Abstract
Background. New immunization programs are dependent on data from surveillance networks and disease burden estimates to prioritize target areas and risk groups. Data regarding invasive Salmonella disease in sub-Saharan Africa are currently limited, thus hindering the implementation of preventive measures. The Typhoid Fever Surveillance in Africa Program (TSAP) was established by the International Vaccine Institute to obtain comparable incidence data on typhoid fever and invasive nontyphoidal Salmonella (iNTS) disease in sub-Saharan Africa through standardized surveillance in multiple countries.
Methods. Standardized procedures were developed and deployed across sites for study site selection, patient enrolment, laboratory procedures, quality control and quality assurance, assessment of healthcare utilization and incidence calculations.
Results. Passive surveillance for bloodstream infections among febrile patients was initiated at thirteen sentinel sites in ten countries (Burkina Faso, Ethiopia, Ghana, Guinea-Bissau, Kenya, Madagascar, Senegal, South Africa, Sudan, and Tanzania). Each TSAP site conducted case detection using these standardized methods to isolate and identify aerobic bacteria from the bloodstream of febrile patients. Healthcare utilization surveys were conducted to adjust population denominators in incidence calculations for differing healthcare utilization patterns and improve comparability of incidence rates across sites.
Conclusions. By providing standardized data on the incidence of typhoid fever and iNTS disease in sub-Saharan Africa, TSAP will provide vital input for targeted typhoid fever prevention programs.
Keywords: typhoid fever, Africa, methodology, surveillance, invasive Salmonella disease
It has been recently estimated that the relative proportion of bacteremia due to invasive Salmonella infections in sub-Saharan Africa has increased dramatically [1], particularly as other major causes of bacteremia, such as Streptococcus pneumoniae and Haemophilus influenzae type b, are decreasing with the implementation of targeted control through immunization programs [2–5]. Furthermore, the disease burden of Plasmodium falciparum malaria is decreasing with successful control measures in many countries in sub-Saharan Africa [6]. However, febrile disease is still commonly misdiagnosed as malaria without ruling out other potential causes of fever, including bacteremia [7]. In light of these changes, there is a need to reevaluate the real contribution of different pathogens as the causes of febrile illnesses in sub-Saharan Africa.
Health policy makers rely on disease burden estimates and data provided by surveillance networks to support decisions on health policies and programs. Although it is widely acknowledged that typhoid fever (TF) and invasive nontyphoidal Salmonella (iNTS) disease are endemic in parts of sub-Saharan Africa, reliable, standardized, age-specific disease burden data across most locations are lacking. Results originating from studies conducted in Asian urban centers have historically shown high incidence rates of TF [8, 9]. These findings had major implications for public health measures in Asia, including the introduction of TF vaccination in school-aged children in Pakistan [10]. A paucity of TF and iNTS disease burden data from Africa limit the ability of health policy makers to evaluate and implement appropriate preventive measures in high-risk groups. Current estimates of the burden of invasive Salmonella disease in sub-Saharan Africa were produced using inaccurate serologic diagnostic approaches to case ascertainment [11], hospital-based investigations (which leave uncertainties about the completeness of capturing cases and the denominator population) [12–15], or postvaccination trial data from Egypt [16] and South Africa [17]. Therefore, existing data are nonspecific or not generalizable except within certain study populations. Prior to the publication of the findings from the Typhoid Fever Surveillance in Africa Program (TSAP) initiative, recent population-based TF incidence data from sub-Saharan Africa were available solely among children 2–8 years of age from an informal urban settlement in Nairobi, Kenya, where incidence was >520 per 100 000 person-years of observation, and the rural village of Agogo, Ghana, where incidence was >200 cases per 100 000 persons per year [18, 19]. A systematic review of the limited data concerning iNTS estimated an incidence in Africa of approximately 227 per 100 000 persons in 2010, with the highest incidence among children and young adults [20]. In addition, uncertainty about increasing resistance to antimicrobials in circulating Salmonella enterica serovar Typhi strains hinders effective treatment, increases treatment costs, and elevates the risk of complications and death [21–23].
In 2009, an international meeting was convened by the International Vaccine Institute (IVI, Seoul, Republic of Korea), the Kenya Medical Research Institute (KEMRI, Nairobi), 5 other international institutions, and 28 investigators from 14 research sites across sub-Saharan Africa, with the aim of establishing a consortium to study invasive salmonelloses in sub-Saharan Africa [24]. Available data on the occurrence of invasive bacterial infections from numerous sites were presented, revealing not only the presence of TF and iNTS disease, but also considerable continent-wide variation in estimated disease prevalence and antimicrobial susceptibility patterns. Such variability was likely dependent on differential disease epidemiology between study sites, but it was hypothesized that variable figures may have been induced by a lack of standardization in study design, data collection, and laboratory techniques. It was surmised that unless standardized methods of data collection and diagnostic procedures were employed across countries and patterns of healthcare utilization were understood and accounted for, the real disease burden of TF and iNTS disease in sub-Saharan African countries would remain unclear. Therefore, to address questions regarding the incidence of TF and iNTS disease in sub-Saharan Africa, meeting members agreed to establish a network assessing disease burden across several sites in sub-Saharan Africa under a standardized protocol.
