze test, light-dark box, novelty suppessed feeding
Introduction
The adipocyte-derived hormone leptin is a pleiotropic hormone that affects multiple physiological processes, including appetite, body weight, neuroendocrine function, and emotional behaviors (Bjorbaek et al., 1998; Friedman and Halaas, 1998; Schwartz et al., 2000; Lu et al., 2006; Lu, 2007; Liu et al., 2010, 2011; Guo et al., 2012, 2013; Guo and Lu, 2014; Wang et al., 2015). Previous studies have shown that systemic administration of leptin elicits anxiolytic-like effects in mice (Liu et al., 2010) and rats (Haque et al., 2013). By contrast, leptin-deficient (ob/ob) mice exhibit increased anxiety behaviors in multiple behavioral tests (Asakawa et al., 2003; Finger et al., 2010). Moreover, clinical research reported that plasma leptin levels are correlated with anxiety states (Lawson et al., 2012; Yoshida-Komiya et al., 2014). These studies support an important role for leptin in the regulation of anxiety-related behaviors.
Leptin exerts its biological effects via the full-length functional leptin receptor (LepRb). Leptin binding to LepRb initiates a cascade of signaling events involving activation of janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3), phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) pathways (Bjorbaek et al., 1997; Yamashita et al., 1998; Myers, 2004). Within the brain, LepRb expression has been observed in limbic brain structures such as the hippocampus, prefrontal cortex, and ventral tegmental area (VTA) (Elmquist et al., 1998; Scott et al., 2009; Guo et al., 2013). Deletion of LepRb specifically in the hippocampus or in glutamate neurons located in the hippocampus and prefrontal cortex causes a depressive-like phenotype, but not an anxiety phenotype (Guo et al., 2012; Guo et al., 2013). Dopamine neurons in the midbrain are direct targets for leptin (Figlewicz et al., 2003; Fulton et al., 2006; Hommel et al., 2006; Scott et al., 2009; Leshan et al., 2010; Liu et al., 2011). We have demonstrated that ablation of LepRb in dopamine neurons results in a robust anxiogenic phenotype (Liu et al., 2011). LepRb-expressing dopamine neurons in the midbrain are found in both the VTA and substantia nigra (Scott et al., 2009). It has been reported that neurotoxin lesions of dopamine neurons in the substantia nigra or tetrodotoxin inactivation of this region results in increased anxiety behavior in the elevated plus-maze and abnormal fear responses (Baldi et al., 2007; Wang et al., 2007; Baldi and Bucherelli, 2010; Ho et al., 2011). Therefore, the specific role of VTA LepRb in the regulation of anxiety remains to be clarified.
In the present study, we assessed the effects of activation of LepRb in the VTA by local infusion of leptin on anxiety-related behaviors. The underlying molecular mechanisms of leptin action on anxiety were explored. Furthermore, we examined the influence of adeno-associated virus (AAV)-Cre-mediated genetic deletion of LepRb in the VTA on anxiety-related behaviors.
Methods
Animals
Adult male C57BL/6J mice (8 weeks) were purchased from the Jackson Laboratory (Bar Harbor, ME) and allowed to acclimate for at least 1 week before the experimental procedures. Leprflox/flox mice on a 129-C57BL/6J-FVB mixed background were obtained from Dr. Streamson Chua (Albert Einstein College of Medicine, Bronx, NY) (McMinn et al., 2004) and maintained by inbreeding for at least 6 generations. All mice were housed in groups of 5 under a 12-h-light/-dark cycle (lights on at 7:00 am) with ad libitum access to food and water except during behavioral tests. All animal procedures were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Behavioral Procedures
Behavioral tests were performed on male C57BL/6J mice (10–12 weeks old) and male Leprflox/flox mice (11–14 weeks old) during the late light phase between 2:00 and 5:00 pm. On the test day, animals were separated into individual cages, transferred to the testing room, and habituated to the room conditions for 3 to 4 hours prior to beginning behavioral experiments. After each test session, the apparatus was thoroughly cleaned with 20% alcohol to eliminate the odor and trace of the previously tested animal. All behaviors were scored and analyzed by the experimenters who were blind to the treatments or genotypes.
Elevated Plus-Maze Test
This test is a widely used anxiety paradigm, which is based on the natural conflict between the drive to explore a new environment and the tendency to avoid a potentially dangerous area (Rodgers and Dalvi, 1997). The elevated plus-maze was made of white acrylic, with 4 arms (30cm long and 5cm wide) arranged in the shape of a “plus” sign and elevated to a height of 70cm from the floor. Two arms have no side or end walls (open arms). The other 2 arms have side walls and end walls (12cm high) but are open on top (closed arms). The open and closed arms intersect, having a central 5×5-cm square platform giving access to all arms. Mice were placed in the central square facing the corner between a closed arm and an open arm and allowed to explore the elevated plus-maze for 5 minutes. Their behaviors on the elevated plus-maze were recorded by a CCD camera positioned directly above the elevated plus-maze. The time spent on the open and closed arms and the number of entries made into each arm were scored. Entry was defined as all 4 paws being positioned within 1 arm. The degree of anxiety was assessed by calculating the percentage of open arm entries (entries into the open arms/total entries into all arms) and percentage of open arm time (time spent in the open arms/total time spent in all arms).
Light-Dark Box Test
This test is based upon a conflict between the innate aversion to brightly illuminated areas and the spontaneous exploratory activity (Crawley and Goodwin, 1980). The apparatus consisted of a polypropylene cage (45 × 27 × 30cm) separated into 2 compartments by a partition, with a rectangular opening (7 × 7cm) at floor level. The larger compartment (27 × 27cm) was open-topped, transparent, and brightly lit (900 lux). The smaller compartment (18×27cm) had black painted sides and was covered at the top with black Plexiglas. For each test, the mouse was placed in the center of the dark compartment facing away from the opening. The latency to the light side, time spent in the light compartment, and the number of transitions between 2 compartments was recorded for 5 minutes.
Novelty Suppressed Feeding Test
The novelty suppressed feeding test is a behavioral model of anxiety based on the conflict between hunger and aversion to a brightly lit, novel environment. Mice were food deprived for 24 hours prior to the test. At the time of testing, one food pellet was placed on a white filter paper located in the middle of an open field (60 × 60 × 40cm) covered with 2cm of fresh bedding. Each mouse was placed in one corner and allowed to explore for a maximum of 10 minutes. The latency to begin eating the food pellet was recorded. Mice were immediately removed from the open field and transferred back to its home cage. The amount of food consumption for 5 minutes was measured.
Locomotor Activity
The locomotor activity was measured using the open field locomotor system (Omnitech Electronics Inc, Columbus, OH). The test apparatus consisted of an open field box (40 × 40 × 30cm) made of transparent acrylic surrounded by 3 sets of 16 photobeam arrays in the horizontal x and y axes as well as in the ventricle z axis. Locomotor movements were determined by breaks in photobeams and converted into distance traveled with the Fusion Software. Total distance traveled for 15 minutes was analyzed using the Fusion Software (Omnitech Electronics).
