Robust increases in tuberculosis-specific polyfunctional (IFN-γ+/IL-2+/TNF-α+) CD4+ T-cells on antiretroviral therapy (ART) are independently associated with paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS). Development of TB-IRIS may be related to coordinated recovery of adaptive and innate immune responses on ART.
Keywords: HIV, tuberculosis, immune reconstitution inflammatory syndrome (IRIS), ART, cellular immune function
Abstract
Background. The immunopathogenesis of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) remains unclear. We determined the association between pathogen-specific T-cell responses and development of paradoxical TB-IRIS on antiretroviral therapy (ART).
Methods. This study was nested within a prospective cohort study of HIV-infected patients with active pulmonary tuberculosis and baseline CD4 counts ≤125 cells/µL initiating ART. T-cell immune activation (CD38, HLA-DR, and PD-1 expression), phenotype, and polyfunctional pathogen-specific cellular immune responses prior to and 4 weeks after ART initiation were determined by flow cytometry. Patients with TB-IRIS were compared to non-IRIS controls using χ2 and rank-sum tests and logistic regression.
Results. TB-IRIS patients and controls had similar CD4 counts, levels of T-cell–associated immune activation, frequencies of T-cell memory subsets, and frequencies of interferon gamma (IFN-γ+)/interleukin 2 (IL-2+)/tumor necrosis factor alpha (TNF-α+) CD4+ T-cells prior to ART initiation. After ART initiation, cellular immune activation and T-cell subsets also were similar in TB-IRIS patients and controls. In contrast, TB-IRIS patients had significantly greater early increases in the frequency of tuberculosis-specific polyfunctional IFN-γ+/IL-2+/TNF-α+ CD4+ T-cells on ART (P = .02); each quartile increase in the percentage of these cells was independently associated with a 2.8-fold increased risk of TB-IRIS (95% confidence interval, 1.1 to 7.5-fold). In a secondary analysis, patients with TB-IRIS had rapid, concomitant increases in tuberculosis-specific adaptive immune responses and interleukin 6 (IL-6) levels, whereas controls with similarly rapid increases in cellular immune function had IL-6 levels that tended to decrease on ART.
Conclusions. Rapid expansion of tuberculosis-specific polyfunctional CD4+ T-cell responses, likely linked to increases in IL-6, is associated with development of paradoxical TB-IRIS.
Initiating antiretroviral therapy (ART) early during tuberculosis treatment in patients with advanced human immunodeficiency virus (HIV)/tuberculosis is associated with improved survival [1, 2]; however, this strategy also increases risk for worsening of tuberculosis symptoms in a condition known as paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) [3–5]. The incidence of TB-IRIS ranges between 8% and 54% [6, 7], and associated morbidity can be significant [3–5]. With the World Health Organization advocating early ART during tuberculosis treatment in highly immunosuppressed HIV/tuberculosis-coinfected patients [8], the incidence of TB-IRIS will likely rise [4].
Immunologically, higher pre-ART levels of immune activation and rapid increases in tuberculosis-specific interferon gamma (IFN-γ)–producing cells after ART initiation in HIV-infected adults have been associated with development of TB-IRIS [7, 9–12]. However, in several recent studies, levels of pre-ART immune activation were actually lower in TB-IRIS patients vs controls [13–15], and multiple reports have indicated that patients without TB-IRIS may also have robust increases in levels of pathogen-specific immune responses on ART [16, 17]. Existing data are therefore inconclusive about whether higher levels of pre-ART immune activation predispose to TB-IRIS and whether tuberculosis-specific T-cells are involved in TB-IRIS pathogenesis.
One important limitation is that previous studies relating immune recovery on ART to TB-IRIS have primarily focused on IFN-γ responses alone [9, 16, 17]; however, multiple cytokines including interleukin 2 (IL-2) and tumor necrosis factor alpha (TNF-α), which are involved in T-cell proliferation and host protection in tuberculosis disease, may also be associated with TB-IRIS [18, 19]. Furthermore, innate immune responses involving rapid increases in proinflammatory cytokines such as interleukin 6 (IL-6) on ART have also been relatively consistently implicated in the pathogenesis of TB-IRIS [11, 20].
To evaluate these issues further, we tested the hypothesis that TB-IRIS is associated with robust recovery of polyfunctional tuberculosis-specific CD4+ T-cells in a relatively large, well-characterized cohort of patients with advanced HIV/tuberculosis coinfection. We also compared levels of T-cell immune activation and memory subsets between IRIS patients and controls.
METHODS
Ethics Statement
The institutional review boards of the University of Pennsylvania, the Botswana Ministry of Health, and the Princess Marina Hospital in Gaborone, Botswana, approved this study.
Study Design
This study was nested within a prospective cohort study investigating the relationship between early immune recovery on ART and early mortality [21]. Here, we investigated the relationship between cellular phenotype and function at baseline and week 4 post–ART initiation and the development of paradoxical TB-IRIS in the first 6 months of ART.
Study Participants
Patients were enrolled from public outpatient clinics and the main public hospital in Gaborone, Botswana, from November 2009 to July 2013, as described elsewhere [15]. Participants had to be HIV-infected, ART-naive adults with pre-ART CD4 counts ≤125 cells/µL who planned to initiate ART within 2 months of commencing antituberculosis therapy for a new diagnosis of WHO-defined smear-positive or smear-negative pulmonary tuberculosis [15, 21, 22]. Pregnant patients, those on immunomodulatory agents, and those with drug-resistant tuberculosis were excluded [15, 21]. For the primary analysis, patients had to have clinical data and peripheral blood mononuclear cells (PBMCs) available from baseline and/or week 4 post–ART initiation. A secondary analysis explored the association between longer-term CD4 count recovery and TB-IRIS among all those in the cohort who had at least a CD4 count measured at 3 and/or 6 months after ART initiation, regardless of PBMC availability.