WHAT IS THE TYPHOID FEVER SURVEILLANCE IN AFRICA PROGRAM?
The TSAP, established in 2009, is a consortium committed to introducing standardized multicountry surveillance for TF and iNTS disease in sub-Saharan Africa. The overarching aim was to produce the evidence required to support policy makers in introducing prevention efforts against invasive Salmonella infections, in particular, the introduction of safe and effective TF vaccines into routine immunization programs.
STUDY SITE SELECTION
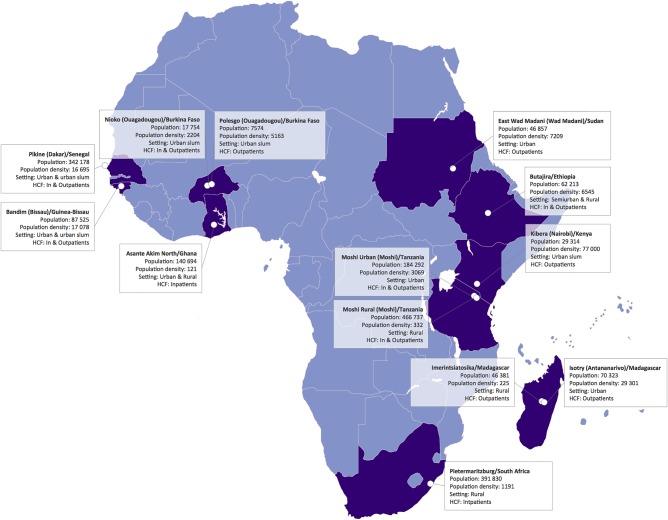
Potential surveillance sites were identified during the 2009 international meeting on invasive salmonelloses, and representatives of the respective bodies on site were contacted. The various international stakeholders discussed available country-specific microbiologic and epidemiologic data at two meetings in Nairobi, Kenya, and Antananarivo, Madagascar. Subsequently, TSAP epidemiologists, data managers, and laboratory specialists evaluated each potential study location through a series of site visits. Assessments of the potential study sites included the demographics of the study population, the logistical suitability for surveillance, the current laboratory infrastructure, and potential for upgrading facilities to conduct febrile disease surveillance, prior experience of local collaborators in surveillance activities, data management capacity, and local and national governmental support of the program. The selection of sites was not random; rather, priority was given to countries and sites that had previously reported TF, and it was ensured that sites were geographically representative. Ultimately, surveillance sites were limited to ten countries: Burkina Faso, Ethiopia, Ghana, Guinea-Bissau, Kenya, Madagascar, Senegal, South Africa, Sudan, and Tanzania (Figure 1). Each country hosted at least one surveillance site, with Burkina Faso, Madagascar, and Tanzania hosting two sites (Table 1). All selected study healthcare facilities were public, serving patients of all ages (except for Ghana, where only children <15 years of age were enrolled), and did not specialize in any particular branch of medical care. Passive surveillance of invasive bacterial diseases was initiated de novo in 8 sites within 6 of the 10 countries. At sites in South Africa, Ghana, Tanzania, and Kenya, the TSAP study protocol was integrated into the existing surveillance infrastructure. The sites in Guinea-Bissau, Burkina Faso, and Ethiopia were part of the International Network for the Demographic Evaluation of Populations and Their Health in Developing Countries (INDEPTH) [45] and were supported by comprehensive Health and Demographic Surveillance Systems (HDSSs). An HDSS provides a platform with a well-characterized population denominator for longitudinal surveillance and evaluation of study populations, allowing for multisite cross-comparison analyses. At the site in Kenya, an HDSS operated by the KEMRI–Centers for Disease Control and Prevention Kenya Collaboration was additionally accessible. At this particular site in Kibera (Nairobi), an active, population-based surveillance component was utilized where home visits were made every two weeks to all HDSS households to screen for febrile patients and encourage visits to the designated sentinel health facility as necessary where free treatment was offered. Once the sentinel facilities were selected, each facility's catchment area boundaries were determined by reviewing facility health records, and catchment populations were determined using census data.
Figure 1.
Locations of the Typhoid Fever Surveillance in Africa Program (TSAP) sites. Abbreviation: HCF, healthcare facility. Population density, /km2
Table 1.