Cannulation and Microinjection
Adult male C57BL/6J mice were anesthetized with 43mg/kg ketamine, 9mg/kg xylazine, and 1.4mg/kg acepromazine in saline. A 26-gauge, stainless-steel, double-guide cannula (C235G; Plastics One) was stereotaxically implanted 2mm above the VTA (coordinates relative to bregma: AP, 3.4mm; ML, ± 0.4mm, and DV, 4.8mm from the surface of the skull). A stainless-steel dummy cannula, extending 0.5mm beyond the guide cannula tip, was used to seal the guide cannula when not in use.
After surgery, the animals were housed individually to avoid damage to guide and dummy cannula. Animals were allowed to recover for 7 days and handled daily to minimize stress caused by the microinjection procedure. On the experimental day, a bilateral injection cannula (33 gauge) was inserted into the guide cannula, extending 2mm beyond the tip of the guide cannula. Based on the effective doses of intra-VTA leptin injection on intracellular signaling and feeding behavior (Hommel et al., 2006; Morton et al., 2009; Verhagen et al., 2011; Bruijnzeel et al., 2013), we chose 2 different doses, 0.05 μg and 0.5 μg per side, to determine the effect of intra-VTA infusion of leptin on anxiety-related behaviors. Recombinant mouse leptin (R&D Systems, Inc., Minneapolis, MN) was freshly dissolved in artificial cerebrospinal fluid (aCSF; CaCl2·2H2O 0.133g/L, MgCl2·6H2O 0.1g/L, KCl 0.2g/L, KH2PO4 0.2g/L, NaCl 8.0g/L, Na2HPO4 1.15g/L) before use. The JAK2/STAT3 inhibitor, AG490 (Selleck Chemicals, Houston, TX), was solubilized in dimethyl sulfoxide. Leptin, AG490, or vehicle in a volume of 0.2 μL were bilaterally infused into the VTA for 2 minutes at a speed of 0.1 μL/min by using an infusion pump through a 5-µL syringe connected to the injection cannula. The injector was held in place for an additional minute after the end of infusion to avoid backflow. Mice were subjected to the elevated plus-maze, light-dark box, novelty suppressed feeding, or locomotor activity tests 30 minutes after intra-VTA microinjection. All microinjections were performed on conscious, unrestrained, freely moving mice in their home cages.
At the end of the experiments, mice were anesthetized, and Indian ink was bilaterally infused into the VTA using the same microinjection procedure as described above. Thirty minutes following ink injection, animals were killed by decapitation and coronal brain sections were cut and stained with toluidine blue. Location of the microinjection cannula tips and the locus of microinjection were identified under light microscopy. Mice with improper cannula placement were excluded from the statistical analysis.
AAV-Cre-Mediated Deletion of LepRb in the VTA of Adult Leprflox/flox Mice
An AAV-Cre/loxP system was used to achieve region-specific deletion of LepRb using AAV2-mediated Cre recombinase expression in adult Leprflox/flox mice, in which exon 17, a critical exon involved in LepRb signaling, is floxed (McMinn et al., 2004). AAV2 has the advantages of high neurotropism and restricted transduction (Tenenbaum et al., 2004). AAV2 was used to mediate expression of the Cre-GFP fusion protein and GFP. The viral vectors were constructed and produced as described in previous studies (Guo et al., 2013). AAV-Cre-GFP and AAV-GFP viruses (with titers of 1 × 1012 infectious units/mL) were injected bilaterally into the VTA of Leprflox/flox mice (coordinates relative to bregma: AP, -3.2mm; ML, ± 1.0mm; DV, -4.6mm from the surface of the skull, at an angle of 7.5° with the sagittal plane). A volume of 0.5 µL of AAV vectors was delivered with a slow injection rate (1.0 µL/10min) through a 33-gauge stainless-steel microinjector attached to a digital stereotaxic arm and connected to an infusion pump. Because AAV-mediated gene transfer became evident 2 weeks after injection, mice were tested for anxiety-like behaviors 3 weeks after intra-VTA injection of AAV-Cre-GFP and AAV-GFP. The elevated plus-maze, light-dark box, and novelty suppressed feeding tests were performed as described above.
At the end of experiments, mice were food deprived overnight and received an i.p. injection of leptin (5mg/kg). Mice were transcardially perfused 2 hours after the injection. Brains were removed, postfixed overnight, and then cryoprotected in 30% sucrose and cut into 40-µm-thick coronal sections. Injection sites and AAV transduction in the VTA were verified by examining GFP expression. Mice with missed injections were excluded from statistical analysis. The effectiveness of AAV-Cre–mediated LepRb deletion in the VTA was examined by assessing leptin-induced p-STAT3. Free-floating sections were pretreated with 1% NaOH, 1% H2O2, 0.3% glycine, blocked by using blocking buffer containing 1% bovine serum albumin, 0.3% normal goat serum, 0.3% Triton X-100, then incubated with a rabbit anti-GFP antibody (1:1000, Chemicon Inc., Temecula, CA) or a rabbit anti-pSTAT3 (Tyr705) antibody (1:1000, Cell Signal Technology, Danvers, MA) for 48 hours at 4°C. Sections were then rinsed and incubated with a secondary biotinylated anti-rabbit antibody (1:1000; Vector Laboratories, Burlingame, CA), labeled with avidin-biotin complex, and then stained with nickel-enhanced diaminobenzidine to visualize the immunoreactivity for GFP or p-STAT3. Every sixth section spaced 240 μm apart throughout the rostral/caudal extent of the VTA was used to access the number of p-STAT3–positive cells. The number of p-STAT3–positive cells was multiplied by 6 to obtain the total number of cells.
Statistical Analysis
Results are expressed as mean ± SEM. Statistical analyses were performed using 1-way ANOVA on leptin treatment with multiple doses and 1-way ANOVA with repeated measures on locomotor activity and body weight, followed by Bonferroni/Dunn posthoc comparisons. Two-tailed Student’s t test was used to compare 2 treatment group means. P < .05 was considered statistically significant.