Data Collection
Clinical data were collected at baseline and monthly for 6 months after ART initiation. CD4 counts, HIV RNA load, and PBMCs were obtained at baseline and week 4 post–ART initiation [21]. CD4 counts were obtained at 3 and/or 6 months post–ART initiation as part of clinical care. A modified International Network for Study of HIV-associated IRIS definition was used to adjudicate TB-IRIS retrospectively [15, 23]. All cases also met the AIDS Clinical Trials Group definition of IRIS [24].
PBMC Preparation and Stimulation
Previously cryopreserved PBMCs were thawed in RPMI 1640 medium (Gibco) containing 10% fetal bovine serum (Gemini Bio-Products), 2 mM l-glutamine (Gibco), 100 U/mL of penicillin and streptomycin (Gibco), and DNase (50 µg/mL, Sigma-Aldrich). PBMCs were rested overnight at 37°C in 5% carbon dioxide. When <4 million PBMCs were available, only surface staining for phenotyping T-cells was done. If a patient had ≥4 million PBMCs, 1 million cells were stimulated with (1) purified anti-CD3 (eBioscience) at 4.0 µg/mL (positive control); (2) HIV consensus C Gag peptide pool (National Institutes of Health AIDS Research and Reference Reagents Program) at 2 µg/peptide/mL for HIV-specific responses; (3) purified protein derivative (PPD; Statens Serum Institute) at 5 µg/mL to measure tuberculosis-specific response; and (4) media alone (negative control). All reactions were co-stimulated with CD28/CD49d (BD FastImmune). After 2 hours of stimulation, monensin and brefeldin A (BD Biosciences) were added and incubated for an additional 4 hours at 37°C in 5% carbon dioxide.
Flow Cytometry
Multicolor flow panels were used to define phenotype and function of T-cells (Supplementary Table 1). LIVE/DEAD fixable aqua stain (Life Technologies) was used for dead cell exclusion. Anti-CD3-BV570 (Biolegend), anti-CD4-Qdot (QD) 705 or QD655, and anti-CD8-QD605 (Life Technologies) were used to identify CD4+ and CD8+ T-cells. Memory T-cell subsets were determined using anti-CD45RO-PE-Texas-Red (Beckman Coulter), anti-CCR7-APC-eFluro780 (eBioscience), and anti-CD27-BV650 (Biolegend). T-cell activation was determined with anti-CD38-PE-Cy7 (Biolegend), anti-HLA-DR-BV785 or FITC (Biolegend, BD Biosciences), and anti-PD1-APC-Cy7 (Biolegend). Intracellular cytokine staining was performed with anti-IFN-γ-Pacific blue (Biolegend), anti-IL-2-AF647 (Biolegend), and anti-TNF-α-AF700 (eBioscience). Stained cells were acquired on an LSR II (BD Biosciences). Flow cytometric data were analyzed using FlowJo version 9.8.2 (TreeStar), Pestle version 1.7, and SPICE version 5.35 [25]. To determine antigen-specific intracellular cytokine levels, the frequency of cytokine-producing cells in the negative control was subtracted from the antigen-stimulated responses. Boolean gating was employed to determine the frequency of poly- and monofunctional IFN-γ, IL-2, and/or TNF-α–containing T-cell subsets.
Statistical Analysis
In the primary analysis, patients with TB-IRIS were compared to patients who survived without TB-IRIS (controls). Participants who died without IRIS were included as controls in a sensitivity analysis of the primary relationship between polyfunctional immune recovery and IRIS. Continuous and categorical variables were compared using Wilcoxon rank-sum and χ2 tests, respectively. All tests were 2-sided and a P < .05 was considered statistically significant. Cells with viability of >50% were used in the analysis; median viability of PBMCs was 72% (interquartile range [IQR], 62%–72%) and did not differ between IRIS patients and controls. Clinical factors associated with TB-IRIS with a P < .20 in the univariate analyses were considered potential confounders in multivariable logistic regression analysis evaluating the association between changes in polyfunctional CD4+ T-cell responses (stratified into quartiles) and TB-IRIS. Factors that changed the unadjusted odds ratio (OR) by >10% were deemed true confounders and were retained in the final model. Furthermore, to evaluate the broader hypothesis that TB-IRIS is associated with greater immune recovery on ART, a secondary analysis used a generalized estimating equation (GEE) model with an independent correlation structure and robust standard error estimators to evaluate the association between TB-IRIS and CD4 count changes on ART during 6 months of follow-up, including all available CD4 counts and adjusting for potential confounders. Additionally, some control patients have rapid recovery of tuberculosis-specific T-cell function similar to TB-IRIS patients, but do not progress to IRIS [15–17]. In an exploratory analysis, we tested the hypothesis that increases in IL-6 levels, which have been associated with IRIS [11, 13, 15, 20], would be less in control patients who also had rapid recovery of cellular immune function compared to TB-IRIS patients using previously determined IL-6 concentrations [15] and rank-sum tests. In this analysis, controls were restricted to those described in this study who had increases at week 4 of ART that were greater than the overall group median for IFN-γ+/IL-2+/TNF-α+ CD4+ T-cells, CD4 cell counts, and tuberculosis-specific IFN-γ responses by enzyme-linked immunospot (ELISpot) assay [15]. Given the focused hypothesis of the primary analysis relating antigen-specific T-cell recovery to TB-IRIS, adjustment for multiple comparisons in the exploratory and secondary analyses was not conducted. Stata software version 11.2 (StataCorp, College Station, Texas) was used for analyses. GraphPad Prism version 5.0 (San Diego, California) and SPICE version 5.35 were used to generate graphs.