Selected Countries and Survey Sites of the Typhoid Fever Surveillance in Africa Program
| Country and Survey Site | Collaborating Institution | Setting | Catchment Population Size, No. and Year | Published Data on TF in TSAP Countries | Further Characteristics of TSAP Survey Sites |
|||
|---|---|---|---|---|---|---|---|---|
| % of Country Population Using Improved [25]: |
DSS Site [26] | GNI Per Capita, US$ [27] | ||||||
| Drinking Water Sources | Sanitation | |||||||
| South Africa, Pietermaritzburg | NICD | Urban | 361 582 (2011) | [28–31] | Total: 91 Urban: 99 Rural: 79 |
Total: 79 Urban: 86 Rural: 67 |
No | 10 790 |
| Kenya, Kibera | KEMRI–CDC Kenya Collaboration | Urban slum | 26 098 | [19, 32,33] | Total: 59 Urban: 82 Rural: 52 |
Total: 32 Urban: 32 Rural: 32 |
Active surveillance |
1720 |
| Tanzania, Moshi | Kilimanjaro Christian Medical Centre/DUKE Collaboration | Rural and urban | 545 168 (2002) | [29, 34–36] | Total: 53 Urban: 79 Rural: 44 |
Total: 10 Urban: 20 Rural: 7 |
No | 1510 |
| Ghana, Asante Akim North Municipal | Kumasi Centre for Collaborative Research in Tropical Medicine | Rural | 80 074 | [18, 37] | Total: 86 Urban: 91 Rural: 80 |
Total: 14 Urban: 19 Rural: 8 |
No | 1820 |
| Ethiopia, Butajira | Armauer Hansen Research Institute | Rural and urban | 61 965 (2012) | [38] | Total: 44 Urban: 97 Rural: 34 |
Total: 21 Urban: 29 Rural: 19 |
Yes | 1110 |
| Madagascar, (1) Isotry and (2) Imerintsiatosika |
University of Antananarivo | (1) Urban slum (2) Rural |
(1) 70 323 (1993) (2) 49 038 (2010) |
[39, 40] | Total: 46 Urban: 74 Rural: 34 |
Total: 15 Urban: 21 Rural: 12 |
No | 950 |
| Senegal, Pikine (Dakar) | Institute Pasteur Senegal | Urban slum | 342 178 (2012) | [41, 42] | Total: 72 Urban: 93 Rural: 56 |
Total: 52 Urban: 70 Rural: 39 |
No | 1960 |
| Burkina Faso, (1) Kossodo and (2) Polesgo |
University of Ouagadougou | Urban | (1) 13 846 (2012) (2) 5738 (2012) |
[43] | Total: 79 Urban: 95 Rural: 73 |
Total: 17 Urban: 50 Rural: 6 |
Yes | 1310 |
| Sudan, WadMedani | University of Gezira | Urban | 47 935 (2013) | [44] | Total: 58 Urban: 67 Rural: 52 |
Total: 26 Urban: 44 Rural: 14 |
No | 2020 |
| Guinea-Bissau, Bandim (Bissau) | Bandim Health Project | Urban | 100 153 (2012) | No published data | Total: 64 Urban: 91 Rural: 53 |
Total: 20 Urban: 44 Rural: 9 |
Yes | 1250 |
Abbreviations: CDC, Centers for Disease Control and Prevention; DSS, Demographic Surveillance System; DUKE, Duke University Medical Center; GNI, Gross National Income; KEMRI, Kenya Medical Research Institute; NICD, National Institute for Communicable Diseases; TF, typhoid fever; TSAP, Typhoid Fever Surveillance in Africa Program.
TSAP NETWORK
The TSAP team was composed of IVI staff and local staff and included a local principal investigator for each country, a project coordinator, epidemiologists, a data management team, and administrative support staff. The team relied on international partnerships as well as collaborations with local and international scientists, medical doctors, epidemiologists, and public health officials. TSAP also benefited from the input of a laboratory consultant for matters regarding diagnostics, laboratory techniques, quality control, and quality assurance, and a medical anthropological consultant for matters concerning ethics and social implications.
The implementation of TSAP was contingent on collaboration with national Ministries of Health in each participating country. The program further benefited from country-specific field support of external partners, including the World Health Organization (WHO). The Bernhard Nocht Institute for Tropical Medicine in Germany served as the reference laboratory for the confirmation, antimicrobial susceptibility testing, and serotyping of all Salmonella enterica isolates. Bacterial isolates other than Salmonella enterica were confirmed at a reference laboratory within the Oxford University Clinical Research Unit in Ho Chi Minh City, Vietnam. The Wellcome Trust Sanger Institute (Cambridge, United Kingdom), provided further support in generating genome sequences of Salmonella isolates and assessing the molecular basis of antimicrobial resistance phenotypes. A further partnership was established with the National Institute for Communicable Diseases, a division of the National Health Laboratory Service located in Johannesburg, South Africa, for supplemental laboratory and technical support and the management of specimens. Finally, for scientific advice and critical analysis of the program and its outcomes, the TSAP team consulted with experts from the Coalition Against Typhoid, a global forum of health and immunization experts on TF vaccination [46], and members of the WHO.