Results
Effects of Intra-VTA Infusion of Leptin on Anxiety-Related Behaviors
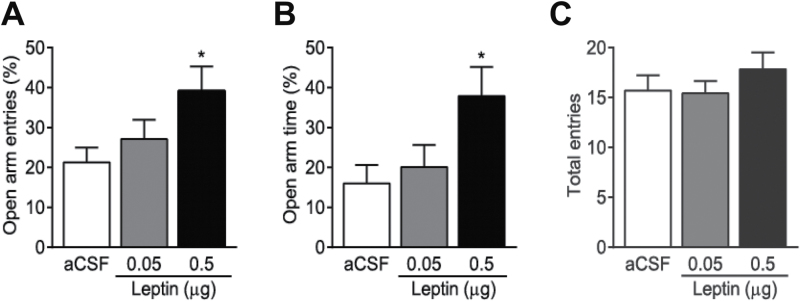
We performed 3 behavioral tests to assess the effects of leptin in the VTA on anxiety-related behaviors. First, the effects of intra-VTA infusions of different doses of leptin on anxiety were examined in the elevated plus-maze test. Thirty-nine mice were weighed and counter-balanced into 3 treatment groups that received bilateral intra-VTA microinjection of aCSF (n = 14), 0.05 µg/side (n = 12), or 0.5 µg/side (n = 13) leptin. Thirty minutes after infusion of leptin or aCSF into the VTA of freely moving mice, mice were allowed to explore on the elevated plus maze for 5 minutes. We found that leptin produced a significant main effect on the percentage of open arm entries [F(2, 36) = 3.493; P < .05] and the percentage of open arm time [F(2, 36) = 3.910; P < .05]. Posthoc comparisons revealed that leptin at the dose of 0.5 µg/side, but not at the lower dose, 0.05 µg/side, induced a significant increase in the percentage of open arm entries (P < .05) and the percentage of open arm time (P < .05) (Figure 1A-B), suggesting a dose-dependent effect. Leptin at either dose had no significant effect on the number of total arm entries [F(2,36) = 0.77; P = .472] (Figure 1C), suggesting that locomotor activity in this test is not altered. Because 0.5 µg/side leptin was effective in the elevated plus maze test, this dosage was chosen to assess the effects of leptin on anxiety in the following behavioral tests.
Figure 1.
Intra-ventral tegmental area (VTA) infusion of leptin dose-dependently induces anxiolytic effects in the elevated plus-maze test. Mice received an intra-VTA infusion of leptin (0.05, 0.5 μg/side) or vehicle (artificial cerebrospinal fluid [aCSF]) 30 minutes before the test. (A) Percent open arm entries. (B) Percent open arm time. (C) The number of total entries made into open and closed arms. n = 12 to 14 each group. *P < .05, compared with the aCSF-injected control group.
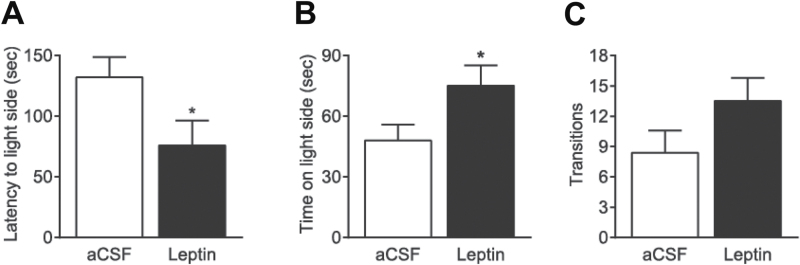
Sixteen mice were weighed and counter-balanced into 2 treatment groups that received aCSF (n = 8) or leptin (0.5 µg/side; n = 8). Thirty minutes after infusion of leptin or aCSF into the VTA, mice were subjected to the light-dark test. Individual mice were placed in the center of the dark compartment facing away from the opening to the light side and allowed to explore for 5 minutes. Intra-VTA infusion of leptin (0.5 µg/side) significantly decreased the latency to enter the light compartment (t (14) = 2.115; P = .05) and increased the total time spent in the light compartment (t (14) = 2.125; P = .05) (Figure 2A-B) but did not affect the number of transitions between the light and dark compartments (t (14) = 1.612; P = 0.129) (Figure 2C).
Figure 2.
Effects of intra-ventral tegmental area (VTA) infusion of leptin on anxiety behavior in the light-dark box test. Mice received an intra-VTA infusion of leptin (0.5 μg/side) or vehicle (artificial cerebrospinal fluid [aCSF]) 30 minutes before the test. The latency to enter the light side (A), total time spent in the light side (B), and number of transition between light and dark compartments (C) were measured during the 5-minute test. n = 8 each group. *P < .05, compared with the aCSF-injected control group.
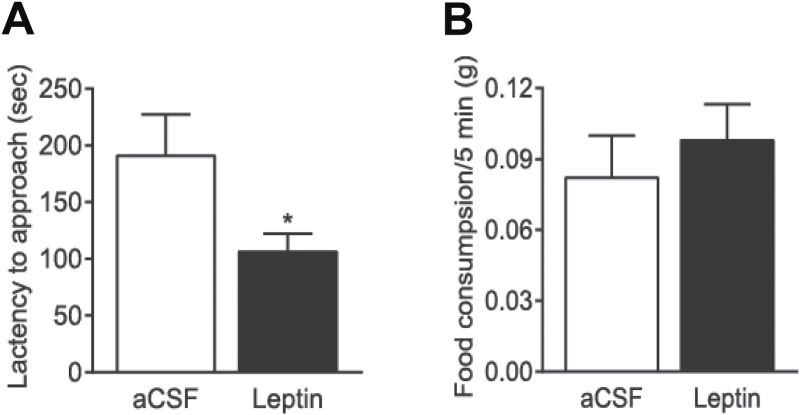
The novelty suppressed feeding test assesses anxiety by measuring the latency of a fasted animal to approach and eat a familiar food in a novel environment (Shephard and Broadhurst, 1982). Following 24-hour food deprivation, mice were weighed and counter-balanced into 2 treatment groups that received a bilateral intra-VTA microinjection of aCSF (n = 6) or leptin (0.5 µg/side; n = 7). Thirty minutes after infusion of leptin or aCSF, mice were placed in one corner of an open-field arena with one pellet of regular mouse food in the center. Leptin significantly decreased the latency to feed (t (11) = 2.243; P = .05), with no significant effect on home-cage food consumption during a 5-minute period (t (11) = 0.6818; P = .5095) (Figure 3).
Figure 3.
Effects of intra-ventral tegmental area (VTA) infusion of leptin on anxiety behavior in the novelty suppressed feeding test. Mice fasted for 24 hours received an intra-VTA infusion of leptin (0.5 μg/side) or vehicle (artificial cerebrospinal fluid [aCSF]) 30 minutes before the test. (A) The latency to feed. (B) Home-cage food consumption within 5 minutes immediately after the test. n = 6–7 each group. * P < .05 compared with the aCSF-injected control group.