RESULTS
Baseline Characteristics of Patients
Of the 201 patients enrolled in the parent study, 170 (85%) initiated ART and had baseline clinical data available for analysis [15]. Of these 170 patients, 157 (92%) had PBMCs at baseline and/or week 4 post–ART initiation available for flow cytometric analysis. Thirteen (8%) patients without samples who were not included in the analysis were similar with respect to age, sex, and baseline CD4 count as those who were included (data not shown). Of the 157 patients, 31 (20%) experienced TB-IRIS, 112 (71%) were non-IRIS controls, and 15 (10%) died, 14 of whom were included as controls in sensitivity analyses. As previously described, 1 patient who had TB-IRIS died, although her IRIS symptoms resolved prior to death [15]. She was considered a TB-IRIS case in the analysis. TB-IRIS patients and controls were similar in terms of pre-ART CD4 count and time between antituberculosis therapy and ART initiation (P > .05; Table 1). TB-IRIS patients were more likely to have initiated a nevirapine-containing ART regimen (P = .04) and appeared less likely to have a body mass index <19 kg/m2 compared with controls (P = .06). IRIS-associated symptoms, described previously [15], were generally mild and involved respiratory worsening occurring at a median of 4 weeks post–ART initiation (IQR, 4–16 weeks). At week 4 post–ART initiation, TB-IRIS patients and controls had similar gains in CD4 count and rates of virologic suppression (Supplementary Table 2).
Table 1.
Baseline Characteristics Associated With Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome Among Patients With Advanced Human Immunodeficiency Virus/Tuberculosis Coinfection Initiating Antiretroviral Therapy
| Characteristic | Controlsa (n = 112) | TB-IRISa (n = 31) | P Value |
|---|---|---|---|
| Female sex, No. (%) | 46 (41) | 14 (45) | .68 |
| Age, y, mean (range) | 36 (22–73) | 37 (22–59) | .99 |
| ATT to ART initiation interval, d, median (IQR) | 27 (18–47) | 30 (20–47) | .56 |
| HIV RNA load at baseline, log10 copies/mL, median (IQR); No. | 5.5 (5.0–6.0); 108 | 5.4 (5.0–5.9); 30 | .56 |
| CD4 T-cell count at baseline, cells/µL, median (IQR) | 66 (32–96) | 62 (26–87) | .77 |
| NVP-based ART, No. (%) | 10 (9) | 7 (23) | .04 |
| AFB smear-negative TB, No. (%) | 30 (27) | 6 (20) | .40 |
| Presence of extrapulmonary TB at baseline, No. (%) | 6 (5) | 2 (6) | .81 |
| Presence of non-TB OI at baseline, No. (%) | 10 (9) | 4 (13) | .51 |
| BMI, kg/m2, median (IQR); No. | 19 (16–22); 111 | 20 (17–23); 31 | .10 |
| BMI, No. <19 kg/m2 (%); No. | 57 (51); 111 | 10 (32); 31 | .06 |
| Hemoglobin, g/dL, median (IQR); No. | 10.3 (9–11.3); 97 | 10.3 (9–12): 29 | .70 |
P values shown are comparing controls to TB-IRIS patients by Wilcoxon rank-sum test for continuous variables and χ2 test for categorical variables.
Abbreviations: AFB, acid-fast bacilli; ART, antiretroviral therapy; ATT, antituberculosis therapy; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; IRIS, immune reconstitution inflammatory syndrome; NVP, nevirapine; OI, opportunistic infection; TB, tuberculosis.
a Indicates the number of non-IRIS survivors (controls) and TB-IRIS patients with any flow cytometric analysis at baseline and/or week 4 post–ART initiation.
Cellular Immune Activation and T-Cell Memory Subsets
TB-IRIS patients and controls had comparable levels of T-cells expressing CD38, HLA-DR, and PD-1, pre– and week 4 post–ART initiation (Table 2). Additionally, TB-IRIS patients and controls were similar with respect to the frequency of memory T-cell subsets at both time points, with the exception of TB-IRIS patients having significantly higher pre-ART frequencies of effector memory CD8+ T-cells than controls (P = .04; Supplementary Figure 1).
Table 2.