TSAP OPERATIONAL PROCEDURES
Ethical Approval
The IVI Institutional Review Board (IRB), the national ethical review bodies in each participating country, and, where required, local research ethics committees approved the final TSAP study protocol. The IVI IRB reviewed the project annually to ensure continued compliance with the International Conference on Harmonisation, the guidelines for good clinical practice, and the ethical principles that have their origins in the Declaration of Helsinki [47].
Training for Clinical and Laboratory Staff
During the TSAP initiation meeting held in Johannesburg in August 2011, discussions, revisions, and training courses were conducted with research teams from all sites with respect to laboratory procedures, patient recruitment, clinical procedures, data collection, and data management. The principal investigators, project coordinators, and laboratory representatives from each country attended the project initiation meeting. In individual training sessions for staff, the aims and objectives of TSAP were discussed. All details regarding screening and enrollment criteria, informed consent procedures, fever measurement techniques, taking clinical histories, physical examination, blood sampling techniques, safety and handling of blood samples, sample transportation, laboratory procedures, interpretation of results, filling of data collection instruments, correcting data errors, and reporting of study results were defined and implemented. To ensure that all procedures were executed in a consistent and standardized manner across the network, the workflow was streamlined according to standard operating procedures (SOPs). Regular follow-up and monitoring visits by TSAP coordinators and field experts were performed throughout the study period.
Study Enrollment Criteria and Clinical Procedures
All patients who resided in the study catchment areas and presenting to any of the healthcare facilities involved in the study were registered and their tympanic or axillary temperature taken with a digital thermometer by study staff to determine their eligibility for inclusion in the study. Outpatients of all ages with a tympanic or axillary temperature of ≥38.0°C or ≥37.5°C, respectively, were eligible for enrollment, as were inpatients with reported fever within the past 72 hours or a tympanic or axillary temperature of ≥38.0°C or ≥37.5°C, respectively. Inpatients were recruited upon admission. The site in Ghana recruited only children aged <15 years. After detailed explanation of the study purpose, written informed consent was sought. For children, written informed consent was obtained from the parent or guardian of the eligible child. Study staff completed the recruitment form, and a study physician took the patient's clinical history, performed the physical examination, and completed the case report form. This form consisted of 5 sections: (1) participant information, (2) history of illness, (3) physical examination, (4) clinical appraisal, and (5) the name and signature of the recruiting physician. Eligible patients were treated according to the national guidelines of each participating country. A member of the study team collected blood samples (5–10 mL for adults; 1–3 mL for children). The designated microbiology laboratory then processed the blood cultures (see below), and results were relayed to the patient care team. The timing of this notification depended on the test result: A negative microbial growth result was reported following a minimum incubation period of five days; positive blood culture results were communicated immediately to prompt discussion of possible implications and treatment options for the patient. Monthly meetings were held with all study staff on site to review results and discuss the study status.
Laboratory Procedures
The sensitivity and reliability of blood culture diagnosis varies on the basis of several parameters, including the pathogen, venipuncture procedures, sample contamination, blood volume inoculated in the culture medium, transport conditions and timing, recent antimicrobial treatment, and laboratory techniques [48–50]. To manage and standardize these parameters across sites, a joint initial training session for laboratory managers was incorporated into the TSAP implementation workshop in Johannesburg, South Africa. Furthermore, we initiated biannual training courses, conducted by a trained microbiologist, at each site. One aerobic blood culture bottle was inoculated per patient and incubated in a continuously monitored blood culture instrument (BACTEC Peds Plus Medium/BACTEC Plus Aerobic-F, BACTEC, Becton-Dickinson, New Jersey; or BacT/ALERT PF Pediatric FAN/BacT/ALERT FA FAN Aerobic, bioMérieux, Marcy l'Etoile, France), with the exception of the site in Sudan, where manual blood culture techniques were used. For blood cultures indicating bacterial growth subcultures were conducted and standard biochemical methods were used for identification. Contaminants were inferred by previous publications and consultations with experienced clinical microbiologists. The refined list of clinically significant or nonsignificant blood culture isolates may not necessarily reflect the clinical observations and may be confounded by human immunodeficiency virus status, malnutrition, or other comorbidities. For example, environmental bacteria and bacteria commonly belonging to the skin flora (eg, coagulase-negative staphylococci, Corynebacterium species, and Bacillus species) were considered as contaminants; however, without combining these organisms with clinical data from every patient, we were unable to discount atypical or opportunistic infections in every case. Suspected Salmonella isolates were identified biochemically by API 20E tests (bioMérieux).