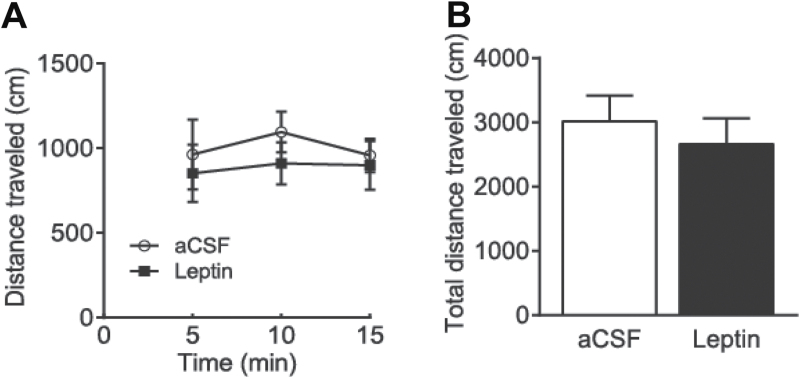
The effects of intra-VTA infusions of leptin (0.5 µg/side) on locomotor activity were evaluated by using the open field locomotor system. Twelve mice were weighed and counter-balanced into 2 treatment groups that received aCSF (n = 6) or leptin (0.5 µg/side; n = 6). Thirty minutes after infusion of leptin or aCSF, total distance traveled was measured for 15 minutes. Distance traveled in 5-minutes bins was analyzed. ANOVA with repeated measures revealed no significant treatment effect on distance traveled (F(1,35) = 0.392, P = .545). Total distance traveled in 15 minutes was not different between vehicle-treated and leptin-treated groups (t (10) = 0.626, P = .5453) (Figure 4).
Figure 4.
Effects of intra-ventral tegmental area (VTA) infusion of leptin on locomotor activity. Mice received an intra-VTA infusion of leptin (0.5 μg/side) or vehicle (artificial cerebrospinal fluid [aCSF]) 30 minutes before the locomotion test. (A) The distance traveled in 5-minute bins. (B) Total distance traveled within the 15 minutes. n = 6 each group.
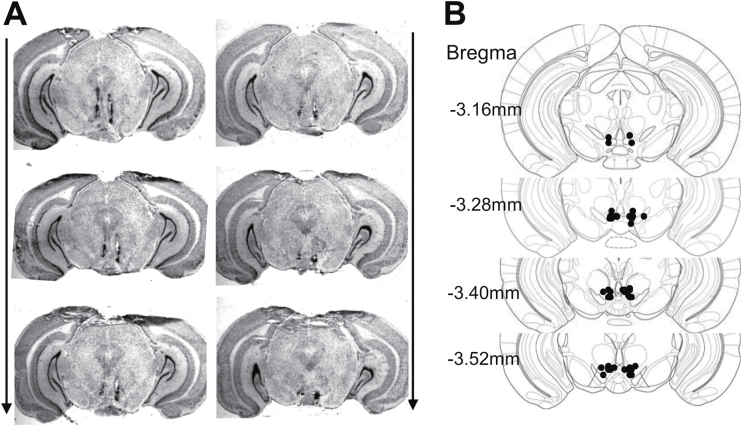
The microinjection sites were confirmed histologically in all mice that were used for behavioral tests described above (Figure 5). Mice with misplaced cannula were excluded from data analysis.
Figure 5.
Histological verification of intra-ventral tegmental area (VTA) injection sites. (A) Representative images showing the deposition sites within the VTA. Arrows indicated the rostral-caudal sequence of the coronal sections. (B) Schematic illustration of bilateral injection sites in the VTA. The drawings of coronal sections were derived from the atlas of Paxinos and Franklin (2001).
Effects of Intra-VTA Infusions of AG490 on Leptin-Induced Anxiolytic Effects
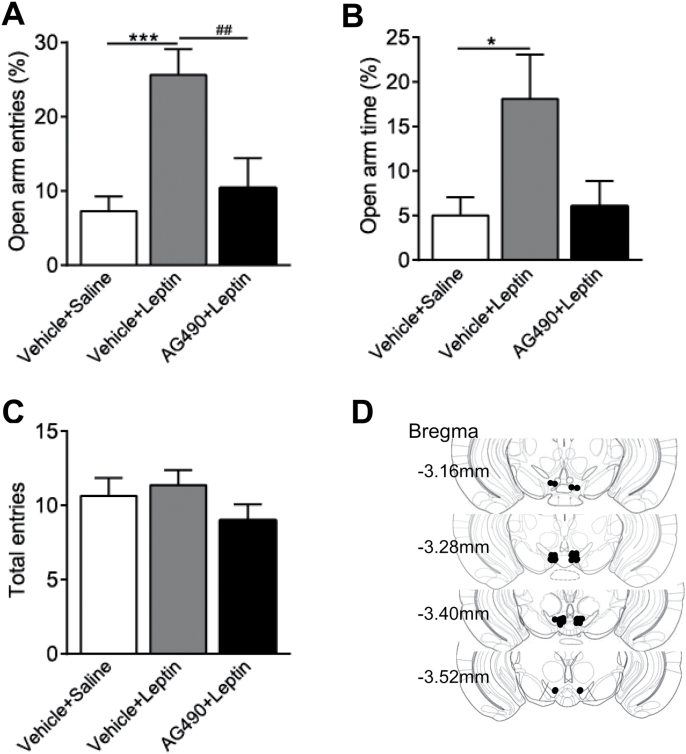
To determine possible molecular mechanisms underlying leptin action on anxiety-related behaviors, mice received bilateral intra-VTA infusions of the JAK2/STAT3 inhibitor, AG490 (0.15 nmol/side), or vehicle (1.67% dimethyl sulfoxide). The dose of AG490 was selected based upon previous reports showing that AG490 at a dose range of 0.01–1.0 nmol is effective to block the central effects of leptin (Morrison et al., 2007; Morton et al., 2009; Roman et al., 2010). Sixty minutes after intra-VTA microinjection, mice were injected with saline or leptin (1mg/kg, i.p.) (Liu et al., 2010). The elevated plus-maze test was performed 30 minutes after leptin injection. Statistical analysis revealed a significant effect of drug treatment on the percentage of open arm entries [F(2,34) = 9.495, P < .001] (Figure 6A) and the percentage of open arm time [F(2,34) = 3.745, P < .05] (Figure 6B). Posthoc analyses showed that leptin treatment significantly increased the percentage of open arm entries; this effect was attenuated by pretreatment with AG490 in the VTA (P < .05). These data indicate that blockade of JAK2/STAT3 signaling in the VTA was able to inhibit leptin’s anxiolytic effects (Figure 6).
Figure 6.
Effects of intra-ventral tegmental area (VTA) infusion of AG490 on leptin’s anxiolytic effects in the elevated plus-maze test. Mice received an intra-VTA infusion of AG490 (0.15 nmol/side) or vehicle 60 minutes before an i.p. injection of leptin (1mg/kg) or saline. The elevated plus-maze test was performed 30 minutes after leptin injection. (A) Percent open arm entries. (B) Percent open arm time. (C) The number of total entries made into open and closed arms. n = 10 to 14 each group. ***P < .001, compared with the Vehicle+Saline control group; ## P < .01, compared with the Vehicle+Leptin group. (D) Schematic illustration of bilateral injection sites in the VTA.
