Abstract
This study sought to evaluate the potential of circulating long non-coding RNAs (lncRNAs) as biomarkers for acute myocardial infarction (AMI). We measured the circulating levels of 15 individual lncRNAs, known to be relevant to cardiovascular disease, using the whole blood samples collected from 103 AMI patients, 149 non-AMI subjects, and 95 healthy volunteers. We found that only two of them, Zinc finger antisense 1 (ZFAS1) and Cdr1 antisense (CDR1AS), showed significant differential expression between AMI patients and control subjects. Circulating level of ZFAS1 was significantly lower in AMI (0.74 ± 0.07) than in non-AMI subjects (1.0 ± 0.05, P < 0.0001), whereas CDR1AS showed the opposite changes with its blood level markedly higher in AMI (2.18 ± 0.24) than in non-AMI subjects (1.0 ± 0.05, P < 0.0001). When comparison was made between AMI and non-AMI, the area under ROC curve was 0.664 for ZFAS1 alone or 0.671 for CDR1AS alone, and 0.691 for ZFAS1 and CDR1AS combination. Univariate and multivariate analyses identified these two lncRNAs as independent predictors for AMI. Similar changes of circulating ZFAS1 and CDR1AS were consistently observed in an AMI mouse model. Reciprocal changes of circulating ZFAS1 and CDR1AS independently predict AMI and may be considered novel biomarkers of AMI.
Acute myocardial infarction (AMI) is the worst threat to human lives and the quality of human life. Early detection of AMI with noninvasive and reliable biomarkers is the foremost step for minimizing ischemic damage to the myocardium. Clinically validated biomarkers like creatine kinase MB (CKMB) and cardiac troponin I (cTnI), currently considered as “gold standard” for AMI diagnosis1,2,3,4, have a number of pitfalls. Search for new biomarkers of AMI, particularly those for early diagnosis, is therefore a top-urgent mission and has actually been an endless effort from fundamental and clinical researchers worldwide.
In addition to protein biomarkers, recent studies have suggested the potential value of RNA biomarkers for AMI, e.g., microRNAs (miRNAs)5,6,7. More recently, long non-coding RNAs (lncRNAs), a new class of functional mRNA-like transcripts lacking significant open reading frames or protein-coding capacity8,9,10,11, are emerging as an important layer in the gene regulatory network. As opposed to miRNAs, another class of regulatory RNAs, that are only 19 ~ 25 nt long, lncRNAs range in length from 200 nts to ~100 kilobases (kb). The growing body of literature has provided ample evidence for the critical role of lncRNAs in controlling a wide spectrum of biological processes through diverse but yet poorly understood molecular mechanisms, despite that only a handful of lncRNAs have been functionally and molecularly characterized10,11,12. LncRNA expression is highly variable, with greater tissue specificity compared to protein-coding mRNAs, and only 1% of the lncRNAs are ubiquitously expressed across all tissues examined. These properties of lncRNAs make them potential new biomarkers for disease diagnosis and prognosis. Indeed, recent studies have unraveled that expression of lncRNAs is temporal- and spatial-dynamically regulated by many factors and aberrant expression of lncRNAs has been increasingly documented in developmental programs, cancers, neuronal disorders, diabetes, etc. Most prominently, lncRNAs are readily detectable in a number of human body fluids such as serum13, plasma14,15,16, saliva17, and urine18,19, making them promising and attractive in the search of novel biomarkers in body fluid samples and noninvasive and rapid diagnostic tool for disease diagnosis and prognosis.
On the basis of available data in the literature, we proposed that circulating lncRNAs are epigenetic biomarkers for AMI and can be used to predict cardiovascular risk. This study was designed to test our hypothesis by identifying circulating lncRNA biomarkers for AMI with human blood samples and in a mouse model with blood and myocardium tissues. We have also analyzed the power of candidate lncRNAs to predict cardiac risk event, correlated them with known biomarkers, and assessed the regulatory role of these lncRNAs in cardiac function.
Results
Clinical Characteristics of the Study Population
Blood samples were collected from a total of 138 AMI patients, 149 non-AMI control subjects, and 95 healthy volunteers. Among the 138 AMI blood samples, 103 were from patients with ischemic time ≤12 h (an average of ischemic time = 3.5 h), 20 with ischemic time ≤12 h but without complete medical records, and 15 with ischemic time ranging from 24–36 h. Therefore, only 103/138 AMI patients were included in our detailed statistical analyses in the following sections to comply with our goal for early detection, despite that the same experimental results held for true the patients with ischemic time longer than 12 h.
AMI patients were aged 60.71 ± 11.05 years, comparable with the healthy volunteers (HV, 54.30 ± 12.69 years) and non-AMI control subjects (57.85 ± 11.92 years). There were no significant differences between AMI and non-AMI in the status hypertension and diabetes mellitus (Table 1).
Table 1. The demographic characteristics and AMI-relevant indicators in AMI patients, non-AMI control subjects and healthy volunteers.