As participating laboratories had differing resources and performance capacities, site-specific training and implementation efforts were undertaken to maintain standards at an uniform level across all sites. SOPs were available for sample collection, blood culture processing including Gram stain, subculturing on blood agar, chocolate agar and MacConkey agar (all Oxoid, Basingstoke, United Kingdom), biochemical reactions for identification and serogrouping if indicated as well as for antimicrobial susceptibility testing performed by agar diffusion tests according to the most current version of the Clinical Laboratory and Standards Institute guidelines [51].
All bacterial isolates were stored (Microbank PL170, Pro-Lab Diagnostics, Round Rock, Texas) at the study sites at −70°C to preserve them for shipment to the reference laboratories for confirmation, further characterization, and additional antimicrobial susceptibility testing.
Quality Assurance
Ongoing internal quality control procedures were implemented at all sites to maintain the integrity of laboratory surveillance, including growth and purity control of self-prepared media, performance of test reagents, and antimicrobial susceptibility testing. Reference strains (American Type Culture Collection, Manassas, Virginia) were provided to all collaborating laboratories to support quality control procedures. The TSAP laboratory coordinator monitored adherence to the SOPs for all laboratory activities and conducted quality assurance assessments during regular site visits, working toward a laboratory quality system management that met international laboratory standards (ISO 15189) [52]. In addition, all national principal investigators and key clinical staff were required to complete basic laboratory training. Local laboratory staff received additional TSAP-specific laboratory training and support from the TSAP laboratory consultant.
Healthcare Utilization Surveys
Healthcare-seeking behavior of febrile patients has substantial implications for developing appropriate disease prevention strategies and more accurately estimating the burden of febrile illnesses. Contemporary hospital records and census data were utilized to estimate the boundaries of each catchment area and the population residing in it. A standardized and pretested HealthCare Utilization Survey (HCUS) was administered to a random sample of households to determine the age-stratified proportions of the respective catchment population that would seek care at a TSAP study healthcare facility during a febrile disease episode [53]. Each facility catchment area was divided into 30 clusters that were allocated proportionally according to population, and then the standard formula of Henderson and Sundaresan [54] was applied to calculate the number of surveys needed to be conducted within the catchment area. A list of randomly selected eligible households was generated from within the catchment area using randomization software (Microsoft Visual Foxpro 7.0, Redmond, Washington) as detailed in by Panzner et al [53]. The HCUS questionnaire focused on three domains: (1) general information about the household, the respondent and household members; (2) healthcare-seeking behavior of household members in general and in case of febrile illness in particular; and (3) socioeconomic information about the household. Further details of this component of the study are described elsewhere [53].
Data Management
Except for the site in Kenya, where data collection was conducted using an electronic Patient Care System, paper-based data collection was used. At the Moshi site, we used HP TeleForms (Hewlett-Packard, Palo Alto, California) including a scanning (entry) stage and a verification stage. For the remaining study sites, the software system for entry, management, and analysis of project data was developed using Microsoft Foxpro (version 7.0, Redmond, Washington). On-site data management staff conducted double data entry. A relational database was designed in which data were stored in several standardized tables. This data storage policy allowed for the storage of all information without redundancy and permitted the rapid retrieval of information. Exploratory data analysis was integrated into the system to detect data errors, including breaking of sequential form number, duplicated records, range errors, inconsistencies, and unlinked data. All information was confidential and participant data were processed anonymously. Access to the hard copy of the data and the electronic database was authorized to senior study personnel only, and the electronic database was password restricted. All data documents including system documents, forms, logs of data flow, error outputs, error resolutions, and the process of data cleaning were retained for future reference. Monthly data summaries were produced and reviewed by investigators to avoid large-scale mistakes or omissions. Multiple backups with at least three previous generations were kept to avoid data loss. One backup was kept on the IVI data server.