Targeted Deletion of LepRb in the VTA Induces an Anxiogenic Phenotype
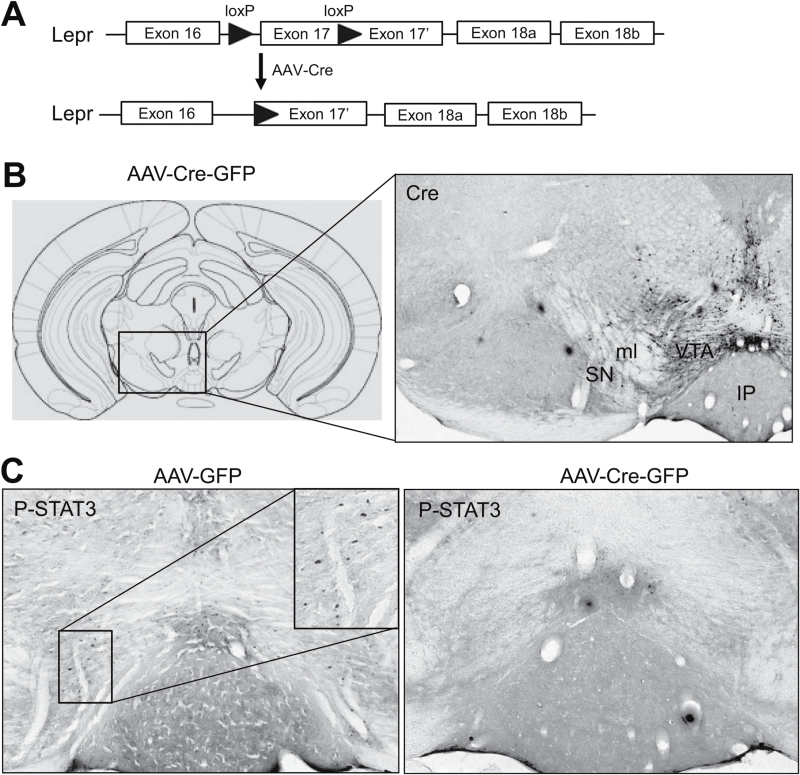
Adult male Leprflox/flox mice received bilateral infusion of AAV-Cre-GFP and AAV-GFP into the VTA (Figure 7A-B). To validate the functional loss of LepRb in the VTA, leptin-induced phosphorylation of STAT3 was evaluated using immunohistochemical staining. p-STAT3–positive cells were found in the VTA of AAV-GFP–injected mice. Only scattered cells showed positive immunostaining for p-STAT3 in the VTA of AAV-Cre-GFP–injected mice (Figure 7C). Cell counting analysis revealed that AAV-Cre-GFP injection greatly reduced the number of p-STAT3–positive cells, an indicator of functional LepRb expression, in the VTA compared to AAV-GFP injection (AAV-GFP: 490 ± 109; AAV-Cre-GFP: 148±26; t (8) = 3.052; P = .016). These data confirmed the loss of LepRb in the VTA induced by AAV-Cre-GFP in Leprflox/flox mice.
Figure 7.
Adeno-associated virus (AAV)-Cre–mediated deletion of leptin receptor (LepRb) in the intra-ventral tegmental area (VTA) of adult Leprflox/flox mice. (A) Schematic diagram depicting the floxed Lepr allele and the Lepr floxed allele after Cre recombination. (B) Left, schematic illustration of AAV injection (Paxinos and Franklin, 2001). Right, Cre-GFP expression in the VTA. (C) Immunohistochemical staining showing leptin-induced STAT3 phosphorylation in the VTA in AAV-GFP (left) and AAV-Cre-GFP (right) injected mice. IP, interpeduncular nucleus; ML, medial lemniscus; SN, substantia nigra.
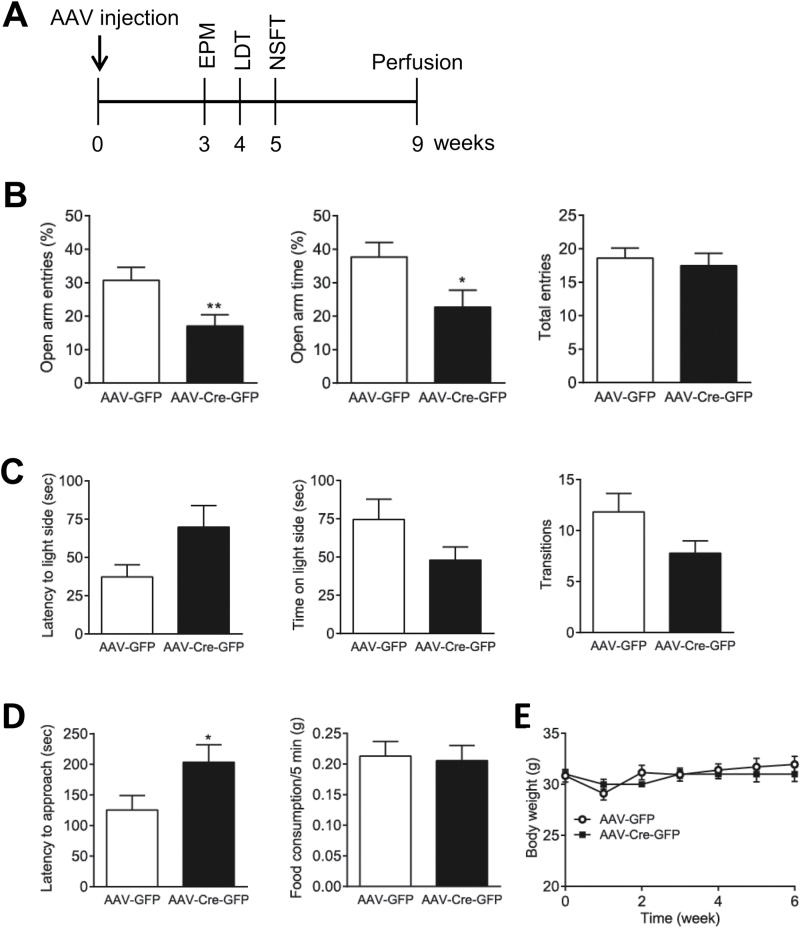
To evaluate the effects of LepRb deletion in the VTA on anxiety-related behaviors, mice were subjected to the elevated plus-maze test 3 weeks after AAV injection. Mice injected with AAV-Cre-GFP in the VTA displayed a decrease in the percentage of open arm entries (t (26) = 2.647; P = .01) and the percentage of open arm time (t (26) = 2.197; P = .04) without affecting the number of total arm entries (t (26) = 0.4711; P = .64). These observations in the elevated plus maze test suggest that LepRb deletion in the VTA causes anxiogenic-like behavior (Figure 8B). Moreover, in the light-dark box test, mice injected with AAV-Cre-GFP tended to explore the light compartment to a lesser extent than mice injected with AAV-GFP (latency to enter the light side: t (24) = 1.908; P = .07; time spent in the light side: t (24) = 1.738; P = .095; number of transitions between the light and dark compartments: t (24) = 1.900; P = .07) (Figure 8C). In the novelty suppressed feeding test, mice injected with AAV-Cre-GFP exhibited significantly longer latencies to feed than did the AAV-GFP–injected mice (t (25) = 2.045; P = .05), while home-cage food consumption in a period of 5 minutes immediately following the behavioral test was not different between 2 treatment groups (t (26) = 0.2202; P = .83) (Figure 8D). Body weight was monitored before and after intra-VTA microinjection of AAV-Cre-GFP and AAV-GFP and showed no difference between 2 treatment groups [F(1, 153) = 0.02, P = .88] (Figure 8E).