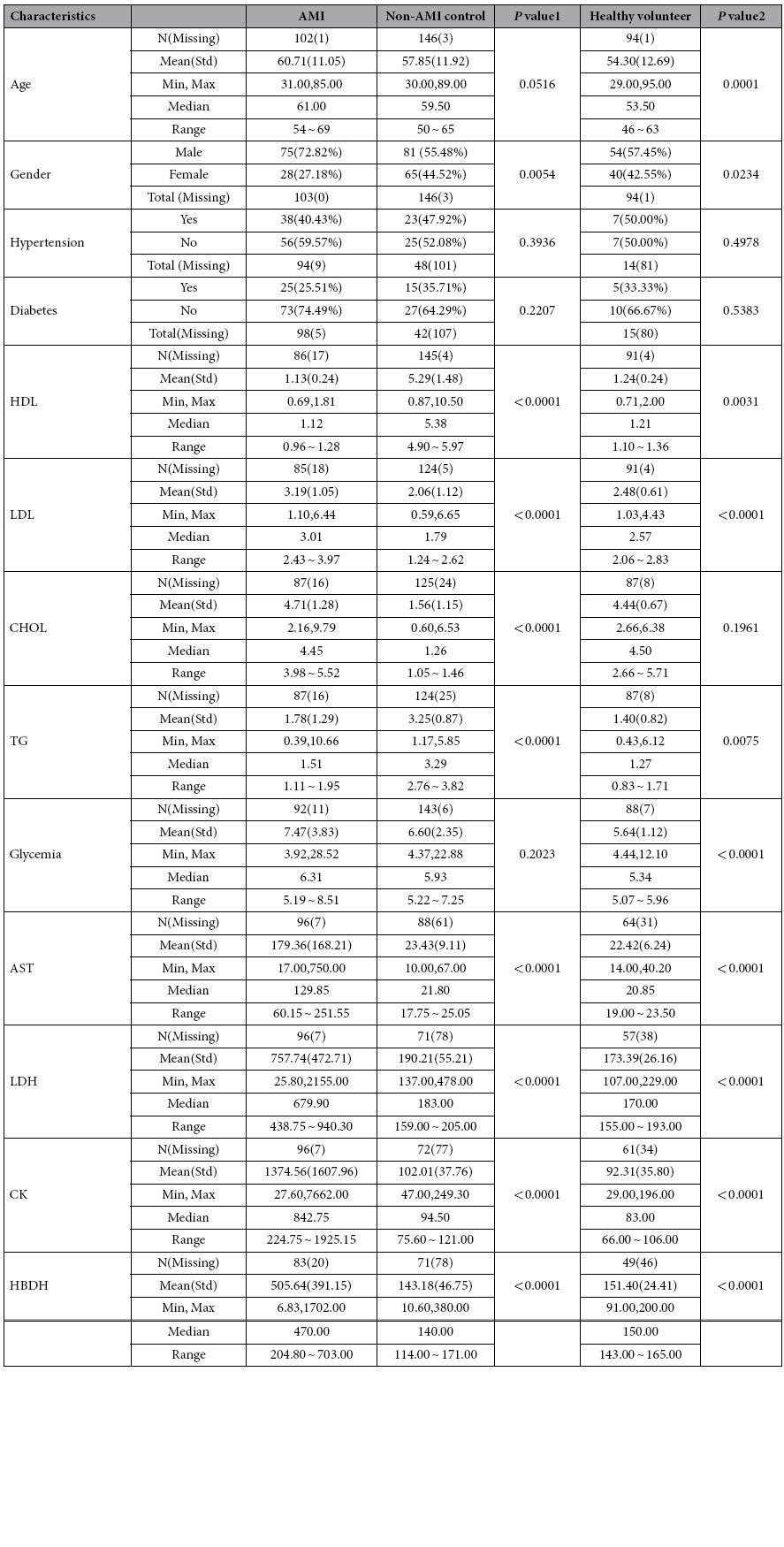
AST: aspartate transaminase; CHOL: total cholesterol; CK: creatine kinase; HBDH: hydroxybutyrate dehydrogenase; HDL: high density cholesterol; LDH: lactic dehydrogenase; LDL: low density cholesterol.
Reciprocal Changes of ZFAS1 and CDR1AS Blood Levels in AMI Patients
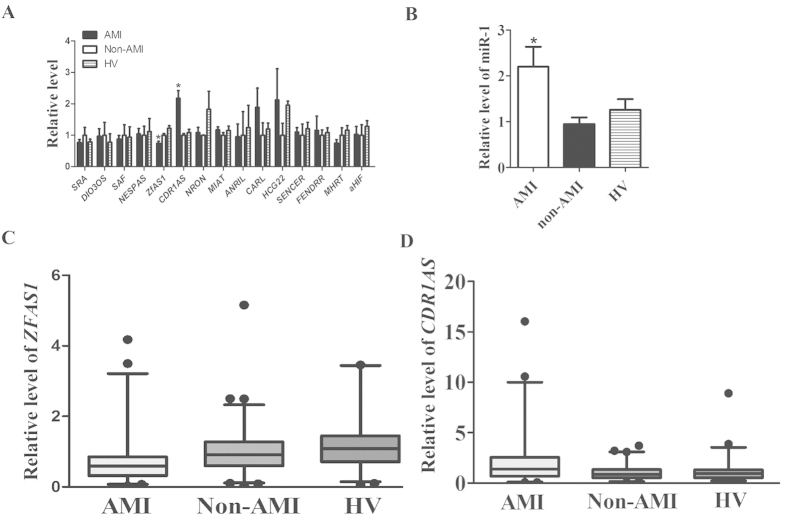
Our initial qPCR analysis included 15 then-known cardiac-specific or cardiac-related lncRNAs (http://cmbi.bjmu.edu.cn/lncrnadisease): SRA, DIO3OS, SAF, NESPAS, MIAT, NRON, ANRIL, ZFAS1, CDR1AS, CARL, HCG22, SENCER, FENDRR, MHRT, aHIF. As illustrated in Fig. 1A, of 15 lncRNAs tested, only ZFAS1 (Zinc finger antisense 1) and CDR1AS (Cdr1 antisense) demonstrated significant differences in the whole blood samples between AMI, non-AMI, and HV. Circulating level of ZFAS1 was significantly lower in AMI (0.74 ± 0.07) than in non-AMI subjects (1.0 ± 0.05, P < 0.0001) and healthy volunteers (1.22 ± 0.08, P < 0.0001), whereas CDR1AS showed the opposite changes with its blood level markedly higher in AMI (2.18 ± 0.24) than in control populations (1.0 ± 0.05 in non-AMI subjects, 1.09 ± 0.10 in healthy volunteers, P < 0.0001). Specifically, circulating level of ZFAS1 was 25.7% and 39.2% lower in AMI than in non-AMI subjects and in volunteers (Fig. 1C, Table 2), whereas CDR1AS showed the opposite changes in the bloodstream with its level 2.2 fold and 2.0 fold higher in AMI than in non-AMI and in healthy subjects (Fig. 1D, Table 2). The median Ct value for ZFAS1 was 23.3, ranging from 20.2–26.2; and the median Ct value for CDR1AS was 22.2 with a range from 17.9–26.6.
Figure 1. Changes of circulating lncRNAs levels in patients with acute myocardial infarction (AMI), with miR-1 as a positive control.
(A) Circulating levels of lncRNAs were determined by quantitative real-time RT-PCR with the whole-blood samples from AMI patients, non-AMI control subjects, and healthy volunteers (HV). Only ZFAS1 and CDR1AS demonstrated significant differences between AMI and healthy patients. P < 0.0001, n = 95 for HV and n = 149 for non-AMI control subjects. (B) Blood level of miR-1. P < 0.05, n = 30 for control and n = 32 for AMI. Data were present as means ± SEM in (A,B). (C,D) Box plot of blood ZFAS1 and CDR1AS levels.