CD38, Human Leukocyte Antigen-DR, and PD-1 Expression on CD4+ and CD8+ T-Cells and Association With Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome Among Patients With Advanced Human Immunodeficiency Virus/Tuberculosis Coinfection Initiating Antiretroviral Therapy
| Cellular Immune Activation | Control | TB-IRIS | P Value |
|---|---|---|---|
| Baseline | |||
| CD4+ CD38+/DR+ | 13.6 (9.67–21); 75 | 15.2 (9.0–22.7); 22 | .79 |
| CD4+ CD38−/DR+ | 6.7 (3.1–10.7); 75 | 3.2 (1.3–10); 22 | .07 |
| CD4+ CD38+/DR− | 42.6 (28.3–51.2); 75 | 39.4 (29.2–51.6); 22 | .79 |
| CD8+ CD38+/DR+ | 23.1 (15.2–32.8); 75 | 24.1 (13.1–32.5); 23 | .67 |
| CD8+ CD38−/DR+ | 2.0 (0.45–6.4); 75 | 0.77 (0.09–4.3); 23 | .17 |
| CD8+ CD38+/DR− | 50.3 (36.1–64.8); 75 | 54.1 (39.5–66.8); 23 | .40 |
| CD4+ PD-1+ | 34.3 (24.9–51.5); 64 | 36.8 (15.9–45.8); 18 | .69 |
| CD8+ PD-1+ | 30.2 (19.6–38.7); 64 | 32.3 (22.3–39.5); 18 | .74 |
| Change from baseline | |||
| CD4+ CD38+/DR+ | −0.1 (−4.9 to 4.4); 64 | 3.5 (−2.3 to 12.8); 15 | .11 |
| CD4+ CD38−/DR+ | 5.1 (1.4–10); 64 | 3.6 (1.6–10); 15 | .93 |
| CD4+ CD38+/DR− | −9.6 (−15.8 to −4.2); 64 | −10.5 (−16.7 to −4.4); 15 | .88 |
| CD8+ CD38+/DR+ | −0.8 (−5.7 to 4.4); 65 | −2.2 (−9.8 to 5.6); 16 | .79 |
| CD8+ CD38−/DR+ | 1.3 (0.26–3.3); 65 | 0.17 (−0.11 to 3.1); 16 | .11 |
| CD8+ CD38+/DR− | −6.8 (−11.6 to −3.1); 65 | −2.1 (−9.2 to 0.64); 16 | .12 |
| CD4+ PD-1+ | 0.6 (−4.0 to 3.5); 54 | 3.2 (−2.3 to 10.3); 14 | .09 |
| CD8+ PD-1+ | −2.2 (−5.2 to 0.70); 54 | −1.8 (−6.6 to 4.7); 14 | .72 |
| Week 4 post-ART | |||
| CD4+ CD38+/DR+ | 17.2 (9.2–26.4); 88 | 18.6 (11.4–29.2); 23 | .58 |
| CD4+ CD38−/DR+ | 11.4 (7.7–20.5); 88 | 11.1 (3.1–23); 23 | .32 |
| CD4+ CD38+/DR− | 29.6 (20–41); 88 | 27.1(19.6–44.3); 23 | .90 |
| CD8+ CD38+/DR+ | 24.6 (17.8–36.9); 89 | 19.6 (15.1–38.4); 23 | .58 |
| CD8+ CD38−/DR+ | 3.5 (1.2–7.0); 89 | 2.7 (0.52–6.6); 23 | .30 |
| CD8+ CD38+/DR− | 43.6 (31.3–54.6); 89 | 42.7 (24.7–62); 23 | .98 |
| CD4+ PD-1+ | 34.1 (19.6–45.9); 81 | 36.5 (22.3–51.9); 20 | .43 |
| CD8+ PD-1+ | 25.1 (14.6–32.1); 81 | 24.7 (19.9–37.7); 20 | .34 |
Data are presented as median (interquartile range) and No.: median frequency of CD38 and/or HLA-DR and PD-1–expressing CD4+ and CD8+ T-cells pre-ART (baseline), change from baseline, and at week 4 post–ART initiation; and number of controls and tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) patients assessed at each time point and those who had paired data to determine change from baseline. P values shown are comparing controls to TB-IRIS patients by Wilcoxon rank-sum test.
Abbreviations: ART, antiretroviral therapy; HLA-DR, human leukocyte antigen-DR.
Tuberculosis-Specific Cellular Immune Responses
A subset of the TB-IRIS (n = 13) patients and controls (n = 72) had PBMCs available for intracellular cytokine staining following antigenic stimulation at baseline and/or week 4 post–ART initiation. Baseline clinical characteristics of these controls and TB-IRIS patients were similar to those described in Table 1.
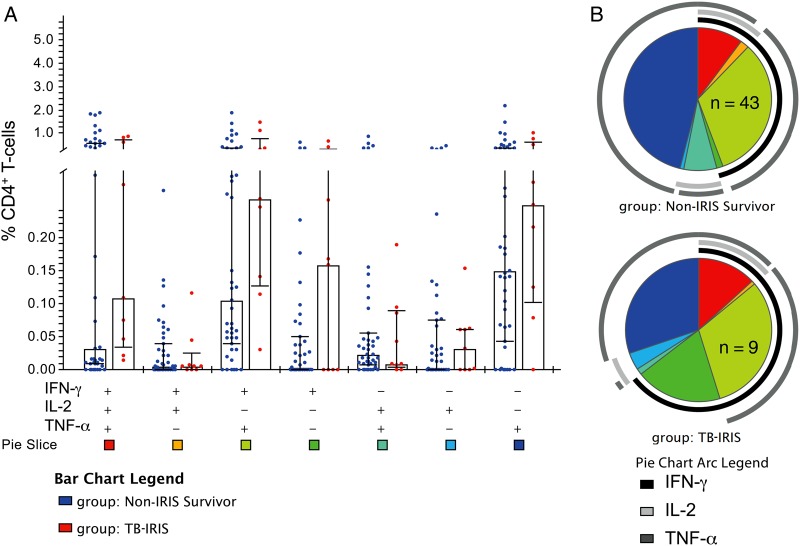
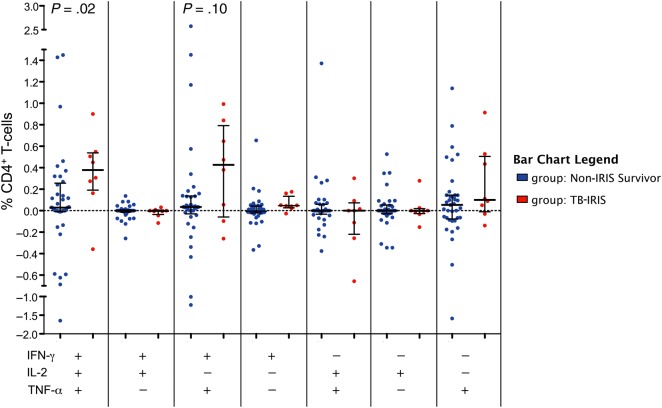
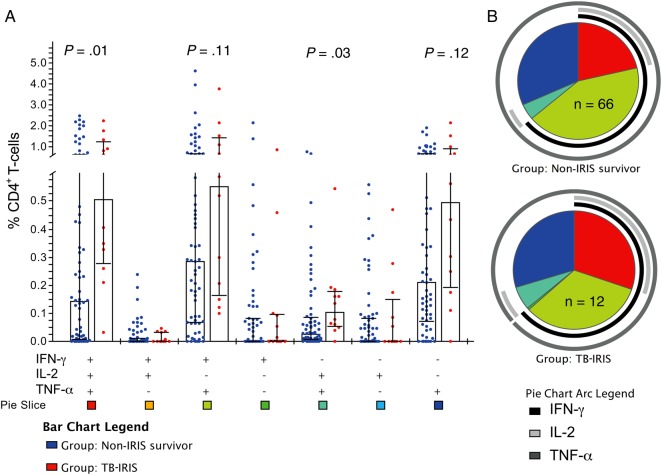
Prior to ART initiation, TB-IRIS patients tended to have higher median frequencies of tuberculosis-specific polyfunctional (IFN-γ+/IL-2+/TNF-α+) CD4+ T-cells vs controls; however, these differences were not statistically significant (Figure 1A). After ART initiation, TB-IRIS patients had significantly greater increases from baseline to week 4 in the frequency of tuberculosis-specific CD4+ T-cells expressing IFN-γ+/IL-2+/TNF-α+ (P = .02; Figure 2). Similarly, tuberculosis-specific IFN-γ+/IL-2+/TNF-α+ and IFN-γ-/IL-2+/TNF-α+ CD4+ T-cell frequencies were higher in TB-IRIS patients compared to controls at week 4 post-ART (P = .01 and P = .03, respectively; Figure 3A). After adjusting for use of nevirapine-based ART regimen and body mass index, every quartile increase in tuberculosis-specific IFN-γ+/IL-2+/TNF-α+ CD4+ T-cells from baseline to week 4 post-ART was independently associated with 2.8-fold (95% confidence interval [CI], 1.1 to 7.5-fold) increased odds of TB-IRIS (Table 3). This association was not affected by adjustment for pre-ART CD4 counts, and was even stronger when week 4 polyfunctional CD4+ T-cells were evaluated (Table 3). Sensitivity analyses, including patients who died as non-IRIS controls, did not meaningfully affect the results (data not shown). Notably, associations between TB-IRIS and greater tuberculosis-specific CD4+ T-cell–associated cytokines, assessed at week 4 or as the change from baseline to week 4, were observed not only for IFN-γ but for IL-2 and TNF-α as well, when assessed as total cytokine expression (Supplementary Figure 2). HIV-specific immune responses did not differ between groups (Supplementary Figure 3).