Figure 8.
Adeno-associated virus (AAV)-Cre–mediated leptin receptor (LepRb) deletion in the ventral tegmental area (VTA) results in anxiogenic-like behaviors. (A) Timeline of experimental procedures and behavioral tests. (B) Elevated plus-maze test. Left, percent open arm entries; middle, percent open arm time; right, number of total entries. n = 13 to 15 each group. (C) Light-dark box test. Left, latency to enter the light side; middle, time spent in the light compartment; right, number of transitions between light and dark compartments. n = 12 to 14 each group. (D) Novelty suppressed feeding test. Left, latency to feed; right, home-cage food consumption within 5 minutes immediately after the test. n = 12 to 15 each group. (E) Body weight before or after AAV injection. n = 10 to 13 each group. *P < .05; **P < .01 compared with the AAV-GFP control group.
Discussion
In the present study, we provided evidence that leptin-LepRb signaling in the VTA participates in mediating anxiety-related behaviors. Our previous studies have demonstrated that systemic administration of leptin produces anxiolytic effects and deletion of LepRb in dopamine neurons causes anxiogenic-like behaviors in mice (Liu et al., 2010, 2011). The present findings demonstrate that activation of LepRb in the VTA produces anxiolytic-like effects in multiple behavioral tests, and inactivation of LepRb in the VTA by a Cre-loxP system results in an anxiogenic phenotype. The Jak/STAT3 signaling pathway seems to mediate leptin action in the VTA on anxiety-related behaviors. Our findings emphasize the importance of leptin and its interaction with LepRb in the VTA in controlling emotional behaviors.
The VTA is an anatomically and functionally heterogeneous brain structure. It is well known that the VTA plays an important role in motivation and reward processing but is also involved in processing aversive stimuli (Bromberg-Martin et al., 2010; Ungless et al., 2010). First, stress, a major risk factor for the development of anxiety disorders, increases c-Fos expression in the VTA neurons, mainly in dopamine neurons (Deutch et al., 1991; Beck and Fibiger, 1995; Campeau et al., 1997; Morrow et al., 2000; Berton et al., 2006; Hoffman et al., 2013). It has been reported that a considerable number of dopamine neurons are excited by aversive stimuli (Anstrom and Woodward, 2005; Anstrom et al., 2009; Brischoux et al., 2009; Cao et al., 2010; Razzoli et al., 2011; Valenti et al., 2011). Second, stressful events are associated with mesolimbic dopamine release in the projection sites of VTA dopamine neurons, such as the nucleus accumbens and prefrontal cortex (Thierry et al., 1976; Abercrombie et al., 1989; Kalivas and Duffy, 1995; Butts et al., 2011). Third, fear reactions and anxiogenic responses can be provoked by electrical stimulation of VTA neurons (Stevens and Livermore, 1978), whereas lesions or inactivation of the VTA decreases anxiety-like behavior (Borowski and Kokkinidis, 1996; Munro and Kokkinidis, 1997; Gifkins et al., 2002; de Oliveira et al., 2006; Corral-Frias et al., 2013). The VTA contains predominantly dopaminergic neurons (65%), but GABAergic neurons (~30%) and glutamate neurons (~5%) have also been identified in this region (Swanson, 1982; Margolis et al., 2006; Nair-Roberts et al., 2008; Yamaguchi et al., 2011). Local administration of compounds influencing the dopaminergic, GABAergic, and glutamatergic systems in the VTA modulates anxiety-related behavior and fear responses. Intra-VTA infusions of dopamine D2/3 agonists, D1 antagonists, GABAA receptor agonists, or NMDA receptor antagonists have been reported to elicit anxiolytic effects in the elevated plus-maze test and decrease fear measured with fear-potentiated startle (Borowski and Kokkinidis, 1996; Munro and Kokkinidis, 1997; Gifkins et al., 2002; de Oliveira et al., 2006, 2009; Zarrindast et al., 2012; Corral-Frias et al., 2013). By contrast, local administration of GABAA receptor antagonists has been shown to cause anxiogenic effects in the elevated plus-maze test (Frye and Paris, 2009). Recently, it was demonstrated that stimulation and inhibition of dopamine vs GABA neurons in the VTA affect anxiety-related behaviors and aversive responses (Tan et al., 2012; van Zessen et al., 2012; Jennings et al., 2013; Danjo et al., 2014). These studies suggest that VTA neurons may serve as a neural substrate that modulates different aspects of aversive responses and anxiety-related behaviors.
In the present study, we demonstrated that infusions of leptin into the VTA reduced anxiety-like behaviors in the elevated plus maze, light-dark box, and novelty suppressed feeding tests. These anxiety tests are based on the conflict between innate drive to explore a novel environment or seek foods and aversion to bright illumination, spaciousness, and elevation. Anxiolytic effects in these tests results from either increased drive to explore or decreased aversion to the fearful conditions. In the novelty suppressed feeding test, leptin, as an appetite suppressant, would be expected to decrease the drive to feed in the open field. Indeed, infusions of leptin into the VTA have been reported to decrease food intake (Hommel et al., 2006; Morton et al., 2009; Bruijnzeel et al., 2013; Mietlicki-Baase et al., 2015). A decrease in latencies to feed following intra-VTA infusions of leptin may suggest that the appetite-suppressing effect of leptin is overridden by its fear-reducing, anxiolytic effect. Home-cage food consumption within 5 minutes immediately following the test was not altered by intra-VTA leptin infusion. This seems to be contradictory to the previous reports that leptin in the VTA inhibits food intake (Hommel et al., 2006; Morton et al., 2009; Bruijnzeel et al., 2013; Mietlicki-Baase et al., 2015). However, none of these studies have shown that leptin acutely decreases fasting-induced feeding within a short period of time (< 30 minutes) after intra-VTA infusion. Clinical data indicate that leptin influences energy metabolism but that it does not act as an immediate satiety factor (Sinha et al., 1996; Joannic et al., 1998; Licinio et al., 2014).
