Recently a KSHV-associated inflammatory cytokine syndrome (KICS) distinct from KSHV-MCD was reported. KICS may be an important unrecognized cause of morbidity and mortality, including symptoms previously ascribed to HIV.
Keywords: HIV, Kaposi sarcoma herpesvirus (KSHV), Human Herpesvirus 8 (HHV-8), IL-6, IL-10
Abstract
Background. Kaposi sarcoma herpesvirus (KSHV) is the cause of Kaposi sarcoma (KS), primary effusion lymphoma (PEL), and a form of Castleman disease (KSHV-MCD). Recently a KSHV-associated inflammatory cytokine syndrome (KICS) distinct from KSHV-MCD was reported.
Methods. We prospectively characterized the clinical, laboratory, virologic and immunologic features of KICS by evaluating symptomatic adults with KSHV using a prespecified definition. These features and overall survival were compared with controls from 2 prospectively characterized human immunodeficiency virus (HIV)-infected cohorts, including 1 with KSHV coinfection.
Results. All 10 KICS subjects were HIV infected males; 5 had HIV viral load (VL) suppressed <50 copies mL (median 72, range <50–74 375); all had KS and 2 also had PEL. All had multiple severe symptoms attributable to KICS: median number of symptoms 8 (6–11), median grade of worst symptom 3 (2–4). These included gastrointestinal disturbance (present in 9); edema (9); respiratory (6); and effusions (5). Laboratory abnormalities included anemia (all); hypoalbuminemia (all) and thrombocytopenia (6). None developed KSHV-MCD; 6 died with median survival from KICS diagnosis 13.6 months. KICS subjects compared with controls had more severe symptoms; lower hemoglobin and albumin; higher C-reactive protein; higher KSHV VL; elevated interleukin (IL)-6 and IL-10; and an increased risk of death (all P < .05). Anemia and hypoalbuminemia at presentation were independently associated with early death.
Conclusions. KICS subjects demonstrated diverse severe symptoms, a high rate of KSHV-associated tumors, high mortality, and a distinct IL-6/IL-10 signature. KICS may be an important unrecognized cause of morbidity and mortality, including symptoms previously ascribed to HIV. Exploration of KSHV-directed therapy is warranted.
Kaposi sarcoma herpesvirus (KSHV, or human herpesvirus 8) is the etiologic agent of 3 tumors: Kaposi sarcoma (KS), primary effusion lymphoma (PEL), and a form of multicentric Castleman disease (KSHV-MCD) [1–3]. The KSHV lifecycle exhibits latent and lytic phases, and is notable for its manipulation of host cellular pathways by mechanisms that include induction of host cytokines and production of a virally encoded interleukin-6 homolog, viral IL-6 (vIL-6) [4, 5]. These are implicated particularly in the pathogenesis of KSHV-MCD, a systemic lymphoproliferative inflammatory disorder whose features include the presence in affected nodes of KSHV-infected plasmablasts expressing vIL-6, systemic KSHV viremia, and overproduction of cytokines including vIL-6, IL-6, and IL-10 [5–7].
KSHV-mediated systemic inflammation may also develop in patients without KSHV-MCD. We originally described 6 such patients in a retrospective series [8]. Each had clinical symptoms and systemic inflammation, but evaluation did not reveal KSHV-MCD. They had KSHV viremia and levels of hIL-6, vIL-6, and IL-10 comparable to those seen in active KSHV-MCD, significantly elevated compared with controls with KS only. Mortality was high despite therapies directed at KSHV replication (including valganciclovir) or at KSHV-related tumors (including liposomal doxorubicin). We subsequently named this syndrome the KSHV-inflammatory cytokine syndrome (KICS) and proposed a working case definition to facilitate investigation (Table 1) [9].
Table 1.
Working Case Definition of KSHV-inflammatory Cytokine Syndrome (KICS)
| 1. Clinical manifestations | |
| a. Symptoms | b. Laboratory abnormalities |
| Fever | Anemia |
| Fatigue | Thrombocytopenia |
| Edema | Hypoalbuminemia |
| Cachexia | Hyponatremia |
| Respiratory symptoms | c. Radiographic abnormalities |
| Gastrointestinal disturbance | Lymphadenopathy |
| Athralgia and myalgia | Splenomegaly |
| Altered mental state | Hepatomegaly |
| Neuropathy with or without pain | Body cavity effusions |
| 2. Evidence of systemic inflammation | |
| Elevated C-reactive protein (≥3 g/dL) | |
| 3. Evidence of KSHV viral activity | |
| Elevated KSHV viral load in plasma (≥1000 copies/mL) or peripheral blood mononuclear cells (≥100 copies/106 cells) | |
| 4. No evidence of KSHV-associated multicentric Castleman disease | |
| Exclusion of MCD requires histopathologic assessment of lymphadenopathy if present. | |
The working case definition of KICS requires the presence of at least 2 clinical manifestations drawn from at least 2 categories (1a, b, and c), together with each of the criteria in 2, 3, and 4.
Abbreviations: KSHV, Kaposi sarcoma herpesvirus; MCD, multicentric Castleman disease.
We performed a prospective study whose primary objective was to define the natural history of KICS, including the spectrum of clinical abnormalities seen in affected patients and their relationship to KSHV viremia and implicated cytokines. As some clinical features of KICS resemble those ascribed to uncontrolled human immunodeficiency virus (HIV) infection and HIV viremia was present in some of the initial KICS subjects, we further sought to explore any role played by HIV viremia by comparing subjects with prospectively characterized cohorts of HIV-infected individuals, including one with KSHV coinfection.