Figure 1.
Patients with tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) tended to have elevated levels of TB-specific CD4+ T-cells expressing multiple cytokines prior to antiretroviral therapy (ART) initiation. Peripheral blood mononuclear cells from patients obtained prior to ART initiation were stimulated with purified protein derivative (PPD) to measure TB-specific responses followed by intracellular cytokine staining and flow cytometry. Frequencies of TB-specific CD4+ T-cells expressing interferon gamma (IFN-γ), interleukin 2 (IL-2), and/or tumor necrosis factor alpha (TNF-α) at baseline in controls (n = 43) vs TB-IRIS patients (n = 9) were compared. A, Bar graph shows median frequency and interquartile range of the 7 possible combinations of IFN-γ, IL-2, and TNF-α expression by CD4+ T-cells in response to PPD stimulation in controls and TB-IRIS patients. B, Pie charts show the contribution of each combination of cytokine response to the total TB-specific responses mediated by CD4+ T-cells in controls and TB-IRIS patients. Colors in the pie charts correspond to the bottom of the bar graph, with pie arcs indicating the contribution of IFN-γ, IL-2, and TNF-α to the 3+ (poly), 2+, and 1+ (mono)-functional responses in non-IRIS survivors (controls) and TB-IRIS patients. Data shown are following subtraction of corresponding background responses. P values were >.05 for all comparisons shown in A and B by Wilcoxon rank-sum tests.
Figure 2.
Patients with tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) have a greater increase in polyfunctional TB-specific CD4+ T-cells from baseline to week 4 post–antiretroviral therapy (ART) initiation compared to controls. Peripheral blood mononuclear cells from patients obtained prior to ART initiation and week 4 post–ART initiation were stimulated with purified protein derivative (PPD) to measure TB-specific responses followed by intracellular cytokine staining and flow cytometry. Change from baseline to week 4 post–ART initiation in the frequency of TB-specific CD4+ T-cells expressing interferon gamma (IFN-γ), interleukin 2 (IL-2), and/or tumor necrosis factor alpha (TNF-α) in controls (n = 37) vs TB-IRIS patients (n = 8) with paired data was determined. Change from baseline in frequency of the 7 possible combinations of IFN-γ, IL-2, and TNF-α expression by CD4+ T-cells following PPD stimulation in controls and TB-IRIS patients is depicted. Horizontal lines indicate median frequency with interquartile range. Wilcoxon rank-sum tests were used to determine P values.
Figure 3.
After 4 weeks of antiretroviral therapy (ART), patients with tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) have increased TB-specific production of multiple cytokines by CD4+ T-cells compared to controls. Peripheral blood mononuclear cells from patients obtained at 4 weeks following ART initiation were stimulated with purified protein derivative to measure TB-specific responses. Cells were stained to quantitate cytokine responses by flow cytometry. The frequency of TB-specific CD4+ T-cells expressing interferon gamma (IFN-γ), interleukin 2 (IL-2), and/or tumor necrosis factor alpha (TNF-α) at week 4 post-ART in controls (n = 66) vs TB-IRIS patients (n = 12) is shown. A, Bar graph shows median frequency and interquartile range of the 7 possible combinations of TB-specific IFN-γ, IL-2, and TNF-α–expressing CD4+ T-cells in controls and TB-IRIS patients. B, Pie charts show the contribution of each combination of cytokine responses to the total TB-specific responses meditated by CD4+ T-cells in controls and TB-IRIS patients. Colors in the pie chart correspond to the pie slice colors shown at the bottom of the bar graph, with pie arcs indicating the contribution of IFN-γ, IL-2, and TNF-α to the 3+ (poly), 2+, and 1+ (mono)-functional responses in non-IRIS survivors (controls) and TB-IRIS patients. Data shown are following subtraction of corresponding background responses. Wilcoxon rank-sum tests were used to determine P values.