Our earlier studies have shown that inactivation of LepRb by deleting its coding exon 17 specifically in midbrain dopamine neurons using a transgenic mouse line, in which Cre recombinase expression is driven by the dopamine transporter promoter, causes a robust anxiogenic phenotype (Liu et al., 2011). The present study used an AAV-cre/loxP system to achieve the VTA-specific inactivation of LepRb in adult mice. The advantage of this approach is bypassing potential developmental effects of LepRb. Mice injected with AAV-Cre-GFP in the VTA exhibited increased anxiety-related behaviors in the elevated plus-maze and novelty suppressed feeding tests, suggesting that functional LepRb in the VTA is required for normal anxiety-like behaviors. We noticed that mice injected with AAV-Cre-GFP in the adult VTA showed less severe behavioral deficits than conditional knockout mice lacking LepRb in dopamine neurons induced by the Cre transgenic mouse line (Liu et al., 2011). This could be due to incomplete deletion in the VTA of mice injected with AAV-Cre-GFP and/or the contribution of LepRb outside the VTA in conditional LepRb knockout mice.
JAK2/STAT3 is a major signaling pathway stimulated by leptin in the VTA (Fulton et al., 2006; Hommel et al., 2006; Morton et al., 2009; Liu et al., 2011). To explore the molecular mechanisms underlying leptin action on anxiety, we examined the influence of the pretreatment with the JAK2/STAT3 inhibitor AG-490 in the VTA on systemic leptin injection-induced anxiolytic effects. We found that leptin-induced increase in the percentage of open arm entries in the elevated plus maze test was attenuated by intra-VTA infusions of AG-490. This finding suggests that leptin’s anxiolytic effect is mediated, at least in part, by the JAK2/STAT3 signaling pathway in the VTA. However, other leptin signaling pathways in the VTA may also participate in mediating leptin action on anxiety as AG-490 only partially blocked the effect of leptin.
Effects of leptin on anxiety-related behaviors in the VTA are likely to be mediated by inhibiting activity of dopamine neurons. We have previously shown that the majority of leptin-induced p-STAT3 occurs in dopamine neurons (Liu et al., 2011). Systemic administration of leptin decreases the frequency of dopamine neuron firing in the VTA of anesthetized rats (Hommel et al., 2006). Deletion of LepRb in dopamine neurons increases bust firing of dopamine neurons in the VTA of anesthetized mice (Liu et al., 2011). These studies, together with the current finding of anxiogenic behaviors in mice with LepRb deletion specifically in the VTA, support that VTA dopamine neurons serve as a neural substrate on which leptin acts to reduce anxiety-like behaviors through LepRb. The anxiolytic effects of leptin in the VTA could include both presynaptic and postsynaptic mechanisms. Recent electrophysiological studies have shown that leptin acts on presynaptic terminals, suppressing excitatory synaptic transmission onto VTA dopamine neurons (Thompson and Borgland, 2013). These studies suggest that leptin in the VTA can reduce activity of dopamine neurons, either by directly inhibiting neuron firing and/or indirectly by dampening excitatory input to dopamine neurons. These 2 mechanisms may occur simultaneously in mice receiving intra-VTA injection of leptin. On the other hand, because a moderate number of LepRb neurons (~30%) are nondopaminergic (Scott et al., 2009; Leshan et al., 2010; Liu et al., 2011), it is possible that GABAergic and glutamate neurons in the VTA may also participate in leptin actions on anxiety.
In summary, we have shown that activation and inactivation of LepRb in the VTA result in opposite changes in anxiety levels. Recent clinical studies have identified significant correlations between fluctuations in plasma leptin concentrations and emotional states (Licinio et al., 2014). Increased human leptin levels are suggested to promote positive feelings (Licinio et al., 2014). Our previous studies have shown that leptin regulates depression-related behaviors via interacting with LepRb in the hippocampus (Garza et al., 2012; Guo et al., 2012, 2013; Guo and Lu, 2014; Wang et al., 2015). The present study suggests that leptin regulates anxiety-related behaviors through LepRb in the VTA. Whether LepRb neurons in the VTA and hippocampus represent unique cell populations that participate in the distinct circuits governing depressive and anxious states remains to further elucidated in future studies.
Statement of Interest
None.
Acknowledgments
This work was supported by grants MH096251 and MH076929 from the National Institutes of Health (to X.-Y.L.) and by the Young Investigator Award (to J.L.) from National Alliance for Research on Schizophrenia and Depression and the National Natural Science Foudation of China (81301164, to M.G).
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. (1989) Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52:1655–1658. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. (2005) Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology 30:1832–1840. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. (2009) Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 161:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M. (2003) Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complications 17:105–107. [DOI] [PubMed] [Google Scholar]
- Baldi E, Bucherelli C. (2010) Substantia nigra, nucleus basalis magnocellularis and basolateral amygdala roles in extinction of contextual fear conditioning in the rat. Neurobiol Learn Mem 94:199–205. [DOI] [PubMed] [Google Scholar]
- Baldi E, Mariottini C, Bucherelli C. (2007) Substantia nigra role in fear conditioning consolidation. Neurobiol Learn Mem 87:133–139. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. (1995) Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci 15:709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Uotani S, da Silva B, Flier JS. (1997) Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272:32686–32695. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. (1998) Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1:619–625. [DOI] [PubMed] [Google Scholar]
- Borowski TB, Kokkinidis L. (1996) Contribution of ventral tegmental area dopamine neurons to expression of conditional fear: effects of electrical stimulation, excitotoxin lesions, and quinpirole infusion on potentiated startle in rats. Behav Neurosci 110:1349–1364. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. (2009) Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A 106:4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68:815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Qi X, Corrie LW. (2013) Anorexic effects of intra-VTA leptin are similar in low-fat and high-fat-fed rats but attenuated in a subgroup of high-fat-fed obese rats. Pharmacol Biochem Behav 103:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts KA, Weinberg J, Young AH, Phillips AG. (2011) Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci U S A 108:18459–18464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. (1997) Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience 78:1087–1104. [DOI] [PubMed] [Google Scholar]
- Cao JL, Covington HE, 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH. (2010) Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci 30:16453–16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Frias NS, Lahood RP, Edelman-Vogelsang KE, French ED, Fellous JM. (2013) Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacology 38:350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13:167–170. [DOI] [PubMed] [Google Scholar]
- Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S. (2014) Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc Natl Acad Sci U S A 111:6455–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira AR, Reimer AE, Brandao ML. (2006) Dopamine D2 receptor mechanisms in the expression of conditioned fear. Pharmacol Biochem Behav 84:102–111. [DOI] [PubMed] [Google Scholar]
- de Oliveira AR, Reimer AE, Brandao ML. (2009) Role of dopamine receptors in the ventral tegmental area in conditioned fear. Behav Brain Res 199:271–277. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. (1991) Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex 1:273–292. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. (1998) Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547. [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. (2003) Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964:107–115. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. (2010) Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology (Berl) 210:559–568. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. (1998) Leptin and the regulation of body weight in mammals. Nature 395:763–770. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ. (2009) Infusions of bicuculline to the ventral tegmental area attenuates sexual, exploratory, and anti-anxiety behavior of proestrous rats. Pharmacol Biochem Behav 93:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. (2006) Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822. [DOI] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. (2012) Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Mol Psychiatry 17:790–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifkins A, Greba Q, Kokkinidis L. (2002) Ventral tegmental area dopamine neurons mediate the shock sensitization of acoustic startle: a potential site of action for benzodiazepine anxiolytics. Behav Neurosci 116:785–794. [PubMed] [Google Scholar]