Table 2. The Statistical Analysis of Circulating ZFAS1 and CDR1AS.
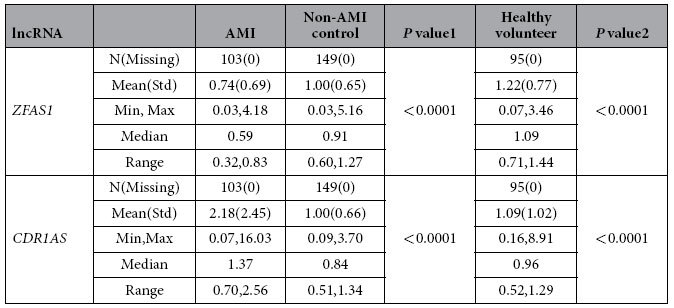
P values 1 are for AMI vs Non-AMI, P values 2 are for AMI vs Healthy volunteer.
Since circulating miR-1, a miRNA belonging to another class of non-coding RNAs, has been documented to be a new miRNA biomarker for AMI by several laboratories7,20,21,22,23,24, we included this miRNA in our analysis for comparison. As depicted in Fig. 1B, miR-1 level was found to be significantly elevated in AMI blood samples, verifying the previous finding and serving as a positive control in this study. These results indicate that ZFAS1 and CDR1AS both exist in the human blood with good stability and high abundance just like miR-1 (known as a highly stable and abundant miRNA species in the circulation).
Evaluation of Circulating ZFAS1 and CDR1AS as New Biomarkers for AMI
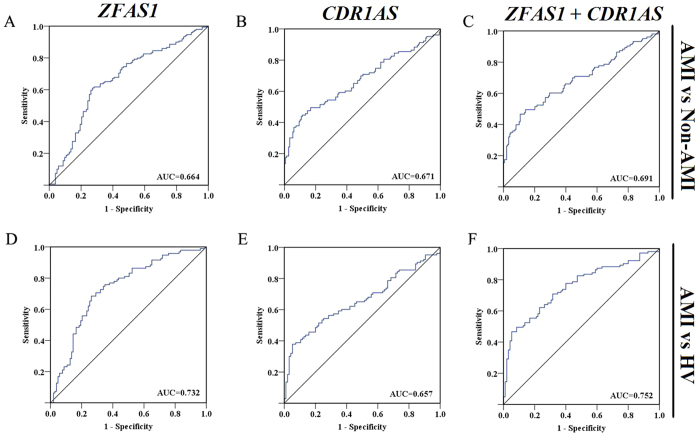
Having established that ZFAS1 and CDR1AS are present in the peripheral circulation and their blood levels are anomaly altered in AMI patients, we sought to determine the potential utility of circulating ZFAS1 and CDR1AS as diagnostic biomarkers of AMI. To this end, ROC analysis was performed to evaluate the predictive power of circulating ZFAS1 or CDR1AS alone and combination of the two for AMI. When comparison was made between AMI and non-AMI, the area under ROC curve (AUC) was 0.664 (95%CI = 0.594 ~ 0.733) for ZFAS1 alone (Fig. 2A), 0.671 (95%CI = 0.600 ~ 0.742) for CDR1AS alone (Fig. 2B), and 0.691 (95%CI = 0.622 ~ 0.760) for ZFAS1 and CDR1AS combination (ZFAS1 + CDR1AS, Fig. 2C). When comparison was made between AMI and HV, the values were 0.732 (95%CI: 0.662 ~ 0.803), 0.657 (0.581 ~ 0.734), and 0.752 (0.684 ~ 0.820) for ZFAS1, CDR1AS, and ZFAS1 + CDR1AS, respectively (Fig. 2D–F).
Figure 2. Receiver–operator characteristic (ROC) analysis of circulating ZFAS1 or CDR1AS alone and in combination for predicting AMI.
The area under ROC curve (AUC) was determined to evaluate the predictive power of circulating lncRNA levels for AMI using non-AMI subjects (A–C) or healthy volunteers (HV; D–F) as control. ZFAS1 + CDR1AS indicates combination of the two lncRNAs.
The univariate analysis with logistic regression showed that ZFAS1and CDR1AS were both predictors for AMI using either non-AMI (Table 3) or HV (Table 5) as control. The multivariate logistic regression analysis further identified ZFAS1 and CDR1AS as an independent predictor for AMI (Tables 3b and 3d). It is noted that the odd ratio (OR), a measure of association between circulating ZFAS1 level and AMI, for univariate analysis was <1 (0.512, 95% CI: 0.324 ~ 0.809, P value = 0.0042; Table 3) whereas the OR value for multivariate analysis was >1 (2.807, 95% CI: 0.559 ~ 14.086, P value = 0.2098; Table 4). The difference was ascribed to the inclusion of CHOL (circulating cholesterol level) in the multivariate analysis. Despite the difference, the fact that the OR values ≠ 1 (either <1 or >1 indicates the existence of an association between circulating ZFAS1 and AMI. Particularly, the fact that P values for both univariate and multivariate analyses were <0.05 (Tables 3a and 3b) strongly support the view about the association between circulating ZFAS1 and AMI. The univariate analysis showed that the odds ratios (OR) were 1.831 (95% CI: 1.425 ~ 2.352) for CDR1AS (P < 0.0001) between AMI and non-AMI (Table 3). The multivariate logistic regression analysis showed that the OR values were 3.989 (95% CI: 0.805 ~ 19.772 for CDR1AS (P = 0.0903) between AMI and non-AMI (Table 4). The univariate analysis showed that the OR values were 0.369 (95% CI: 0.227 ~ 0.597) for ZFAS1 (P < 0.0001) and 1.535 (95% CI: 1.207 ~ 1.952) for CDR1AS (P < 0.0005) between AMI patients and healthy volunteers (Table 5). The multivariate logistic regression analysis showed that the OR values were 0.437 (95% CI: 0.186 ~ 1.028) for ZFAS1 (P = 0.0578) and 2.186 (95% CI: 1.281 ~ 3.730 for CDR1AS (P = 0.0041) between AMI patients and healthy volunteers (Table 6).