METHODS
Clinical Assessment
Participants were seen at the Clinical Center of the US National Institutes of Health, Bethesda, Maryland. Evaluation was performed prospectively with laboratory evaluations the day of clinical assessments. Symptoms severity was graded using Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0 [10]. All participants provided written informed consent. Participants were followed for 2 years beyond symptom resolution or until death.
KICS Protocol and Cohort
We evaluated KSHV-infected adults of any HIV status who had at least 1 symptom and laboratory abnormality potentially attributable to KICS and evidence of systemic inflammation indicated by an elevated C-reactive protein (CRP) using prospectively described criteria (Table 1) including exclusion of any alternate causes of the clinical presentation such as intercurrent infection or KSHV-MCD. Assessment included assay of KSHV viral load in plasma and peripheral blood mononuclear cells (PBMCs); 18F-fluoro deoxyglucose positron emission tomography with computed tomography (18FDG-PET); biopsy of adenopathy if present; bone marrow biopsy; microbiological culture; and other testing directed by symptoms. Those who met the KICS definition with no alternate causes of symptoms were followed prospectively. Subjects reported here are the first 10, accrued from protocol inception in September 2011 through February 2013. The KICS protocol is registered as NCT1419561.
Control Protocols and Cohorts
We employed a stratified selection strategy to identify HIV-infected controls with or without HIV viremia from 2 contemporaneous prospective natural history protocols. Our goal was to explore any influence of HIV viremia including in a cohort with known KSHV coinfection. Controls were selected blind to clinical and other laboratory parameters. Source protocols were a natural history study of HIV and KSHV coinfected adults in whom KSHV infection was established by serology or tissue expression of viral antigens (Cohorts 2 and 3; NCT6171), and natural history studies of HIV infection in subjects not known to be KSHV infected (Cohorts 4 and 5; NCT00286767 and NCT00101374). Each contributed ten controls with HIV viral load (VL) <50 copies/mL and 10 with HIV VL >1000, for total of 40.
Laboratory Assessment
Viral Assays
All viral load assays were performed using quantitative real-time polymerase chain reaction (PCR). Assessment of KSHV VL in PBMCs was performed as previously using primers for the K6 gene region [8]. KSHV VL in plasma was performed using DNA extracted from EDTA whole blood using primers for the conserved region of ORF26 (Focus Diagnostics, Cypress, California). EBV, CMV, and HHV6 VLs were performed using DNA extracted from EDTA whole blood as previously described [11–13]. Primers for CMV were 203-bp segment from the glycoprotein-B gene; for EBV 192-bp segment from the BamHI-W gene; and for HHV6 533-bp region of the polymerase gene. Melt curve analysis was performed to differentiate HHV-6A from -6B.
Cytokine Assays
Serum levels of hIL-6, IL-1β, IL-8, IL-10, IL-12 p70, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) were evaluated using the Mesoscale Discovery (MSD) Multiarray Proinflammatory 7-plex Assay (Meso-Scale Discovery, Gaithersburg, Maryland) with the Sector Imager. The Meso-Scale hIL-6 assay did not detect vIL-6 at concentrations up to 20 000 pg/mL. Viral IL-6 was measured using a sandwich enzyme-linked immunosorbent assay as previously described [5, 7]. The lower limit of detection was 1560 pg/mL, and the assay did not detect hIL-6 at concentrations up to 10 000 pg/mL.
Other Assays
High sensitivity CRP was assayed using the Siemens Dimension Vista platform (Siemens AG, Munich, Germany). CD4 positive T-lymphocyte counts were assessed by fluorescent-activated cell sorting using the BlueOcean platform (Beckman Coulter, Carlsbad, California). Plasma HIV-1 mRNA was measured by quantitative RNA PCR using Roche Amplicor HIV-1 Monitoring Kits (Roche Diagnostics, Branchburg, New Jersey).
Statistical Analysis
Statistical comparisons between groups were made by Wilcoxon rank sum test for continuous parameters and Cochran–Armitage trend test for the ordered categorical variable symptom severity. For vIL-6, levels were considered detectable or not and compared using Fisher exact test. Survival analysis was performed using the Kaplan–Meier method, with a log-rank test to compare curves. Patients were monitored from enrollment until 1 February 2014. P-values are 2-tailed; in view of the number of comparisons, P < .005 was considered significant and .005 < P < .05 a trend.
RESULTS
KICS Subjects
Baseline characteristics of KICS subjects are summarized in Table 2. All were HIV coinfected, with 8 (80%) receiving antiretroviral therapy at presentation; 5 (50%) had suppressed HIV VL. Median time from HIV diagnosis was 52 months (7–183) and from KSHV diagnosis 10 months (2–72). All had at least 1 KSHV-related tumor: all had biopsy-proven KS, advanced (T1 by AIDS Clinical Trial Group criteria) [14] in 9 (90%), whereas 2 (20%) had primary effusion lymphoma. None was referred primarily to evaluate for KICS; 7 were referred for KS and 2 for PEL and then found to have inflammatory symptoms; 1 was referred with possible MCD but found to have KICS.
Table 2.