Table 3.
Logistic Regression Model Assessing Relationship Between Polyfunctional Response and Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome Among Patients With Advanced Human Immunodeficiency Virus/Tuberculosis Coinfection Initiating Antiretroviral Therapy
| TB-Specific IFN-γ+/IL-2+/TNF-α+ CD4+ T-cells | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Baseline | 1.7 (.82–3.4) | 1.7 (.83–3.5) |
| Change from baseline | 2.5 (1.1–6.0)a | 2.8 (1.1–7.5)a |
| Week 4 post-ART | 2.3 (1.2–4.5)a | 3.5 (1.4–8.8)a |
Baseline (n = 52), change from baseline (n = 45), and week 4 post–ART initiation (n = 78) frequencies of polyfunctional (IFN-γ+/IL-2+/TNF-α+) CD4+ T-cell response to purified protein derivative stimulation from unadjusted analyses shown in Figures 1A, 2, and 3A were stratified into quartiles and assessed for association with tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) in a logistic regression model. Median frequencies of polyfunctional CD4+ T-cells corresponding to each quartile are detailed in Supplementary Table 3. Table shows the ORs and 95% CIs for TB-IRIS for every quartile increase in polyfunctional IFN-γ+/IL-2+/TNF-α+ CD4+ T-cells. For adjusted analyses, nevirapine-based ART, body mass index, and pre-ART CD4 counts were included in the model.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; IFN-γ, interferon gamma; IL-2, interleukin 2; OR, odds ratio; TB, tuberculosis; TNF-α, tumor necrosis factor alpha.
a Indicates independent association between polyfunctional tuberculosis-specific CD4+ T-cell response and TB-IRIS.
More rapid functional tuberculosis-specific CD4+ T-cell recovery at week 4 was also reflected in overall CD4 cell recovery during the first 6 months after ART initiation among those with available CD4 count data beyond week 4 (n = 151). In the GEE model, adjusting for baseline CD4 count, TB-IRIS patients had higher average follow-up CD4 counts on ART (25 cells/µL [95% CI, 3–47 cells/µL]; P = .03).
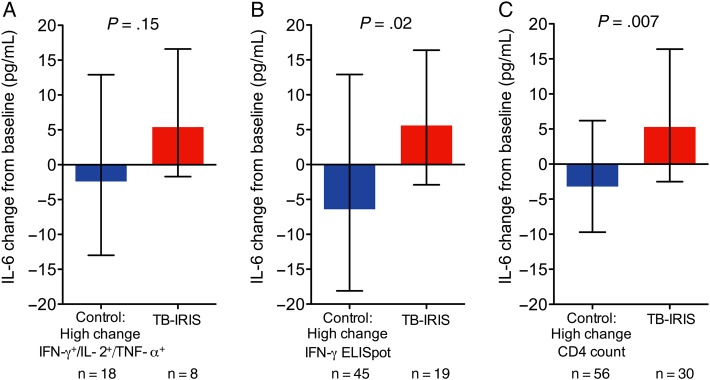
We also assessed the relationship between change in cellular immune function and IL-6 (innate immune response) on ART among controls and TB-IRIS patients (Supplementary Figure 4). A substantial number of controls had rapid increases in tuberculosis-specific IFN-γ+/IL-2+/TNF-α+ CD4+ T-cells (median change, 0.23% [IQR, 0.11%–0.42%]) following ART initiation that were comparable to those in TB-IRIS patients (median change, 0.38% [IQR 0.22%–0.53%]; P = .40). However, while TB-IRIS patients had early increases in IL-6 (median change, 5.4 pg/mL [IQR, −1.7 to 16.6 pg/mL]), controls tended to have IL-6 concentrations that decreased (median change, −2.4 pg/mL [IQR, −13 to 12.9 pg/mL]; P = .15; Figure 4A). This relationship became significant when assessing larger sample sizes afforded by assessment of cellular immune recovery by ELISpot assay, where the median IL-6 change was significantly higher in those who developed IRIS vs controls (5.6 pg/mL [IQR, −2.9 to 16.4 pg/mL] vs −6.4 pg/mL [IQR, −18.1 to 12.9 pg/mL]; P = .02; Figure 4B). Changes in IL-6 on ART were also higher among TB-IRIS patients when controls with rapid increases in CD4 counts were examined (median change, 5.3 pg/mL [IQR, −2.5 to 16.4 pg/mL] vs −3.2 pg/mL [IQR, −9.7 to 6.2 pg/mL]; P = .007; Figure 4C).
Figure 4.
Linked tuberculosis (TB)–specific T-cell and innate immune responses as measured by systemic interleukin 6 (IL-6) characterized TB-associated immune reconstitution inflammatory syndrome (TB-IRIS). A, Non-IRIS controls with change in TB-specific polyfunctional (interferon gamma [IFN-γ+]/interleukin 2 [IL-2+]/tumor necrosis factor alpha [TNF-α+]) CD4+ T-cell responses post–antiretroviral therapy (ART) initiation that were above the median for the group were identified from Figure 2. Control patients with above-median TB-specific IFN-γ responses measured previously by enzyme-linked immunospot (ELISpot) assay [15] (B), as well as higher than median increase in CD4 count on ART (C), were identified. Change in circulating IL-6 levels from baseline to week 4 post-ART were compared between controls with high adaptive cellular immune response to all TB-IRIS patients with corresponding data. Bars indicate median change in IL-6 concentration with interquartile range, which was previously quantitated by Luminex assay [15]. Wilcoxon rank-sum tests were used to determine P values.