Table 3. Univariate regression analysis for the association of ZFAS1 and CDR1AS with demographic characteristics between AMI patients and non-AMI control subject.
| Parameter | Estimate | SE | Chi-Square | P value | OR | 95%CI |
|
|---|---|---|---|---|---|---|---|
| lower | upper | ||||||
| ZFAS1 | −0.6695 | 0.2337 | 8.2109 | 0.0042 | 0.512 | 0.324 | 0.809 |
| CDR1AS | 0.6046 | 0.1279 | 22.3575 | <0.0001 | 1.831 | 1.425 | 2.352 |
| Age | 0.0215 | 0.0114 | 3.5890 | 0.0582 | 1.022 | 0.999 | 1.045 |
| Gender | 0.7651 | 0.2771 | 7.6238 | 0.0058 | 2.149 | 1.249 | 3.699 |
| Blood sugar | 0.0958 | 0.0473 | 4.0985 | 0.0429 | 1.101 | 1.003 | 1.207 |
| HDL | 1.4455 | 0.1718 | 70.8076 | <0.0001 | 4.244 | 3.031 | 5.943 |
| LDL | −1.5691 | 0.2116 | 55.0011 | <0.0001 | 0.208 | 0.138 | 0.315 |
| TG | 0.8834 | 0.1444 | 37.4498 | <0.0001 | 2.419 | 1.823 | 3.210 |
| CHOL | −2.6585 | 0.7385 | 12.9578 | 0.0003 | 0.070 | 0.016 | 0.298 |
OR: odds ratio; CI: confidence interval.
Table 5. Univariate regression analysis for the association of ZFAS1 and CDR1AS with demographic characteristics between AMI patients and healthy volunteers.
| Parameter | Estimate | SE | Chi-Square | Pvalue | OR | 95%CI |
|
|---|---|---|---|---|---|---|---|
| lower | upper | ||||||
| ZFAS1 | −0.9980 | 0.2464 | 16.4092 | <0.0001 | 0.369 | 0.227 | 0.597 |
| CDR1AS | 0.4283 | 0.1227 | 12.1781 | 0.0005 | 1.535 | 1.207 | 1.952 |
| Age | 0.0459 | 0.0129 | 12.5689 | 0.0004 | 1.047 | 1.021 | 1.074 |
| Gender | 0.6852 | 0.3042 | 5.0717 | 0.0243 | 1.984 | 1.093 | 3.602 |
| Blood sugar | 0.4420 | 0.1161 | 14.5044 | 0.0001 | 1.556 | 1.239 | 1.953 |
| HDL | −1.9724 | 0.6868 | 8.2479 | 0.0041 | 0.139 | 0.036 | 0.535 |
| LDL | 1.0480 | 0.2207 | 22.5423 | <0.0001 | 2.852 | 1.850 | 4.395 |
| TG | 0.4165 | 0.1967 | 4.4828 | 0.0342 | 1.517 | 1.031 | 2.230 |
| CHOL | 0.2641 | 0.1554 | 2.8875 | 0.0893 | 1.302 | 0.960 | 1.766 |
OR: odds ratio; CI: confidence interval.
Table 4. Multivariate regression analysis for the association of ZFAS1 and CDR1AS with demographic characteristics between AMI patients and non-AMI control subjects.
| Parameter | Estimate | SE | Chi-Square | P value | OR | 95%CI | |
|---|---|---|---|---|---|---|---|
| lower | upper | ||||||
| ZFAS1 | 1.0321 | 0.8230 | 1.5726 | 0.2098 | 2.807 | 0.559 | 14.086 |
| CDR1AS | 1.3835 | 0.8167 | 2.8694 | 0.0903 | 3.989 | 0.805 | 19.772 |
| Age | −0.0512 | 0.0536 | 0.9122 | 0.3395 | 0.950 | 0.855 | 1.055 |
| Gender | 0.9524 | 1.0404 | 0.8380 | 0.3600 | 2.592 | 0.337 | 19.915 |
| Blood sugar | 0.0979 | 0.1911 | 0.2623 | 0.6085 | 1.103 | 0.758 | 1.604 |
| HDL | −1.7646 | 0.9086 | 3.7717 | 0.0521 | 0.171 | 0.029 | 1.016 |
| LDL | −0.1440 | 0.2734 | 0.2772 | 0.5985 | 0.866 | 0.507 | 1.480 |
| TG | 1.6917 | 0.9918 | 2.9093 | 0.0881 | 5.429 | 0.777 | 37.924 |
| CHOL | −4.3830 | 1.3252 | 10.9388 | 0.0009 | 0.012 | <0.001 | 0.168 |
OR: odds ratio; CI: confidence interval.
Table 6. Multivariate regression analysis for the association of ZFAS1 and CDR1AS with demographic characteristics between AMI patients and healthy volunteers.
| Parameter | Estimate | SE | Chi-Square | P value | OR | 95%CI |
|
|---|---|---|---|---|---|---|---|
| lower | upper | ||||||
| ZFAS1 | −0.8270 | 0.4358 | 3.6001 | 0.0578 | 0.437 | 0.186 | 1.028 |
| CDR1AS | 0.7822 | 0.2726 | 8.2341 | 0.0041 | 2.186 | 1.281 | 3.730 |
| Age | 0.0499 | 0.0224 | 4.9911 | 0.0255 | 1.051 | 1.006 | 1.098 |
| Gender | 0.6747 | 0.6435 | 1.0996 | 0.2943 | 1.964 | 0.556 | 6.930 |
| Blood sugar | 0.2792 | 0.1484 | 3.5423 | 0.0598 | 1.322 | 0.989 | 1.768 |
| HDL | 2.1829 | 1.4824 | 2.1684 | 0.1409 | 8.872 | 0.486 | 162.108 |
| LDL | 4.4150 | 0.9635 | 20.9973 | <0.0001 | 82.679 | 12.510 | 546.407 |
| TG | 1.1201 | 0.4272 | 6.8758 | 0.0087 | 3.065 | 1.327 | 7.080 |
| CHOL | −3.6087 | 0.9621 | 14.0687 | 0.0002 | 0.027 | 0.004 | 0.179 |
OR: odds ratio; CI: confidence interval.
Relation of ZFAS1 and CDR1AS to Conventional Prognostic Markers
In order to further evaluate the usefulness of circulating ZFAS1 and CDR1AS as AMI biomarkers, we tested whether their levels correlated with cardiac risk factors, conventional AMI markers, and cardiac function parameters. The data summarized in Table 7 show that ZFAS1 was negatively correlated with AST, LDH, and CK, HBDH, whereas CDR1AS was positively correlated with AST, LDH, and CK. Neither ZFAS1 nor CDR1AS was correlated with diabetes mellitus, hypertension, smoking history, or cardiac contractile and electrophysiological functions.