Baseline Characteristics of KSHV-associated Inflammatory Cytokine Syndrome Subjects and Controls
| KICS Subjects (10) | KSHV and HIV Coinfected Subjects |
HIV Infected Subjects |
|||
|---|---|---|---|---|---|
| HIV Uncontrolled (10) | HIV Controlled (10) | HIV Uncontrolled (10) | HIV Controlled (10) | ||
| Male sex | 10 (100%) | 9 (90%) | 9 (90%) | 8 (80%) | 9 (90%) |
| Age (years) | 36 (22–60) | 38 (29–50) | 46 (34–61) | 37 (24–51) | 37 (29–51) |
| Receiving ART | 8 (80%) | 0 | 10 (100%) | 0 | 10 (100%) |
| HIV VL (copies/mL) | 72 (<50–74 375) | 61 600 (12 100–1 190 000) | <50 (<50–<50) | 35 686 (1425–500 000) | <50 (<50–459) |
| CD4 (cells/µL) | 88 (7–1308) | 324 (75–568) | 568 (261–972) | 375 (12–860) | 307 (100–577) |
For age and laboratory values, figures given are median and range; for other characteristics number and percentage.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; KSHV, Kaposi sarcoma herpesvirus; VL, viral load.
Symptoms in KICS Subjects
Symptoms of KICS subjects are summarized in Table 3, and the features and course of a typical subject are shown in Figure 1. The most common symptoms included fatigue (present in all), gastrointestinal symptoms including anorexia, nausea, and diarrhea (present in 9, 90%); edema (9, 90%); respiratory symptoms including dyspnea and cough (6, 60%); anasarca (4, 40%); and effusions or ascites (5, 50%, including the 2 with PEL). Fever was present in 2 (20%). Each subject had multiple symptoms, and they were commonly severe: median number of symptoms was 8 (range 6–11) and median severity of the worst symptom was CTCAE grade 3 (range 2–4). Patients were commonly critically ill, with 8 (80%) requiring inpatient management, 5 (50%) requiring management in the intensive care unit; 2 (30%) requiring invasive ventilation; and 3 (30%) requiring renal replacement therapy (RRT).
Table 3.
Selected Clinical Abnormalities in Patients With KSHV-associated Inflammatory Cytokine Syndrome Compared With Controls
| KICS Subjects (10) |
KSHV and HIV Coinfected Subjects |
HIV Infected Subjects |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV Uncontrolled (10) |
HIV Controlled (10) |
HIV Uncontrolled (10) |
HIV Controlled (10) |
|||||||
| Present (N, %) | Severity Grade (Median, Range) | Present (N, %) | Severity Grade (Median, Range) | Present (N, %) | Severity Grade (Median, Range) | Present (N, %) | Severity Grade (Median, Range) | Present (N, %) | Severity Grade (Median, Range) | |
| Symptoms | 10 (100%) | 3 (2–4)a | 4 (40%) | 0 (0–2)a P < .0001b |
2 (20%) | 0 (0–1)a P < .0001b |
4 (40%) | 0 (0–2)a P = .0002b |
2 (20%) | 0 (0–1)a P < .0001b |
| Intercurrent KSHV-associated tumor | 10 (100%) | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| Fever | 2 (20%) | 0 (0–2); 36.6°C (36.5–38.2) |
0 | 0 (0–0); 36.7°C (36.3–37.2) |
0 | 0 (0–0); 36.6°C (36.0–37.1) |
0 | 0 36.3°C (36.0–37.1) |
0 | 0 (0–0); 36.5°C (35.6–37) |
| Fatigue | 10 (100%) | 2 (2–3) | 1 (10%) | 0 (0–1) | 0 | 0 (0–0) | 1 (10%) | 0 (0–1) | 0 | 0 (0–0) |
| Respiratory | 6 (60%) | 1 (0–4) | 0 | 0 (0–0) | 1 (10%) | 0 (0–1) | 1 (10%) | 0 (0–2) | 0 | 0 (0–0) |
| Gastrointestinal | 9 (90%) | 2 (0–3) | 3 (30%) | 0 (0–2) | 0 | 0 (0–0) | 0 | 0 | 0 | 0 (0–0) |
| Rheumatologic | 0 | NA | 1 (10%) | 0 (0–1) | 0 | 0 (0–0) | 1 (10%) | 0 (0–1) | 0 | 0 (0–0) |
| Dermatologic | 1 (10%) | 0 (0–2) | 0 | 0 (0–0) | 0 | 0 (0–0) | 1 (10%) | 0 (0–1) | 1 (10%) | 0 (0–2) |
| Neurologic | 3 (30%) | 0 (0–2) | 1 (10%) | 0 (0–1) | 1 (10%) | 0 (0–1) | 2 (20%) | 0 (0–2) | 1 (10%) | 0 (0–1) |
| Edema | 9 (90%) | 2 (0–3) | 0 | 0 (0–0) | 0 | 0 (0–0) | 0 | 0 (0–0) | 0 | 0 (0–0) |
| Effusions | 5 (50%) | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| Adenopathy | 4 (40%) | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
Abbreviations: HIV, human immunodeficiency virus; KICS, KSHV-associated inflammatory cytokine syndrome; KSHV, Kaposi sarcoma herpesvirus; NA, not applicable; NCI, National Cancer Institute.
a Symptom severity graded using NCI Common Toxicity Criteria for Adverse Events (CTCAE) v4.0; body cavity effusions and adenopathy are not graded in CTCAE; the most severe symptom present was used for summary and comparisons.
b P values are for each control group separately compared with KICS subjects. Italicized values are significant.
Figure 1.