Incidence Calculations
Adjusted incidence rates (IRs) were estimated per 100 000 person-years of observation (PYO) from confirmed invasive Salmonella infections and catchment populations for comparability across study sites and to previously published incidences. In HDSS sites, where households were routinely visited (ie, every three months), each resident contributed to PYO during the study recruitment period; the time contributed by a resident ended if he/she died or was no longer registered in the applicable area for any reason. For simplicity, all patients in the catchment area contributed to PYO for the respective period of surveillance—before, during, and after any illness attributed to TF or iNTS disease. In non-HDSS sites, PYO was calculated by projecting the catchment population from the start to the end of the study recruitment period based on annualized growth rates. The calculated average population was then multiplied by the number of years of surveillance. The population within the catchment areas determined by review of health facility records was obtained from national census databases, Ministries of Health, and Departments of Health and Social Services. Based on the HCUS findings, age-stratified healthcare utilization adjustment factors were applied to catchment populations to determine the number of people within each age group consulting sentinel healthcare facilities in case of fever. Adjusted populations contributing to PYO were calculated and used as denominators for incidence calculations. A subsequent adjustment factor was applied to invasive Salmonella cases (numerator) to account for the proportion of eligible patients of recruited patients that were enrolled in the study. The 95% confidence interval (CI) for the adjusted IR was derived on the log scale and the antilog was used to give a CI for the adjusted rate. The error factor (EF) formula was used (EF = exp[1.96/√adjusted cases]) and the result applied to calculate the lower (adjusted rate/EF) and upper (adjusted rate × EF) 95% CI for a rate.
The capture of accurate incidence data for TF and iNTS disease is difficult given the limited diagnostic sensitivity of blood culture [49], a methodology that is already difficult to broadly implement as a consequence of cost, equipment, and technical expertise. Statistical adjustments for this, as well as for blood culture volume inadequacy or antimicrobial pretreatment, were not considered. Adjusted IRs for TF and iNTS were assessed for sites in Guinea-Bissau, Ghana, Kenya, Burkina Faso, Madagascar, and Tanzania. Differences in proportions were assessed using the χ2 test (SAS software, version 9.3, SAS Institute, Cary, North Carolina).
CONCLUSIONS
The TSAP, a network of 13 sentinel sites in ten sub-Saharan African countries, was created to generate high-quality, contemporary, standardized data on the incidences of TF and iNTS disease in sub-Saharan Africa. TSAP and its collaborators have strengthened local surveillance capacities at multiple sites across sub-Saharan Africa using the methods described, creating a system that has improved bacterial disease diagnostics, disease surveillance, reporting, and analysis systems. Based on these data, the TSAP network is an ideal group of strategically based healthcare centres in sub-Saharan Africa that can be expanded and utilized for additional infectious diseases research including the evaluation and introduction of new or underutilized vaccines.
Notes
Acknowledgments. The International Vaccine Institute (IVI) is grateful to all field staff collaborating on the project in the ten countries where surveillance is conducted. The IVI also acknowledges the invaluable support of the medical anthropology consultant, Alfred Pach, as well as the extensive institutional administrative support given by Soo-Young Kwon, Hye-Jin Seo and Hyon-Jin Jeon. The IVI also thanks members from the Coalition Against Typhoid, Gavi, the Sabine Vaccine Institute, the Bill & Melinda Gates Foundation, Fondation Mérieux, the Oxford University Clinical Research Unit (Vietnam), and the World Health Organization for their support and expertise provided to TSAP.
Disclaimer. The findings and conclusions contained within this publication are those of the authors and do not necessarily reflect the positions or policies of the Bill & Melinda Gates Foundation or the US Centers for Disease Control and Prevention.
Financial support. The publication is based on research funded by the Bill & Melinda Gates Foundation (OPPGH5231). IVI acknowledges its donors, including the Republic of Korea and the Swedish International Development Cooperation Agency. Research infrastructure at the Moshi site was supported by the US National Institutes of Health (grant numbers R01TW009237; U01 AI062563; R24 TW007988; D43 PA-03-018; U01 AI069484; U01 AI067854; P30 AI064518), and by the UK Biotechnology and Biological Sciences Research Council (grant number BB/J010367). S. B. is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (100087/Z/12/Z). This publication was made possible by a grant from the Bill & Melinda Gates Foundation (OPP1129380).
Supplement sponsorship. This article appears as part of the supplement “Typhoid Fever Surveillance in Africa Program (TSAP)” sponsored by the International Vaccine Institute.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gordon MA, Graham SM, Walsh AL et al. Epidemics of invasive Salmonella enterica serovar Enteritidis and S. enterica serovar Typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis 2008; 46:963–9. [DOI] [PubMed] [Google Scholar]
- 2.Scott JA. The preventable burden of pneumococcal disease in the developing world. Vaccine 2007; 25:2398–405. [DOI] [PubMed] [Google Scholar]
- 3.Lucero MG, Dulalia VE, Nillos LT et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev 2009; 4:CD004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessner BD. Haemophilus influenzae type b vaccine impact in resource-poor settings in Asia and Africa. Expert Rev Vaccines 2009; 8:91–102. [DOI] [PubMed] [Google Scholar]
- 5.Morris SK, Moss WJ, Halsey N. Haemophilus influenzae type b conjugate vaccine use and effectiveness. Lancet Infect Dis 2008; 8:435–43. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization, Global Malaria Programme. World malaria report 2012. Available at: http://www.who.int/malaria/publications/world_malaria_report_2012/report/en/index.html Accessed 1 June 2015.