Table 7. Spearman’s rank correlation analysis for the association of ZFAS1 and CDR1AS with cardiac risk factors, AMI biomarkers and cardiac function parameters in AMI patients.
|
ZFAS1 |
CDR1AS |
|||
|---|---|---|---|---|
| Coefficient | P | Coefficient | P | |
| Cardiovascular risk factors | ||||
| Age | −0.14543 | 0.0071 | 0.00941 | 0.8624 |
| Gender | −0.01964 | 0.7170 | −0.27672 | <0.0001 |
| Diabetes | 0.06353 | 0.4322 | −0.07592 | 0.3478 |
| Hypertension | 0.05540 | 0.4921 | 0.04995 | 0.5358 |
| Smoking | 0.07969 | 0.4684 | −0.14758 | 0.1777 |
| HDL | 0.07340 | 0.1889 | −0.13414 | 0.0160 |
| LDL | −0.01136 | 0.8396 | 0.09472 | 0.0907 |
| CHOL | −0.02210 | 0.7035 | 0.15780 | 0.0063 |
| TG | 0.01888 | 0.7455 | −0.11924 | 0.0397 |
| Cardiac biomarkers | ||||
| cTnI | −0.08305 | 0.6201 | −0.23340 | 0.1585 |
| AST | −0.31188 | <0.0001 | 0.22618 | 0.0003 |
| LDH | −0.28928 | <0.0001 | 0.28244 | <0.0001 |
| CK | −0.16315 | 0.0134 | 0.17946 | 0.0065 |
| CKMB | −0.12912 | 0.1748 | 0.11498 | 0.2274 |
| HBDH | −0.14003 | 0.0463 | 0.16458 | 0.0190 |
| Cardiac function | ||||
| E/A | 0.12059 | 0.2930 | 0.09910 | 0.3880 |
| EF | 0.06547 | 0.5375 | −0.09906 | 0.3502 |
| FS | −0.00606 | 0.9569 | −0.06679 | 0.5510 |
| Electrocardiogram | ||||
| QRS | 0.00586 | 0.9613 | −0.00245 | 0.9838 |
| QT | −0.00074 | 0.9951 | 0.09224 | 0.4442 |
| QTc | −0.01337 | 0.9119 | −0.01778 | 0.8830 |
| PR | 0.00360 | 0.9784 | −0.17636 | 0.1815 |
| P | 0.04044 | 0.7510 | 0.00693 | 0.9567 |
| RR | −0.01216 | 0.9216 | 0.05646 | 0.6474 |
| PP | 0.12888 | 0.2949 | 0.03728 | 0.7628 |
| P (o) | −0.14728 | 0.2494 | −0.10992 | 0.3911 |
| QRS (o) | −0.13512 | 0.2647 | −0.02287 | 0.8509 |
| T (o) | −0.10579 | 0.3906 | −0.09140 | 0.4585 |
| Heart rate | −0.01139 | 0.9232 | −0.07593 | 0.5202 |
| ST-segment elevation | −0.02231 | 0.8230 | −0.00080 | 0.9936 |
HDL: high density cholesterol; LDL: low density cholesterol; CHOL: total cholesterol; TG: triglycerides; cTnI: cardiac troponin I; AST: aspartate transaminase; LDH: lactic dehydrogenase; CK: creatine kinase ; CKMB: isoenzyme creatine kinase; HBDH: hydroxybutyrate dehydrogenase; E/A: ratio of E velocity to A velocity; EF: ejection fraction; FS: left ventricular shortening fraction; QRS, QT, QTc, PR, P, RR, PP(ms): interval of QRS, QT, QTc, PR, P, RR, PP; P (o), QRS (o), T (o): axis for P, QRS and T.
Reciprocal Changes of Circulating and Myocardial ZFAS1 and CDR1AS Levels in a Mouse Model of AMI
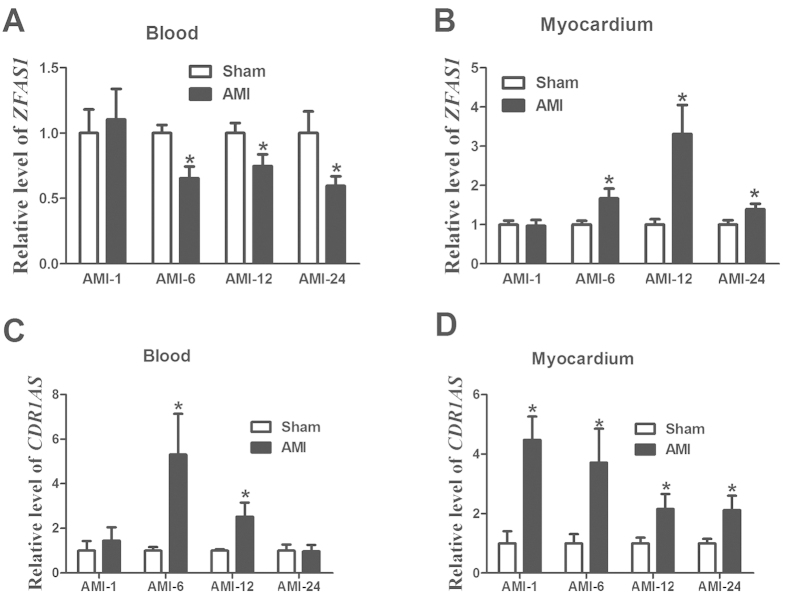
Next, we sought to see if the alterations of circulating ZFAS1 and CDR1AS in AMI patients could be reproduced in an experimental model of AMI with minimal confounding factors. RNA samples from whole blood and left ventricular myocardium of AMI mice and sham-operated control littermates were prepared at varying time points (1 h, 6 h, 12 h, 24 h). Real-time RT-PCR was carried out for changes of ZFAS1 and CDR1AS in AMI samples relative to control samples. As illustrated in Fig. 3, the blood ZFAS1 levels in AMI mice were decreased at all time points tested in our study and in contrast CDR1AS levels were increased, consistent with the findings in AMI patients. Notably, statistically significant decreases in circulating ZFAS1 began as early as from 6 hour following LAD ligation, and similarly, significant elevation of CDR1AS occurred from 6 h after AMI.
Figure 3.