Clinical manifestations and clinical course of a representative patient with Kaposi sarcoma herpesvirus (KSHV)-associated inflammatory cytokine syndrome (KICS). Note: Functional and structural imaging, histopathology, and laboratory abnormalities of a representative subject are illustrated. Presenting symptoms included respiratory failure, gastrointestinal symptoms, and generalized hemorrhage in the setting of thrombocytopenia. Kaposi sarcoma (KS) was present with limited cutaneous involvement (B) but severe pulmonary involvement shown in bronchoscopy and computed tomography (C) together with hepatic and lymph node involvement demonstrated by histopathology (D). There were bilateral pleural effusions (C), ascites, and severe soft tissue edema. 18F-fluoro deoxyglucose positron emission tomography with computed tomography (18FDG-PET) (A) demonstrated tracer uptake at sites of KS, including limited lymph nodes, without adenopathy or splenomegaly. Laboratory abnormalities (E) included markedly elevated C-reactive protein (CRP), severe thrombocytopenia, severe hypoalbuminemia and anemia. KSHV viral load (VL) in PBMCs at presentation was 20 250 copies/106 peripheral blood mononuclear cells (PBMCs) and in plasma 3.99 × 106 copies/mL. There was no evidence of intercurrent infection, no evidence of KSHV-MCD on lymph node biopsy (D) and no evidence of primary effusion lymphoma. The patient was treated with liposomal doxorubicin and antiretroviral therapy together intravenous immunoglobulin and a single dose of rituximab and made a gradual improvement (E) before succumbing to a pulmonary complication. Abbreviation: MCD, multicentric Castleman disease.
Laboratory Findings in KICS Subjects
Laboratory findings in KICS subjects are summarized in Table 4. The most common abnormalities were anemia and hypoalbuminemia (both present in all). Thrombocytopenia was also common (6, 60%), and was severe (<50 cells/mL) in 3, each of whom had significant bleeding. In contrast, leukopenia and electrolyte disturbances were uncommon. Notably 3 subjects with hypoalbuminemia and anasarca manifested renal dysfunction considered to be due at least in part to hypoperfusion and required RRT.
Table 4.
Selected Laboratory Abnormalities in Patients With KICS Compared With Controls
| KICS Subjects (10) |
KSHV and HIV Coinfected Subjects |
HIV Infected Subjects |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV Uncontrolled (10) |
HIV Controlled (10) |
HIV Uncontrolled (10) |
HIV Controlled (10) |
|||||||
| Abnormal (N, %) | Median, Range | Abnormal (N, %) | Median, Range | Abnormal (N, %) | Median, Range | Abnormal (N, %) | Median, Range | Abnormal (N, %) | Median, Range | |
| KSHV VL (copies/106 PBMCs) | NA | 1569 (0–90 909)a | NA | 0 (0–1) P = .0001b |
NA | 0 (0–1) P = .0002b |
NA | 0 (0–1) P = .0001b |
NA | 0 (0–1) P = .0001b |
| C-reactive protein (g/dL) | 10 (100%) | 37.8 (4.9–185.0) | 3 (30%) | 1.2 (0.5–36.3) P = .0003b |
3 (30%) | 1.1 (0.16–5.9) P < .0001b |
5 (50%) | 2.0 (0.16–7.6) P < .0001b |
1 (10%) | 1.9 (0.2–5.9) P < .0001b |
| Hemoglobin (g/dL) | 10 (100%) | 9.0 (6.5–10.2) | 2 (20%) | 14.1 (9.5–15.4) P = .0001b |
3 (30%) | 14.3 (9.5–15.6) P = .0001b |
6 (60%) | 13.3 (7.4–16.1) P = .0009b |
4 (40%) | 14.1 (11.1–15.6) P < .0001b |
| White Cell Count | 4 (40%) | 5.2 (2.5–13.9) | 3 (30%) | 5.0 (2.5–7.8) P = .8b |
4 (40%) | 5.2 (3.5–8.9) P = .91b |
4 (40%) | 5.7 (1.7–6.6) P = .48b |
2 (20%) | 5.0 (2.6–8.0) P = .85b |
| Platelet Count | 6 (60%) | 138 (27–371) | 0 | 204 (161–286) P = .22b |
1 (10%) | 211 (156–253) P = .29b |
3 (30%) | 194 (47–314) P = .74b |
1 (10%) | 244 (154–291) P = .15b |
| Albumin | 10 (100%) | 2.4 (1.6–3.1) | 4 (40%) | 3.8 (2.5–4.1) P = .0002b |
3 (30%) | 3.9 (3.3–4.4) P < .0001b |
4 (40%) | 3.7 (2.2–4.2) P = .0002b |
0 | 4.0 (3.7–4.3) P < .0001b |
| Sodium | 1 (10%) | 136 (126–143) | 0 | 138 (135–141) P = .58b |
1 (10%) | 138 (133–140) P = .34b |
0 | 139 (135–144) P = .29b |
0 | 139 (135–140) P = .42b |
Abbreviations: HIV, human immunodeficiency virus; KICS, KSHV-associated inflammatory cytokine syndrome; KSHV, Kaposi sarcoma herpesvirus; NA, not applicable; PBMC, peripheral blood mononuclear cell; VL, viral load.
a One patient whose KSHV VL was not detected in PBMCs had an elevated KSHVL VL in pleural effusions (1509 copies/106 cells) and in plasma.
b P values are for each control group separately compared with KICS subjects. Italicized values are significant.
Intercurrent infections were not common, and a causal relationship to the observed symptoms was unlikely. One subject had genital herpes simplex; 1 Escherichia coli urinary tract infection; 1 colonization of a pleural effusion with Staphylococcus epidermis and Corynebacterium jeikeium; 2 (20%) had active asymptomatic hepatitis C; whereas the others had no active infection.