- Guo M, Lu XY. (2014) Leptin receptor deficiency confers resistance to behavioral effects of fluoxetine and desipramine via separable substrates. Transl Psychiatry 4:e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, Lu B, Lu XY. (2012) Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transl Psychiatry 2:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Huang TY, Garza JC, Chua SC, Lu XY. (2013) Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int J Neuropsychopharmacol 16:857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque Z, Akbar N, Yasmin F, Haleem MA, Haleem DJ. (2013) Inhibition of immobilization stress-induced anorexia, behavioral deficits, and plasma corticosterone secretion by injected leptin in rats. Stress 16:353–362. [DOI] [PubMed] [Google Scholar]
- Ho YJ, Ho SC, Pawlak CR, Yeh KY. (2011) Effects of D-cycloserine on MPTP-induced behavioral and neurological changes: potential for treatment of Parkinson’s disease dementia. Behav Brain Res 219:280–290. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Anouti DP, Lacagnina MJ, Nikulina EM, Hammer RP, Jr., Conrad CD. (2013) Experience-dependent effects of context and restraint stress on corticolimbic c-Fos expression. Stress 16:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. (2006) Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. (2013) Distinct extended amygdala circuits for divergent motivational states. Nature 496:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannic JL, Oppert JM, Lahlou N, Basdevant A, Auboiron S, Raison J, Bornet F, Guy-Grand B. (1998) Plasma leptin and hunger ratings in healthy humans. Appetite 30:129–138. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. (1995) Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675:325–328. [DOI] [PubMed] [Google Scholar]
- Lawson EA, Miller KK, Blum JI, Meenaghan E, Misra M, Eddy KT, Herzog DB, Klibanski A. (2012) Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf) 76:520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Munzberg H, Myers MG., Jr (2010) Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci 30:5713–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Negrao AB, Wong ML. (2014) Plasma leptin concentrations are highly correlated to emotional states throughout the day. Transl Psychiatry 4:e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Perez SM, Zhang W, Lodge DJ, Lu XY. (2011) Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Mol Psychiatry 16:1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. (2010) Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 207:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY. (2007) The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol 7:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. (2006) Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A 103:1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. (2006) The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577:907–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, Chua SC., Jr (2004) An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome 15:677–685. [DOI] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Olivos DR, Jeffrey BA, Hayes MR. (2015) Cooperative interaction between leptin and amylin signaling in the ventral tegmental area for the control of food intake. Am J Physiol Endocrinol Metab 308:E1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, Zhang ZY, Gettys TW. (2007) Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology 148:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Lee EJ, Roth RH. (2000) Divergent effects of putative anxiolytics on stress-induced fos expression in the mesoprefrontal system of the rat. Synapse 36:143–154. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP. (2009) The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab 297:E202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro LJ, Kokkinidis L. (1997) Infusion of quinpirole and muscimol into the ventral tegmental area inhibits fear-potentiated startle: implications for the role of dopamine in fear expression. Brain Res 746:231–238. [DOI] [PubMed] [Google Scholar]
- Myers MG., Jr (2004) Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 59:287–304. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (2001) The Mouse Brain in Stereotaxic Coordinates.
- Razzoli M, Andreoli M, Michielin F, Quarta D, Sokal DM. (2011) Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behav Brain Res 218:253–257. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. (1997) Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev 21:801–810. [DOI] [PubMed] [Google Scholar]
- Roman EA, Reis D, Romanatto T, Maimoni D, Ferreira EA, Santos GA, Torsoni AS, Velloso LA, Torsoni MA. (2010) Central leptin action improves skeletal muscle AKT, AMPK, and PGC1 alpha activation by hypothalamic PI3K-dependent mechanism. Mol Cell Endocrinol 314:62–69. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. (2000) Central nervous system control of food intake. Nature 404:661–671. [DOI] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. (2009) Leptin targets in the mouse brain. J Comp Neurol 514:518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RA, Broadhurst PL. (1982) Hyponeophagia and arousal in rats: effects of diazepam, 5-methoxy-N,N-dimethyltryptamine, d-amphetamine and food deprivation. Psychopharmacology (Berl) 78:368–372. [DOI] [PubMed] [Google Scholar]
- Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. (1996) Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest 97:1344–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Livermore A., Jr (1978) Kindling of the mesolimbic dopamine system: animal model of psychosis. Neurology 28:36–46. [DOI] [PubMed] [Google Scholar]
- Swanson LW. (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9:321–353. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. (2012) GABA neurons of the VTA drive conditioned place aversion. Neuron 73:1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. (2004) Recombinant AAV-mediated gene delivery to the central nervous system. J Gene Med 6 Suppl 1:S212–222. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. (1976) Selective activation of mesocortical DA system by stress. Nature 263:242–244. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Borgland SL. (2013) Presynaptic leptin action suppresses excitatory synaptic transmission onto ventral tegmental area dopamine neurons. Biol Psychiatry 73:860–868. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Argilli E, Bonci A. (2010) Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci Biobehav Rev 35:151–156. [DOI] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. (2011) Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci 31:4280–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. (2012) Activation of VTA GABA neurons disrupts reward consumption. Neuron 73:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen LA, Luijendijk MC, Adan RA. (2011) Leptin reduces hyperactivity in an animal model for anorexia nervosa via the ventral tegmental area. Eur Neuropsychopharmacol 21:274–281. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nabeshima T. (2007) Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav Brain Res 178:262–273. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang D, Lu XY. (2015) Dentate gyrus-CA3 glutamate release/NMDA transmission mediates behavioral despair and antidepressant-like responses to leptin. Mol Psychiatry 20:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. (2011) Mesocorticolimbic glutamatergic pathway. J Neurosci 31:8476–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Murakami T, Otani S, Kuwajima M, Shima K. (1998) Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem Biophys Res Commun 246:752–759. [DOI] [PubMed] [Google Scholar]
- Yoshida-Komiya H, Takano K, Fujimori K, Niwa SI. (2014) Plasma levels of leptin in reproductive-aged women with mild depressive and anxious states. Psychiatry Clin Neurosci 68:574–581. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Eslahi N, Rezayof A, Rostami P, Zahmatkesh M. (2012) Modulation of ventral tegmental area dopamine receptors inhibit nicotine-induced anxiogenic-like behavior in the central amygdala. Prog Neuropsychopharmacol Biol Psychiatry 41:11–17. [DOI] [PubMed] [Google Scholar]