Changes of circulating (A,C) and myocardium (B,D) ZFAS1 and CDR1AS levels in a mouse model of AMI at different time point (1 h, 6 h,12 h, and 24 h), determined by real-time RT-PCR methods. The number of blood samples ranged from 6 to 16 for different groups. AMI represent acute myocardial infarction with LAD. Data were present as means ± SEM.
Intriguingly, the changes of the myocardial expression of ZFAS1 demonstrated the opposite direction to its circulating levels: the myocardium ZFAS1 level was markedly increased in AMI at all four time points examined (Fig. 3). It is noted that the increases did not reach a statistical significant level until 6 h after AMI which lagged far behind its changes in the bloodstream. By comparison, the abundance of the myocardial CDR1AS expression followed the same direction of changes as its blood concentration, being upregulated in AMI relative to sham-operated control samples from 1 h after AMI (Fig. 3).
Discussion
Main Findings of the Study
We present here a study using whole blood samples collected from AMI patients at an average ischemic time of 3.5 h (~200 minutes) for early detection of circulating lncRNAs as potential biomarkers for AMI, by detecting the levels of 15 lncRNAs that are known to be relevant to cardiac development and cardiovascular disease. There a number of new findings in this study. (1) Of 15 known cardiac-relevant lncRNAs examined, ZFAS1 and CDR1AS are the only two that demonstrated significant differences in their expressions between AMI patients and healthy subjects, as well as between experimental AMI and sham-operated mice. Blood ZFAS1 and CDR1AS show reciprocal changes with ZFAS1 being decreased and CDR1AS increased in the settings of AMI. (2) Significant changes of ZFAS1 and CDR1AS in the bloodstream were detected at an average ischemic time of 3.5 h. (3) While either ZFAS1 or CDR1AS was found to be well correlated with AMI, the combination of the two or the reciprocal changes of the two gives higher power of sensitivity and specificity of prediction, representing the superior biomarker for AMI. (4) ZFAS1 and CDR1AS levels in the myocardium of AMI mice were both remarkably upregulated. Our findings indicate that circulating ZFAS1 and CDR1AS are predictors of AMI and expression deregulation of lncRNAs may be a new molecular mechanism for cardiac disorders.
Previous Studies on Circulating LncRNAs and Cardiovascular Disease
LncRNAs are newly discovered class of gene expression regulators. These long non-coding RNAs have garnered tremendous research interest worldwide, not only because they have been shown to participate in a wide spectrum of biological processes but also because they have been characterized as potential biomarkers for human disease. Indeed, lncRNAs have been documented to be high stable and readily detectable in a number of human body fluids such as serum13, plasma14,15,16, saliva17, and urine18,19. These properties of lncRNAs make them promising and attractive in the search of novel biomarkers in body fluid samples and noninvasive and rapid diagnostic tool for disease diagnosis and prognosis25,26.
During the course of the present study, a study on circulating lncRNAs as biomarkers for AMI was published27. This study compared the expression levels of 5 lncRNAs: aHIF, ANRIL, KCNQLOT1, MIAT and MALAT1. The levels of aHIF, KCNQLOT1 and MALAT1 were found increased in AMI relative to HV, whereas ANRIL level was decreased in AMI patients. In the present study, we focused our analysis on a selected set of lncRNAs (SRA, DIO3OS, SAF, NESPAS, MIAT, NRON, ANRIL, ZFAS1, CDR1AS, CARL, HCG22, SENCER, FENDRR, MHRT, aHIF) according to their cardiac-specific or cardiac-enriched expression using 103 blood samples from AMI patients, 149 samples from non-AMI control subjects, and 95 samples from healthy volunteers, in conjunction with blood samples from AMI mice. Among these lncRNAs, only ZFAS1 and CDR1AS were found significantly altered with ZFAS1 decreased whereas CDR1AS increased in their circulating levels in both human and mouse samples. The changes of circulating ZFAS1 and CDR1AS levels in a mouse model of AMI at 12 hours can be detected with confidence after AMI. By comparison, among the lncRNAs selected for examination only two are overlapped between our study and the study by Vausort et al.: MIAT and ANRIL. We did not find any statistical significant or biologically meaningful difference of MIAT levels between AMI and healthy subjects, which is consistent with the finding in the study by Vausort et al. On the other hand, Vausort et al. reported lower levels of ANRIL in AMI patients, but our study failed to identify any significant changes of blood ANRIL level in AMI. The discrepancy could be explained by the difference of timing for blood sample collection: in the study by Vausort et al. the blood samples were harvested at the time of reperfusion with an average of 5 h after chest pain onset while in our study the samples were collected at an average ischemic time of 3.5 h for early detection of lncRNAs for AMI. The difference in the ischemic time may give rise to different levels of a given lncRNA as Vausort et al. found that lncRNA levels are dynamically regulated after MI. An alternative explanation would be the different samples used for lncRNA detection: Vausort et al. used leukocytes while we employed whole blood samples. Furthermore, under our experimental conditions, ANRIL level in bloodstream was extremely low with PCR cycle number over 32 which was virtually null. Another possible explanation is that the patients enrolled into the study by Vausort et al. were taking a variety of preadmission medications, whereas the patients included in our study were all on the first visit to our hospitals in emergency due to their chest pain and subsequent diagnosis of AMI prior to any medications. Thus, our results were presumably unaffected by medications.
ZFAS1, zinc finger antisense 1, is a transcript antisense to the 5’ end of the protein-coding gene Znfx1 and intronically hosts three previously undescribed C/D box snoRNAs (SNORDs): Snord12, Snord12b, and Snord12c. ZFAS1 was found extremely stable with a half-life of >32 hrs in neuroblastoma cells28. ZFAS1 is highly expressed in the mammary gland and is down-regulated in breast tumors compared to normal tissue. CDR1AS is an antisense to the cerebellar degeneration-related protein 1, belonging to the class of circular RNAs29,30. It is highly expressed in human brain, spinal cord, heart, lung, thymus and thyroid. In our samples, both ZFAS1 and CDR1AS are fairly abundant RNA species in myocardium and blood as well, according to the Ct values from qPCR assays. Either of these two lncRNAs was found to be a good predictor of AMI though the changes of their levels went the opposite directions in blood, and combination of the two increased the power of prediction.