Imaging and Histopathological Findings in KICS Subjects
18FDG-PET/CT imaging identified expected tracer uptake at sites of known tumors. Mild adenopathy at limited anatomic sites proximate to areas of known KS involvement was present in 4 subjects (40%), all with maximal nodal diameter <1.5 cm. No subject exhibited the widespread marked adenopathy characteristic of KSHV-MCD, and none had splenomegaly, also a characteristic of KSHV-MCD [15].
Lymph node biopsies were performed in 6 patients guided by 18FDG-PET/CT findings, including 3 of those with adenopathy (1 with adenopathy had no surgically accessible nodes). Nodal involvement by KS was found in all. In 2 cases no residual lymphoid tissue was present; in the other 4 reactive hyperplasia was noted without morphological features of multicentric Castleman disease. Latency-associated nuclear antigen staining for KSHV was performed in all cases and nuclear stain was present in the spindle cell proliferation and rare mononuclear cells only. Bone marrow biopsies in 8 subjects demonstrated nonspecific changes characterized increased cellularity with reactive plasmacytosis and scattered KSHV-infected plasma cells, similar to findings previously reported [16].
Systemic Inflammation in KICS Subjects
The working KICS case definition requires the presence of systemic inflammation demonstrated by an elevated CRP (>3.0 g/L). Notably, KICS subjects demonstrated marked inflammation, with median CRP 37.8 (range 4.9–185.0) (Table 4). Other evidence of systemic inflammation included elevated circulating immunoglobulins and serum free light chains (FLCs; assayed in 9): 8 each (89% of tested) had elevated immunoglobulin G (median 1670 mg/dL, range 293–1900), elevated kappa FLC (median 7.84 mg/dL, range 1.37–25.8), and elevated lambda FLC (median 5.98 mg/dL, range 1.42–16.2). The kappa:lambda ratio was abnormal in one, whereas immunoglobulin M was normal in all (median 47 g/dL, range 6–184). Fibrinogen was elevated in 3 subjects (median 335 mg/dL, range 204–810) and lactate dehydrogenase in 3 including 1 with PEL (median 232 units/L, range 102–527).
KSHV and Other Viral Replication in KICS Subjects
The threshold of KSHV viremia required by the case definition was relatively low (>100 copies/cell in PBMCs or >1000 copies/mL in plasma), however as with CRP, subjects who met the full definition manifested marked systemic KSHV replication. Median KSHV VL in PBMCs was 1569 copies/cell (0–90 909) and in whole blood 81 968 copies/mL (0–3 987 997) (Table 4). One subject with KSHV viremia in whole blood and an effusion did not have PBMC viremia, whereas 1 had PBMC but not plasma KSHV viremia; in all other cases PBMC and plasma findings were concordant.
Systemic replication of other herpesviruses was uncommon. Four (40%) had low level detectable CMV replication, with median CMV VL 0 copies/mL (range 0–1050); 7 (70% including both with PEL) had EBV replication, with median EBV VL 4850 copies/mL (0–94 850); and none had detectable HHV6 replication. Only 1 subject had an elevated HHV6 VL (1.37 million copies/mL), which was found to be due to chromosomally integrated rather than replicating HHV6 by previously published methods [17].
Comparative Clinical Features of KICS Subjects and Controls
Baseline characteristics of each control group are summarized in Table 2; these were similar to KICS subjects with the exception of differences in CD4 counts. After evaluation, no control would have met the KICS case definition. By contrast with KICS subjects, systemic symptoms were uncommon in all control groups including the 2 where subjects had uncontrolled HIV viremia (Table 4): only 40% of patients in the 2 HIV viremic control groups and only 20% of the 2 control groups with suppressed HIV had any symptoms. Both the proportion with symptoms and severity of the worst symptom present were significantly worse for KICS subjects compared with each control group (Table 3).
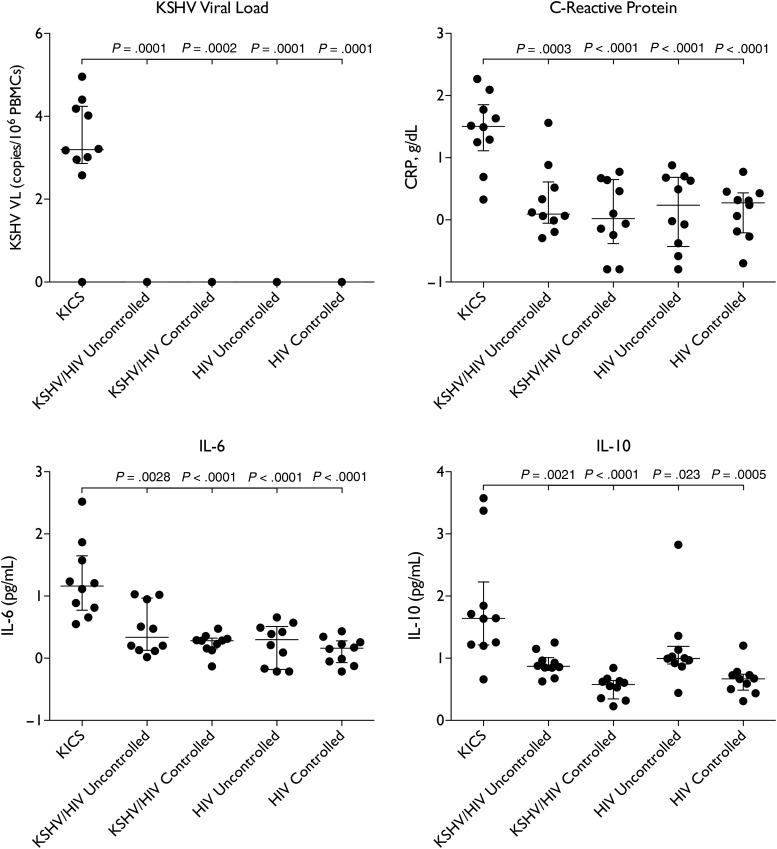
Similarly, laboratory abnormalities were uncommon in controls, including those with uncontrolled HIV viremia, and when present they were mild (Table 4). Hemoglobin and albumin were significantly lower in KICS subjects compared with each control group (Table 4), whereas CRP and KSHV viral load in PBMCs were each significantly elevated in KICS subjects compared with each control group (Figure 2). No control had detectable PBMC KSHV viremia.