DISCUSSION
We utilized a large cohort of patients with advanced HIV/tuberculosis coinfection to evaluate the association between cellular phenotype and function prior to and shortly after ART initiation and the risk of paradoxical TB-IRIS. Despite similar cellular phenotype and immune activation profiles, TB-IRIS patients demonstrated significantly greater increases in polyfunctional tuberculosis-specific CD4+ T-cell responses that are considered correlates of host protection from tuberculosis [26, 27]. Improved cellular immune recovery in those with TB-IRIS is further suggested by the significantly greater increases in CD4 counts on ART during longer-term follow-up.
Most immunologic investigations of TB-IRIS have focused on IFN-γ responses, yielding inconsistent results [9, 16, 17]. Although very few studies have evaluated polyfunctional T-cell responses, our finding that increases in tuberculosis-specific IFN-γ+/IL-2+/TNF-α+ CD4+ T-cells on ART are independently associated with paradoxical TB-IRIS is consistent with a report where patients with several different types of opportunistic infections (including 2 with paradoxical TB-IRIS) were evaluated [28]. As in previous studies [9, 10, 16, 28], these responses were pathogen-specific. Additional insights into the pathogenesis of TB-IRIS were obtained by measuring, in parallel, multiple cytokines that are important in tuberculosis disease [29]. Specifically, the assessment of polyfunctional responses indicates that development of TB-IRIS on ART may involve several interrelated aspects of the recovering host immune system. For example, IFN-γ and TNF-α are important in monocyte/macrophage activation and the recruitment of these cells to the site of infection [30, 31], whereas IL-2 can promote T-cell proliferation in response to antigen exposure, particularly when produced in combination with IFN-γ and TNF-α [32]. Thus, robust recovery of polyfunctional tuberculosis-specific CD4+ T-cells may simultaneously activate innate and adaptive immune cells, triggering systemic inflammation to a greater extent than recovery of less functional cells.
Previous studies noted that many patients without TB-IRIS have similarly rapid increases in pathogen-specific immune responses on ART, questioning the role of these cells in development of the syndrome [16, 17]. Our exploratory analysis of changes in IL-6 levels, which is consistently implicated in IRIS immunopathology [11, 20], in non-IRIS controls who also had rapid cellular immune recovery needs confirmation, but preliminarily suggests that development of TB-IRIS may be dependent on the coordinated recovery of both adaptive and innate immune function. We have previously documented that granulocyte colony-stimulating factor and TNF-α increase on ART in TB-IRIS patients in this cohort, whereas these biomarker levels (and granulocyte macrophage colony-stimulating factor) decrease in non-IRIS controls [15]. These factors, involved in the maturation and activation of monocyte/macrophages, may lead to pathologic inflammation by boosting tuberculosis antigen presentation in the setting of rapidly expanding pathogen-specific polyfunctional CD4+ T-cells in TB-IRIS [33]. Collectively, these data suggest that the link between reconstitution of both adaptive and innate immune responses after ART initiation and TB-IRIS should be investigated further.
Evaluation of other cytokines in addition to IFN-γ may also illuminate potential treatment strategies. For example, TB-IRIS in our study was generally associated not only with increases in multiple CD4+ T-cell subsets containing TNF-α, but also with increases in total CD4+ T-cell–derived TNF-α (Figures 2 and 3; Supplementary Figure 2) and systemic levels of TNF-α [15], after ART initiation. These observations are in line with a report including 5 TB-IRIS patients documenting an association between IRIS and CD4+ T-cells expressing both IFN-γ and TNF-α [10]; with case reports documenting paradoxical reactions in patients with autoimmune disease who develop tuberculosis and discontinue infliximab, a monoclonal antibody against TNF-α [34]; and with a case series of patients with mycobacterial IRIS who improved after infliximab administration [35]. Trials of immunotherapies with approved drugs targeting TNF-α or drugs that decrease TNF-α expression should be considered [35, 36].
We found minimal evidence to support the hypothesis that higher pre-ART levels of cellular immune activation (PD-1 [28, 37], HLA-DR, and CD38 [12]) increased risk of subsequent TB-IRIS. These findings are similar to several other investigations [14, 16] and are generally consistent with our previous report documenting reduced pre-ART systemic inflammation in those who later developed TB-IRIS [15]. Furthermore, we found no enrichment in late differentiation stage CD4+ T-cells in TB-IRIS. We were able to compare paradoxical (and not unmasking) IRIS cases and controls in the setting of similar times to ART initiation after tuberculosis diagnosis, thereby eliminating a major potential confounder associated with higher levels of immune activation at ART initiation [7, 11, 12, 38].
Our data cannot elucidate why some individuals recovered polyfunctional responses more rapidly than others. One possibility is that the lower levels of circulating markers of pre-ART systemic inflammation in the TB-IRIS patients compared to controls in this cohort, documented previously [15], may have facilitated greater redistribution of sequestered polyfunctional and total CD4+ T-cells from lymphoid tissue soon after ART initiation [39]. While the study may be generalizable to similar settings, findings from this study were restricted to relatively mild cases of paradoxical pulmonary TB-IRIS but may not be generalizable to those with more severe disease including that involving the central nervous system. Furthermore, a greater number of patients with TB-IRIS would have enabled greater power for statistical comparisons. Finally, we did not characterize monocyte function to integrate more comprehensive analyses [11].