- 7.Crump JA, Morrissey AB, Nicholson WL et al. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis 2013; 7:e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochiai RL, Acosta CJ, Agtini M et al. The use of typhoid vaccines in Asia: the DOMI experience. Clin Infect Dis 2007; 45(suppl 1):S34–8. [DOI] [PubMed] [Google Scholar]
- 9.Ochiai RL, Acosta CJ, Danovaro-Holliday MC et al. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ 2008; 86:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochiai RL, Khan MI, Soofi SB et al. Immune responses to Vi capsular polysaccharide typhoid vaccine in children 2 to 16 years old in Karachi, Pakistan, and Kolkata, India. Clin Vaccine Immunol 2014; 21:661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frimpong EH, Feglo P, Essel-Ahun M, Addy PA. Determination of diagnostic Widal titres in Kumasi, Ghana. West Afr J Med 2000; 19:34–8. [PubMed] [Google Scholar]
- 12.Mills-Robertson F, Addy ME, Mensah P, Crupper SS. Molecular characterization of antibiotic resistance in clinical Salmonella Typhi isolated in Ghana. FEMS Microbiol Lett 2002; 215:249–53. [DOI] [PubMed] [Google Scholar]
- 13.Wilkens J, Newman MJ, Commey JO, Seifert H. Salmonella bloodstream infection in Ghanaian children. Clin Microbiol Infect 1997; 3:616–20. [DOI] [PubMed] [Google Scholar]
- 14.Gross U, Amuzu SK, de Ciman R et al. Bacteremia and antimicrobial drug resistance over time, Ghana. Emerg Infect Dis 2011; 17:1879–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obaro S, Lawson L, Essen U et al. Community acquired bacteremia in young children from central Nigeria—a pilot study. BMC Infect Dis 2011; 11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahdan MH, Sérié C, Cerisier Y, Sallam S, Germanier R. A controlled field trial of live Salmonella Typhi strain ty 21a oral vaccine against typhoid: three-year results. J Infect Dis 1982; 145:292–5. [DOI] [PubMed] [Google Scholar]
- 17.Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella Typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine 1996; 14:435–8. [DOI] [PubMed] [Google Scholar]
- 18.Marks F, Adu-Sarkodie Y, Hünger F et al. High incidence of typhoid fever among ghanaian children. Emerg Infect Dis 2010; 16:1796–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breiman RF, Cosmas L, Njuguna H et al. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 2012; 7:e29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ao TT, Feasey NA, Gordon MA et al. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg Infect Dis 2015; 21:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunguya O, Lejon V, Phoba M-F et al. Antimicrobial resistance in invasive non-typhoid Salmonella from the Democratic Republic of the Congo: emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLoS Negl Trop Dis 2013; 7:e2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutterloh E, Likaka A, Sejvar J et al. Multidrug-resistant typhoid fever with neurologic findings on the Malawi-Mozambique border. Clin Infect Dis 2012; 54:1100–6. [DOI] [PubMed] [Google Scholar]
- 23.Wong VK, Baker S, Pickard DJ et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 2015; 47:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemens JD. Meeting on establishment of consortium to study invasive salmonelloses in sub-Saharan Africa. Emerg Infect Dis 2009; 5:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization/United Nations Children's Fund Joint Monitoring Programme for Water Supply and Sanitation. Progress on drinking water and sanitation—2012 update. Available at: http://www.wssinfo.org/fileadmin/user_upload/resources/JMP-report-2012-en.pdf Accessed 2 April 2015.
- 26.INDEPTH Network. Demographic surveillance site (DSS). Available at: http://www.indepth-network.org Accessed 2 April 2015.
- 27.The World Bank. GNI per capita, PPP (current international $). Available at: http://data.worldbank.org/indicator/NY.GNP.PCAP.PP.CD Accessed 2 April 2015.