Potential Significance of the Study
The discovery of deregulated lncRNAs not only sheds light on a new layer of regulatory network of human diseases, but it also opens up new opportunities for using these molecules as diagnostic markers and therapeutic targets. Our data suggest that lncRNAs as biomarkers can offer a number of advantages. First, they are sufficiently stable and can be readily detected in blood samples. Second, they can be quantified by highly sensitive methods such as PCR. Third, changes of lncRNAs in the blood may reflect the underlying mechanisms for the disease under detection. Our findings thus add that expression profiling and quantification of the lncRNA complement of the cardiac transcriptome in the systemic circulation will conceivably provide new venues for early diagnosis and treatment of the heart disease.
Possible Limitations of the Study
Our measurement was limited to only a subset of lncRNAs without global transcriptome profiling. The data acquired thus do not provide us the overall picture of lncRNA presentation in blood and myocardium of AMI patients, nor do they define the lncRNA signature in AMI patients. There is well a possibility that we have missed out other important lncRNA markers for AMI. Nonetheless, the lncRNAs selected for our study are those that have been shown to be able to cause cardiac disorders or are abundantly expressed in heart cells. Fortunately, we were able to identify from this small group two lncRNAs that offer reliable detection with high-sensitivity and reproducibility. There is a hope that using high-throughput methods would allow for identification of better lncRNA biomarkers for AMI in future studies.
Another limitation of the study is the unknown sources of ZFAS1 and CDR1AS in the blood stream. The release of RNA into the blood is thought to be related to the apoptosis and necrosis of cells and/or is the result of secretion from cells. LncRNAs are detectable in the serum and plasma, being surprisingly stable in spite of the fact that high amounts of RNases circulate in the blood stream, implying that these molecules may be protected from degradation by its packaging into microparticles, such as exosomes, microvesicles, apoptotic bodies, and apoptotic microparticles31. The reported RNA content of microvesicles and exosomes thus far includes primarily small miRNAs and long protein-coding mRNAs32.
Finally, the control subjects (including both non-AMI and healthy populations) in our study were not examined for any AMI biomarkers. While such a criterion for inclusion of controls is expected to give rise to minimal confounding results, a concomitant limitation is the inability to compare with the established risk markers in our analyses. Nevertheless, comparison between our results and the published data on cTnI33, an established risk marker of AMI, indicates that ZFAS1/CDR1AS and cTnI share a similar predictive power as early markers for AMI. In our study, the area under ROC curve (AUC) between AMI and HV was 0.732, 0.657 and 0.752 for ZFAS1, CDR1AS, and ZFAS1 + CDR1AS, respectively; it is nearly identical to the AUC of 0.74 for cTnI.
Summary
Collectively, we detected 15 known cardiac - relevant lncRNAs for their levels in the blood samples of AMI patients, and found that only two of them, ZFAS1 and CDR1AS, demonstrated significant differences in their circulating levels between AMI patients and control subjects. Circulating ZFAS1 and CDR1AS showed opposite changes with ZFAS1 being decreased and CDR1AS increased in AMI. Moreover, while either ZFAS1 or CDR1AS was found to be well correlated with AMI, the combination of the two or the reciprocal changes of the two gives higher power of sensitivity and specificity of prediction, representing the superior biomarker for AMI. Our findings suggest circulating ZFAS1 + CDR1AS as a new biomarker of AMI.
Materials and Methods
Participants
Between February 2013 and November 2014, 138 AMI patients and 95 healthy volunteers (HV) and 149 non-AMI control subjects presented to the First Affiliated Hospital and the Second Affiliated Hospital of Harbin Medical University (Harbin, China). AMI was previously described34,35: see supplementary methods. The healthy volunteers were recruited at the time of regular annual medical checkup and the non-AMI subjects were recruited from the patients who visited the First Affiliated Hospital and the Second Affiliated Hospital of Harbin Medical University with conditions other than AMI. The non-AMI subjects were recruited from the patients without AMI. All patients and control subjects included in our study belong to Han people (Han nationality). These patients were all on the first visit to our hospitals in emergency due to their chest pain and subsequent diagnosis of AMI prior to any medications to avoid their possible influence on study results. The clinical characteristics of the study population are summarized in Table S1.
Ethical Approval of Studies and Informed Consent
All experimental protocols were approved by the Ethnic Committee for Use of Human Samples of the Harbin Medical University and the methods were carried out in accordance with the approved guidelines. All human investigators procedures were approved by the Institutional Research Board of Harbin Medical University (No. HMUIRB-20140026). For investigations of humans, written informed consent was obtained from the study participants and participants received a stipend.
All animal procedures were approved by the Institutional Animal Care and Use Committee at Harbin Medical University (No. HMUIRB-2008-06) and the Institute of Laboratory Animal Science of China (A5655-01).
Collection and Handling of Human Blood Samples
For lncRNA detection, whole blood (WB) samples (1 mL per patient) were drawn from the study subjects via a direct venous puncture into tubes containing sodium citrate. For AMI, peripheral blood samples were collected within an average ischemic time of 3.5 h prior to blood draw.
Quantitative Real-Time Reverse Transcription (RT)-Polymerase Chain Reaction (PCR)
Total RNA was isolated from 1 mL whole blood sample using phenol/chloroform extraction procedures and real-time RT-PCR was performed as described before20,21. The PCR primer pairs are listed in Table S2.
Animals
C57BL/6 mice ranging from 10 weeks to 12 weeks in age and weighed between 25–30 g each were provided by the experimental animal Center of Harbin Medical University. All experimental protocols were approved by the Ethnic Committee for Use of Animals Samples of the Harbin Medical University and the methods were carried out in accordance with the approved guidelines. And all the experiments were conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Acute Myocardial Infarction Model (AMI)
AMI was induced by left anterior descending coronary artery (LAD) ligation, as described in our previous study21.
Collection and Handling of Mouse Blood Samples
Blood samples were drawn directly from mouse hearts, and the hearts were then dissected post AMI 12 h. These samples were immediately used for total RNA isolation as previously described20.
Statistical Analysis
Categorical data were presented with count and percentile. Continuous variables were described as means ± SD, min, max, median and interquartile range. The statistical analyses were described in detail in supplementary methods. All analyses were carried out with SAS 9.1 (Serial No. 989155) except that ROC was done with SPSS v17.0 software. The significant level was set at 0.05 and two-tailed P values < 0.05 were considered statistically significant.
Additional Information
How to cite this article: Zhang, Y. et al. Reciprocal Changes of Circulating Long Non-Coding RNAs ZFAS1 and CDR1AS Predict Acute Myocardial Infarction. Sci. Rep. 6, 22384; doi: 10.1038/srep22384 (2016).