Figure 2.
Systemic inflammation, Kaposi sarcoma herpesvirus (KSHV) activation and human interleukin (IL)-6 and IL-10 elevation in patients with KSHV-associated inflammatory cytokine syndrome (KICS) compared with controls. Note: Systemic inflammation measured by C-reactive protein (CRP), KSHV replication measured by viral load (VL) in peripheral blood mononuclear cells (PBMCs), and circulating IL-6 and IL-10 were each markedly elevated in KICS subjects compared with each of the 4 control groups, including the 2 groups with uncontrolled human immunodeficiency virus (HIV) viremia. P values are by Wilcoxon rank sum test for comparison to KICS patients. Note: all values are log transformed.
Cytokines in KICS Subjects and Controls
KICS subjects demonstrated marked elevations of IL-6 and IL-10 (Figure 2), significant compared with each control group. Median IL-6 in KICS subjects was 14.6 (range 3.6–330.3) and median IL-10 was 36.5 ng/mL (4.6–2357.2). By contrast, although we detected circulating viral IL-6 in 4 KICS cases (40%), there was no difference in the proportion positive compared with KSHV-infected controls (P = .66 for HIV viremic and P = 1.0 for HIV suppressed groups). There were no consistent significant differences in other cytokines (Supplementary Figure 1).
Outcomes in KICS Subjects and Controls
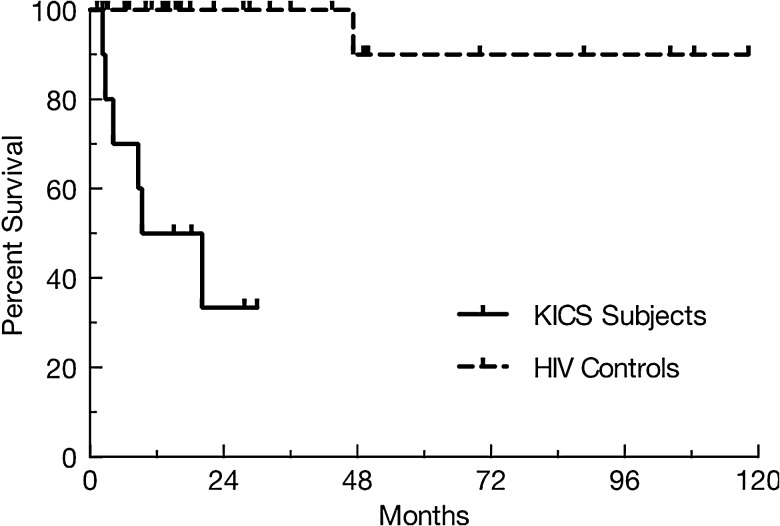
Despite therapies directed at intercurrent tumors and/or KSHV replication, 6 (60%) of KICS subjects succumbed, with median survival 13.6 months (Figure 3). Four deaths were from KSHV-associated tumors (2 KS and 2 PEL), whereas in 2 the cause could not be established. Among the 40 controls, only 1 death (unrelated to KSHV or HIV) occurred at 44 months. Survival was thus significantly worse among KICS subjects (P < .0001). No KICS subject developed KSHV-MCD during follow-up, and the 1 who underwent autopsy had no evidence of KSHV-MCD. No control developed KICS during follow-up.
Figure 3.
Overall survival in Kaposi sarcoma herpesvirus-associated inflammatory cytokine syndrome (KICS) subjects compared with controls. Note: Survival was significantly worse among KICS subjects than controls (P < .0001). Note that the 4 control cohorts are grouped here for the purposes of analysis, as only 1 death occurred among all 40 patients in those 4 groups. Abbreviation: HIV, human immunodeficiency virus.
We explored factors associated with early (<12 months) death among KICS subjects (n-5) and found anemia and hypoalbuminemia at presentation were each associated with trends for early death (P = .040 and P = .056, respectively); other parameters including HIV VL, KSHV VL, CD4, and implicated cytokines were not associated.
DISCUSSION
This is the first prospective effort to our knowledge to study patients with KICS. It extends our original description in using a prespecified case definition and structured clinical and laboratory evaluation to exclude alternate explanations of the symptoms [8, 9]. The use of 18FDG-PET to guide evaluation, together with the lack of KSHV-MCD during follow-up or at autopsy, provide strong evidence that KICS patients did not have unrecognized KSHV-MCD. We used prospectively defined controls to explore contributions of HIV and KSHV and validate the ability of the case definition to identify patients distinguished by their presentations, cytokine milieu, and clinical outcomes.