- 28.Coovadia YM, Gathiram V, Bhamjee A et al. An outbreak of multiresistant Salmonella Typhi in South Africa. Q J Med 1992; 82:91–100. [PubMed] [Google Scholar]
- 29.Keddy KH, Sooka A, Letsoalo ME et al. Sensitivity and specificity of typhoid fever rapid antibody tests for laboratory diagnosis at two sub-Saharan African sites. Bull World Health Organ 2011; 89:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keddy KH, Sooka A, Ismail H et al. Molecular epidemiological investigation of a typhoid fever outbreak in South Africa, 2005: the relationship to a previous epidemic in 1993. Epidemiol Infect 2011; 139:1239–45. [DOI] [PubMed] [Google Scholar]
- 31.Keddy KH, Smith AM, Sooka A, Ismail H, Oliver S. Fluoroquinolone-resistant typhoid, South Africa. Emerg Infect Dis 2010; 16:879–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feikin DR, Olack B, Bigogo GM et al. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One 2011; 6:e16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kariuki S, Revathi G, Kiiru J et al. Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in Southeast Asia. J Clin Microbiol 2010; 48:2171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crump JA, Ramadhani HO, Morrissey AB et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health 2011; 16:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crump JA, Ramadhani HO, Morrissey AB et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis 2011; 52:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biggs HM, Lester R, Nadjm B et al. Invasive Salmonella infections in areas of high and low malaria transmission intensity in Tanzania. Clin Infect Dis 2014; 58:638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen MV, Sarpong N, Krumkamp R et al. Incidence and characteristics of bacteremia among children in rural Ghana. PLoS One 2012; 7:e44063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worku B. Typhoid fever in an Ethiopian children's hospital: 1984–1995. Ethiop J Health Dev 2000; 14:311–5. [Google Scholar]
- 39.Charieras JL, de Broca A. Typhoid fever in children in Tananarive (Madagascar). Comments on 97 cases [in French]. Med Trop (Mars) 1985; 45:413–22. [PubMed] [Google Scholar]
- 40.Vieu JF, Binette H, Leherissey M. Absence of the antigen H:z66 in 2355 strains of Salmonella Typhi from Madagascar and several countries of tropical Africa [in French]. Bull Soc Pathol Exot Filiales 1986; 79:22–6. [PubMed] [Google Scholar]
- 41.Lefebvre N, Gning SB, Nabeth P et al. Clinical and laboratory features of typhoid fever in Senegal. A 70-case study [in French]. Med Trop (Mars) 2005; 65:543–8. [PubMed] [Google Scholar]
- 42.Dromigny JA, Perrier-Gros-Claude JD. Antimicrobial resistance of Salmonella enterica serotype Typhi in Dakar, Senegal. Clin Infect Dis 2003; 37:465–6. [DOI] [PubMed] [Google Scholar]
- 43.Zida M, Ouedraogo T, Bandre E et al. Primary ileostomy for typhoid-related ileal perforation: a 62-case series in Ouagadougou, Burkina Faso [in French]. Med Trop (Mars) 2010; 70:267–8. [PubMed] [Google Scholar]
- 44.Ibrahim SA, Asakir SF, Idris AA, Martinez-Urtaza J, Hag Elsafi HE. Prevalence of Salmonella species among asymptomatic food handlers in Khartoum State, Sudan. Br J Biomed Sci 2013; 70:88–9. [DOI] [PubMed] [Google Scholar]
- 45.Sankoh O, Byass P. The INDEPTH Network: filling vital gaps in global epidemiology. Int J Epidemiol 2012; 41:579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson CB, de Quadros C. Coalition against Typhoid (CaT): a new, global initiative to advance typhoid vaccination. Vaccine 2011; 29:6443. [DOI] [PubMed] [Google Scholar]
- 47.World Medical Association. WMA declaration of Helsinki: ethical principles for medical research involving human subjects. 2013. Available at: http://www.wma.net/en/30publications/10policies/b3/ Accessed 1 Jun 2015. [DOI] [PubMed]
- 48.Hoffman SL, Edman DC, Punjabi NH et al. Bone marrow aspirate culture superior to streptokinase clot culture and 8 mL 1:10 blood-to-broth ratio blood culture for diagnosis of typhoid fever. Am J Trop Med Hyg 1986; 35:836–9. [DOI] [PubMed] [Google Scholar]
- 49.Gilman RH, Terminel M, Levine MM et al. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella Typhi in typhoid fever. Lancet 1975; 1:1211–3. [DOI] [PubMed] [Google Scholar]
- 50.Wain J, Bay PVB, Vinh H et al. Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. J Clin Microbiol 2001; 39:1571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial suceptibility testing. CLSI M100-S-23, ed Wayne, PA: CLSI, 2013. [Google Scholar]
- 52.Minnick RA. ISO 15189 in use 'round the world. MLO Med Lab Obs 2010; 42:6. [PubMed] [Google Scholar]
- 53.Panzner U, Pak GD, Aaby P et al. Utilization of healthcare in the Typhoid Fever Surveillance in Africa Program. Clin Infect Dis 2016; 62(suppl 1):S56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henderson RH, Sundaresan T. Cluster sampling to assess immunization coverage: a review of experience with a simplified sampling method. Bull World Health Organ 1982; 60:253–60. [PMC free article] [PubMed] [Google Scholar]