In conclusion, rapid increases in tuberculosis-specific polyfunctional CD4+ T-cells following ART initiation characterized TB-IRIS. Studies investigating immune mechanisms of TB-IRIS should characterize multiple relevant cytokines, and links between adaptive and innate immune function may reveal new preventive and treatment interventions for this syndrome.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the Botswana Harvard AIDS Institute Partnership for providing laboratory space for PBMC isolation in Botswana; the clinical care providers who referred patients; and the patients who volunteered to participate in this study.
Financial support. This work was supported by grants from the National Institutes of Health (grant number R01AI080337) and the Penn Center for AIDS Research (grant number AI045008).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Abdool Karim SS, Naidoo K, Grobler A et al. . Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlir DV, Kendall MA, Ive P et al. . Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luetkemeyer AF, Kendall MA, Nyirenda M et al. . Tuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programs. J Acquir Immune Defic Syndr 2014; 65:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naidoo K, Yende-Zuma N, Padayatchi N et al. . The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Intern Med 2012; 157:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laureillard D, Marcy O, Madec Y et al. . Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in a randomized clinical trial. AIDS 2013; 27:2577–86. [DOI] [PubMed] [Google Scholar]
- 6.Muller M, Wandel S, Colebunders R et al. . Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narendran G, Andrade BB, Porter BO et al. . Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One 2013; 8:e63541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. June 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/ Accessed 2 March 2015. [PubMed]
- 9.Bourgarit A, Carcelain G, Martinez V et al. . Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006; 20:F1–7. [DOI] [PubMed] [Google Scholar]
- 10.Bourgarit A, Carcelain G, Samri A et al. . Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gammadelta T cells. J Immunol 2009; 183:3915–23. [DOI] [PubMed] [Google Scholar]
- 11.Andrade BB, Singh A, Narendran G et al. . Mycobacterial antigen driven activation of CD14++CD16- monocytes is a predictor of tuberculosis-associated immune reconstitution inflammatory syndrome. PLoS Pathog 2014; 10:e1004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haridas V, Pean P, Jasenosky LD et al. . TB-IRIS and remodelling of the T-cell compartment in highly immunosuppressed HIV+ patients with TB: the CAPRI-T (ANRS-12614) study. AIDS 2015; 29:263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goovaerts O, Jennes W, Massinga-Loembe M et al. . LPS-binding protein and IL-6 mark paradoxical tuberculosis immune reconstitution inflammatory syndrome in HIV patients. PLoS One 2013; 8:e81856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goovaerts O, Jennes W, Massinga-Loembe M et al. . Lower pre-treatment T cell activation in early- and late-onset tuberculosis-associated immune reconstitution inflammatory syndrome. PLoS One 2015; 10:e0133924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravimohan S, Tamuhla N, Steenhoff AP et al. . Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis 2015; 15:429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meintjes G, Wilkinson KA, Rangaka MX et al. . Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med 2008; 178:1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goovaerts O, Jennes W, Massinga-Loembe M et al. . Antigen-specific interferon-gamma responses and innate cytokine balance in TB-IRIS. PLoS One 2014; 9:e113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn JL, Goldstein MM, Chan J et al. . Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995; 2:561–72. [DOI] [PubMed] [Google Scholar]
- 19.Lindenstrom T, Knudsen NP, Agger EM, Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol 2013; 190:6311–9. [DOI] [PubMed] [Google Scholar]
- 20.Barber DL, Andrade BB, McBerry C, Sereti I, Sher A. Role of IL-6 in Mycobacterium avium–associated immune reconstitution inflammatory syndrome. J Immunol 2014; 192:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravimohan S, Tamuhla N, Steenhoff AP et al. . Early immunologic failure is associated with early mortality among advanced HIV-infected adults initiating antiretroviral therapy with active tuberculosis. J Infect Dis 2013; 208:1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. TB/HIV: a clinical manual. 2nd ed Available at: http://www.who.int/tb/publications/who_htm_tb_2004_329/en/. Accessed 11 June 2012.
- 23.Meintjes G, Lawn SD, Scano F et al. . Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008; 8:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant PM, Komarow L, Andersen J et al. . Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PLoS One 2010; 5:e11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011; 79:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes EK, Sander C, Ronan EO et al. . Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008; 181:4955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beveridge NE, Price DA, Casazza JP et al. . Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol 2007; 37:3089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahnke YD, Greenwald JH, DerSimonian R et al. . Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood 2012; 119:3105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol 2014; 5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin PL, Plessner HL, Voitenok NN, Flynn JL. Tumor necrosis factor and tuberculosis. J Investig Dermatol Symp Proc 2007; 12:22–5. [DOI] [PubMed] [Google Scholar]
- 31.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993; 178:2249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day CL, Abrahams DA, Lerumo L et al. . Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 2011; 187:2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev 2008; 226:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivoisy C, Amrouche L, Carcelain G, Sereni D, Bourgarit A. Paradoxical exacerbation of tuberculosis after TNFalpha antagonist discontinuation: beware of immune reconstitution inflammatory syndrome. Joint Bone Spine 2011; 78:312–5. [DOI] [PubMed] [Google Scholar]
- 35.Hsu DC, Faldetta KF, Pei L et al. . A paradoxical treatment for a paradoxical condition: infliximab use in three cases of mycobacterial IRIS. Clin Infect Dis 2016; 62:258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallis RS, Kyambadde P, Johnson JL et al. . A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS 2004; 18:257–64. [DOI] [PubMed] [Google Scholar]
- 37.Antonelli LR, Mahnke Y, Hodge JN et al. . Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood 2010; 116:3818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eller MA, Blom KG, Gonzalez VD et al. . Innate and adaptive immune responses both contribute to pathological CD4 T cell activation in HIV-1 infected Ugandans. PLoS One 2011; 6:e18779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bucy RP, Hockett RD, Derdeyn CA et al. . Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Investig 1999; 103:1391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.