As in our original series, KICS subjects were critically ill, with multiple clinical and laboratory abnormalities [8]. The outcomes here demonstrate that these inflammatory symptoms and KSHV replication are associated with clinically significant consequences, including a markedly elevated risk of death compared with controls. There were some differences from the original series, including a stronger association with KSHV-associated tumors. The relatively uncommon detection of viral IL-6 in this series, in contrast to KSHV-MCD and the original series, may reflect a difference in the distribution of KSHV-infected cells as well as the relatively low sensitivity of this assay [7, 8]. There was no evidence that any subject was undergoing KSHV seroconversion, although interestingly previous reports of KSHV seroconversion illness bear similarities to KICS symptoms [18]. Similarly, unlike a prior report, we found no evidence that other herpesviruses were contributing to the presentations [19].
Although the clinical manifestations and cytokine signature of KICS resemble those of KSHV-MCD, this series reveals several differences. In particular, all KICS subjects had a high KSHV-associated tumor burden, whereas KS and PEL complicate a smaller proportion of cases of KSHV-MCD [8, 20]. This may in part reflect referral patterns to NCI and will be important to study in other contexts. Importantly too, the widespread adenopathy and splenomegaly that characterize KSHV-MCD were absent in KICS [15]. Also, HIV viremia and advanced CD4 lymphocytopenia were common in KICS subjects, whereas KSHV-MCD is most common in patients with suppressed HIV and preserved CD4 counts [8, 20]. The reason for this difference remains unclear; however, there is known to be a cellular interplay between HIV and KSHV that may be contributing to KSHV replication [21]. It is also possible that establishment of the clinical and pathological characteristics of KSHV-MCD is less likely in the setting of severe CD4 lymphocytopenia. Further delineation of KICS and KSHV-MCD is warranted and would be aided by establishment of a consensus clinicopathological definition of KSHV-MCD.
The source of circulating systemic IL-6, IL-10, and vIL-6 in KICS is unclear and may differ among patients and between KICS and KSHV-MCD. It is probable that KSHV replication is driving their production in infected cells and perhaps uninfected cells. This process may be most significant in patients with the highest burden of KSHV-infected cells, such as those with extensive tumors. These cytokines are known to enhance the growth of KS and PEL [22], and KICS may thus contribute to tumor progression. Interestingly, systemic “B” symptoms have consistently been associated with an increased risk of death for patients with KS [14, 23, 24]. This evaluation of inflammatory abnormalities in patients with KS strongly suggests that KSHV-mediated inflammation is a driver of these symptoms and the observed adverse clinical outcomes.
Symptoms and laboratory abnormalities were uncommon even in controls with HIV viremia, and levels of systemic inflammation were generally low with no evidence of KSHV replication. This provides evidence that HIV viremia is not making a significant contribution to clinical manifestations of KICS, and that those manifestations including KSHV replication are not an epiphenomenon of uncontrolled HIV. The severe CD4 lymphocytopenia in KICS subjects may be a risk factor for KICS development or additionally a manifestation of the cytokine dysregulation. Intriguingly, some features of KICS including cytopenias do resemble those reported in uncontrolled HIV since early in the epidemic [25]. This raises the possibility that some abnormalities previously attributed to uncontrolled HIV were in fact unrecognized manifestations of KSHV-associated inflammation.
A number of aspects of KICS remain to be defined. These include the viral and host factors that influence its development, including perhaps viral susceptibility to lytic induction and host cytokine promoter polymorphisms [26]. The potential intersection in pathogenesis between KICS and the clinical syndrome of Kaposi sarcoma associate immune reconstitution inflammatory syndrome (KS-IRIS) also warrants consideration [8, 9, 27]. Given the poor clinical outcomes, the utility of therapies that target KSHV replication, its cellular reservoirs, or the implicated cytokines should be explored [8, 28–30]. Finally, study of KICS in other contexts is desirable. In particular, few cases of KSHV-MCD have been reported from sub-Saharan Africa despite a high prevalence of KSHV [27]. It is possible that KICS is a similarly underappreciated cause of morbidity and mortality in that setting.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors gratefully acknowledge participating patients and their families; Kirsta Waldon and Qinghua (Roger) Ge of the HIV and AIDS Malignancy Branch, Center for Cancer Research, National Cancer Institute; Randy Stevens and Adam Rupert of the AIDS Monitoring Laboratory, Frederick National Laboratory for Cancer Research; Gary Fahle of the Department of Laboratory Medicine, Clinical Center, National Institutes of Health (NIH); and Prof Peter Medveczky of the University of South Florida for performing assays for chromosomally integrated HHV6. M. N. P. gratefully acknowledges the mentorship of Dr Merrole Cole-Sinclair of St Vincent's Hospital, Melbourne, Australia.
Author Contributions. M. N. P., T. S. U., and R. Y. designed the study; M. N. P., T. S. U., K. A., K. M. W., R. F. L., I. S., and R. Y. cared for patients; M. N. P., T. S. U., K. A., K. M. W., S. P., E. S. J., C. M., R. F. L., I. S., and R. Y. collected and interpreted data; V. M. and D. W. developed and performed the KSHV VL assay; V. W., G. T., and R. Y. developed and performed the viral IL-6 assay; M. N. P., S. M. S., and R. Y. analyzed data; M. N. P. and R. Y. wrote the manuscript. All authors reviewed and approved the final manuscript.
Financial support. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute. This work has also been funded in part with federal funds from the National Cancer Institute, NIH, under contract no. HHSN261200800001E.