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Funds for Creative Research Groups of the National Natural Science Foundation of China (81421063), the Key Project of National Science Foundation of China (81230081 & 81130088), the National Basic Research Program of China (973 program, 2013CB531104), and the Natural Science Foundation of China (81170219, 81371211, 81470490).
Footnotes
Author Contributions Y.Z., L.H.S., L.N.X., Y.J.L. and B.F.Y.: designed, performed study, supervised all aspects of the research and analysis. Y.Z., L.H.S. and B.F.Y. finalized the manuscript. Y.Z., L.H.S., L.N.X., K.L., Z.W.P., S.S.L., Y.C.H., X.Y.Z., L.H.H., Z.G.W. and Y.H. assisted in research, data analysis and interpretation. J.N.L., Y.T., J.H.Y., H.H., Y.H.L., F.G., Y.Z., S.W., Z.M.D. and Y.J.L. were responsible for design of the study, collect blood samples and for the final approval of the manuscript. K.L., Y.H. and J.N.L. was responsible for the statistical analysis involved in the study. Y.T., J.H.Y., S.W. and Z.M.D. reviewed the clinical aspects and writing of manuscript.
References
- Jaffe A. S., Babuin L. & Apple F. S. Biomarkers in acute cardiac disease: the present and the future. Journal of the American College of Cardiology 48, 1–11 (2006). [DOI] [PubMed] [Google Scholar]
- Wang T. J. et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. The New England journal of medicine 350, 655–663 (2004). [DOI] [PubMed] [Google Scholar]
- Gu Y. L. et al. Comparison of the temporal release pattern of copeptin with conventional biomarkers in acute myocardial infarction. Clinical research in cardiology: official journal of the German Cardiac Society 100, 1069–1076 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agewall S., Giannitsis E., Jernberg T. & Katus H. Troponin elevation in coronary vs. non-coronary disease. European heart journal 32, 404–411 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Circulating miR-499 are novel and sensitive biomarker of acute myocardial infarction. Journal of thoracic disease 7, 303–308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J. et al. Circulating microRNA-19a as a potential novel biomarker for diagnosis of acute myocardial infarction. International journal of molecular sciences 15, 20355–20364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K. et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. European heart journal 31, 659–666 (2010). [DOI] [PubMed] [Google Scholar]
- Maass P. G., Luft F. C. & Bahring S. Long non-coding RNA in health and disease. J Mol Med (Berl) 92, 337–346 (2014). [DOI] [PubMed] [Google Scholar]
- Roberts T. C., Morris K. V. & Weinberg M. S. Perspectives on the mechanism of transcriptional regulation by long non-coding RNAs. Epigenetics: official journal of the DNA Methylation Society 9, 13–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonrock N., Harvey R. P. & Mattick J. S. Long noncoding RNAs in cardiac development and pathophysiology. Circulation research 111, 1349–1362 (2012). [DOI] [PubMed] [Google Scholar]
- Papait R., Kunderfranco P., Stirparo G. G., Latronico M. V. & Condorelli G. Long noncoding RNA: a new player of heart failure? Journal of cardiovascular translational research 6, 876–883 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarswamy R. & Thum T. Non-coding RNAs in cardiac remodeling and heart failure. Circulation research 113, 676–689 (2013). [DOI] [PubMed] [Google Scholar]
- Yuan S. X. et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology 56, 2231–2241 (2012). [DOI] [PubMed] [Google Scholar]
- Xie H., Ma H. & Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. BioMed research international 2013, 136106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S. et al. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer 49, 2949–2959 (2013). [DOI] [PubMed] [Google Scholar]
- Reis E. M. & Verjovski-Almeida S. Perspectives of Long Non-Coding RNAs in Cancer Diagnostics. Frontiers in genetics 3, 32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Wu Z., Zhang J. & Su B. Salivary lncRNA as a potential marker for oral squamous cell carcinoma diagnosis. Molecular medicine reports 7, 761–766 (2013). [DOI] [PubMed] [Google Scholar]
- Tinzl M., Marberger M., Horvath S. & Chypre C. DD3PCA3 RNA analysis in urine-a new perspective for detecting prostate cancer. European urology 46, 182–186, discussion 187 (2004). [DOI] [PubMed] [Google Scholar]
- Fradet Y. et al. uPM3, a new molecular urine test for the detection of prostate cancer. Urology 64, 311–315, discussion 315–316 (2004). [DOI] [PubMed] [Google Scholar]
- Ai J. et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochemical and biophysical research communications 391, 73–77 (2010). [DOI] [PubMed] [Google Scholar]
- Yang B. et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nature medicine 13, 486–491 (2007). [DOI] [PubMed] [Google Scholar]
- Cheng Y. et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci (Lond) 119, 87–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsten M. F. et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circulation. Cardiovascular genetics 3, 499–506 (2010). [DOI] [PubMed] [Google Scholar]
- Kuwabara Y. et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circulation. Cardiovascular genetics 4, 446–454 (2011). [DOI] [PubMed] [Google Scholar]
- Li D. et al. Transcriptome analysis reveals distinct patterns of long noncoding RNAs in heart and plasma of mice with heart failure. PloS one 8, e77938 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K. C. et al. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation 129, 1009–1021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vausort M., Wagner D. R. & Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circulation research 115, 668–677 (2014). [DOI] [PubMed] [Google Scholar]
- Askarian-Amiri M. E. et al. SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA 17, 878–891 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. B. et al. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. The EMBO journal 30, 4414–4422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. B. et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013). [DOI] [PubMed] [Google Scholar]
- Orozco A. F. & Lewis D. E. Flow cytometric analysis of circulating microparticles in plasma. Cytometry. Part A: the journal of the International Society for Analytical Cytology 77, 502–514 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M., Subra C., Silvente-Poirot S. & Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochemical pharmacology 81, 1171–1182 (2011). [DOI] [PubMed] [Google Scholar]
- Kavsak P. A., Wang X., Ko D. T., MacRae A. R. & Jaffe A. S. Short- and Long-Term Risk Stratification Using a Next-Generation, High-Sensitivity Research Cardiac Troponin I (hs-cTnI) Assay in an Emergency Department Chest Pain Population. Clin Chem 55, 1809–1815 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert J. S., Thygesen K., Antman E. & Bassand J. P. Myocardial infarction redefined-a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Journal of the American College of Cardiology 36, 959–969 (2000). [DOI] [PubMed] [Google Scholar]
- Morrow D. A. et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 115, e356–375 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.