Potential conflicts of interest. R. Y. reports other from Celgene Corp., nonfinancial support from Genentech, outside the submitted work; In addition, R. Y. has a patent Peptide vaccine against HIV pending and G. T., a coauthor and the spouse of R. Y., is a coinventor on a patent describing the measurement of KSHV vIL-6. This invention was made when G. T. was an employee of the US Government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the US Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (PL 99–502). R. Y. is the spouse of G. T.; R. Y. has a provisional patent application filed, only slightly related to the subject of the article; it will be assigned to US Government if it issues. T. S. U. reports other from Genentech, other from Celgene Corporation, other from Bayer Corporation, outside the submitted work; In addition, T. S. U. has a patent 62/185 265 pending. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chang Y, Cesarman E, Pessin MS et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994; 266:1865–9. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995; 332:1186–91. [DOI] [PubMed] [Google Scholar]
- 3.Soulier J, Grollet L, Oksenhendler E et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 1995; 86:1276–80. [PubMed] [Google Scholar]
- 4.Moore PS, Gao SJ, Dominguez G et al. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J Virol 1996; 70:549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki Y, Tosato G, Fonville TW, Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood 2001; 97:2526. [DOI] [PubMed] [Google Scholar]
- 6.Oksenhendler E, Carcelain G, Aoki Y et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood 2000; 96:2069. [PubMed] [Google Scholar]
- 7.Polizzotto MN, Uldrick TS, Wang V et al. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2013; 122:4189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uldrick TS, Wang V, O'Mahony D et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without multicentric Castleman disease. Clin Infect Dis 2010; 51:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polizzotto MN, Uldrick TS, Hu D, Yarchoan R. Clinical manifestations of Kaposi sarcoma herpesvirus lytic activation: multicentric Castleman disease (KSHV-MCD) and the KSHV inflammatory cytokine syndrome. Front Microbiol 2012; 3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Common Toxicity Criteria for Adverse Events version 4.0, 2009. Department of Health and Human Services, Bethesda, MD. [Google Scholar]
- 11.Cortez KJ, Fischer SH, Fahle GA et al. Clinical trial of quantitative real-time polymerase chain reaction for detection of cytomegalovirus in peripheral blood of allogeneic hematopoietic stem-cell transplant recipients. J Infect Dis 2003; 188:967–72. [DOI] [PubMed] [Google Scholar]
- 12.Feng WH, Cohen JI, Fischer S et al. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst 2004; 96:1691–702. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JI, Fahle G, Kemp MA, Apakupakul K, Margolis TP. Human herpesvirus 6-A, 6-B, and 7 in vitreous fluid samples. J Med Virol 2010; 82:996–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasti G, Talamini R, Antinori A et al. AIDS-related Kaposi's sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS Clinical Trial Group Staging System in the HAART Era. J Clin Onc 2003; 21:2876–82. [DOI] [PubMed] [Google Scholar]
- 15.Polizzotto MN, Millo C, Uldrick TS et al. 18FDG PET in KSHV-associated multicentric Castleman disease: correlation with activity, severity, inflammatory, and virologic parameters. J Infect Dis 2015; 212:1250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkataraman G, Uldrick TS, Aleman K et al. Bone marrow findings in HIV-positive patients with Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Am J Clin Pathol 2013; 139:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantry SN, Medveczky MM, Arbuckle JH et al. Persistent human herpesvirus-6 infection in patients with an inherited form of the virus. J Med Virol 2013; 85:1940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oksenhendler E, Cazals-Hatem D, Schulz TF et al. Transient angiolymphoid hyperplasia and Kaposi's sarcoma after primary infection with human herpesvirus 8 in a patient with human immunodeficiency virus infection. N Engl J Med 1998; 338:1585–90. [DOI] [PubMed] [Google Scholar]
- 19.Tamburro KM, Yang D, Poisson J et al. Vironome of Kaposi sarcoma associated herpesvirus-inflammatory cytokine syndrome in an AIDS patient reveals co-infection of human herpesvirus 8 and human herpesvirus 6A. Virology 2012; 433:220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bower M, Newsom-Davis T, Naresh K et al. Clinical features and outcome in HIV-associated multicentric Castleman's disease. J Clin Oncol 2011; 29:2481–6. [DOI] [PubMed] [Google Scholar]
- 21.Aoki Y, Tosato G. HIV-1 Tat enhances Kaposi sarcoma-associated herpesvirus (KSHV) infectivity. Blood 2004; 104:810–4. [DOI] [PubMed] [Google Scholar]
- 22.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 1999; 94:2871–9. [PubMed] [Google Scholar]
- 23.Krown SE, Metroka C, Wernz JC. Kaposi's sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1989; 7:1201–7. [DOI] [PubMed] [Google Scholar]
- 24.Mosam A, Shaik F, Uldrick TS et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr 2012; 60:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fauci AS, Macher AM, Longo DL et al. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med 1984; 100:92–106. [DOI] [PubMed] [Google Scholar]
- 26.Ray A, Marshall V, Uldrick T et al. Sequence analysis of Kaposi sarcoma-associated herpesvirus (KSHV) microRNAs in patients with multicentric Castleman disease and KSHV-associated inflammatory cytokine syndrome. J Infect Dis 2012; 205:1665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polizzotto MN, Uldrick TS, Yarchoan R. Kaposi sarcoma herpesvirus associated inflammatory syndromes. N Eng J Med 2014; 370:87–8. [Google Scholar]
- 28.Casper C, Nichols WG, Huang ML, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood 2004; 103:1632–4. [DOI] [PubMed] [Google Scholar]
- 29.van Rhee F, Fayad L, Voorhees P et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman's disease. J Clin Oncol 2010; 28:3701–8. [DOI] [PubMed] [Google Scholar]
- 30.Uldrick TS, Polizzotto MN, Aleman K et al. Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood 2014; 124:3544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.