Abstract
Cardiomyocyte apoptosis contributes to ischemic cardiac injury and the development of heart failure. Higenamine is a key component of the Chinese herb aconite root that has been prescribed for treating symptoms of heart failure for thousands of years in the oriental Asian countries. It has been shown that higenamine has anti-apoptotic effects in a few cell types including cardiomyocytes. However, the pharmacological target and molecular mechanism of higenamine in the heart are still not fully illustrated. Herein, we report that higenamine protected myocyte apoptosis and ischemia/reperfusion (I/R) injury through selective activation of beta2-adrenergic receptor (β2-AR). In particular, we show that higenamine significantly reduced I/R-induced myocardial infarction in mice. In both primary neonatal rat and adult mouse ventricular myocytes, we show higenamine inhibited cell apoptosis and also reduced biochemical markers of apoptosis such as cleaved caspase 3 and 9. More importantly, we show that the anti-apoptotic effects of higenamine in cardiomyocytes were completely abolished by β2-AR but not β1-AR antagonism. Furthermore, we confirmed that higenamine attenuated I/R-induced myocardial injury and reduced cleaved caspases in a β2-AR dependent manner in intact mouse hearts. Higenamine stimulated AKT phosphorylation and required PI3K activation for the anti-apoptotic effect in cardiomyocytes. These findings together suggest that anti-apoptotic and cardiac protective effects of higenamine are mediated by the β2-AR/PI3K/AKT cascade.
Keywords: Higenamine, Ischemia/reperfusion, Beta2-adrenergic receptor, Cardiomyocyte, Apoptosis
1. Introduction
Circulating catecholamines play fundamental roles in cardiac function by activating myocardial β-adrenergic receptors (β-AR)/adenylyl cyclase (AC)/cyclic adenosine monophosphate (cAMP)/cAMP-dependent protein kinase A (PKA) signaling cascade. Long-term activation of β-AR signaling regulates myocyte hypertrophy and survival. It has been known that β1-AR activation is pro-apoptotic while β2-AR activation is anti-apoptotic to cardiac myocytes [1–3]. Stimulation of both β1-AR and β2-AR can activate Gs and AC, which increases intracellular cAMP and PKA [4–6]. PKA phosphorylates multiple substrates that influence contraction/relaxation through regulating excitation-contraction coupling (ECC) and Ca2+ handling [7,8]. Different from β1-AR, stimulation of β2-AR can also activate pertussis toxin (PTX)-sensitive Gi signaling, which is believed to activate phosphatidyl inositol-3 kinase (PI3K)/AKT signaling cascade that promotes myocyte survival [9,10]. Therefore, pharmacological therapies targeting the β2-AR/Gi/PI3K/AKT pathway have been considered as new strategies to protect myocyte apoptosis and cardiac injury.
Higenamine (1-[(4-hydroxyphenyl)methyl]-1,2,3,4- tetrahydroisoquinoline-6,7-diol), was first purified from traditional Chinese herb aconite root in 1976 [11]. Aconite has been prescribed as principal herb combined with other components to treat patients with symptoms of heart failure for thousands of years in the oriental Asian countries. Previous studies have shown that higenamine can activate α-AR signaling and in turn inhibit platelet aggregation [12]. Higenamine also exerts positive chronotropic and inotropic effects likely through activating β-AR signaling [13]. Moreover, higenamine functions as a β2-AR agonist to antagonize bronchoconstriction [14]. It has been also shown that higenamine inhibits apoptosis of neurons in the brain and C6 glial cells through the activation of PI3K/AKT pathway [15]. In the heart, higenamine can attenuate doxorubicin-induced neonatal rat cardiomyocytes apoptosis and protect ischemia/reperfusion (I/R)-induced cardiac injury in rat heart through upregulating heme oxygenase-1 (HO-1) [16,17]. However, the molecular target and mechanism responsible the cardiac protective effect of higenamine is still unknown. In this study, we demonstrate that β2-AR but not β1-AR mediates the protective effect of higenamine against myocyte apoptosis in isolated myocytes as well as myocardial injury in intact hearts.
2. Materials and methods
2.1. Animal care
All animal procedures were performed in accordance with the National Institutes of Health (NIH) and University of Rochester institutional guidelines.
2.2. Isolation and culture of neonatal rat ventricular myocytes (NRVMs)
Primary cultures of NRVMs were prepared from 2 to 3 day old Sprague-Dawley rat pups as previously described [18]. Briefly, hearts were excised and ventricles separated and rinsed in Hank’s balanced salt solution (HBSS) prior to digestion with multiple rounds of collagenase type II (Worthington) containing HBSS. Cells were collected by centrifugation, resuspended in Dulbecco’s Modified Eagle Medium (DMEM) containing 5% fetal bovine serum (FBS), 5% horse serum, 100 IU/ml penicillin, 100 μg/ml streptomycin and non-myocytes were removed by preplating the cells at 37 °C for 1 h. Cells were then cultured in DMEM (described above) containing 10 μM cytosine arabinoside (Ara-C) for 24 h on gelatinized plates before switching to serum-free DMEM medium containing 0.2× insulin–transferrin–selenium (ITS).
2.3. Isolation and culture of adult mouse ventricular myocytes (AMVMs)
Adult mouse cardiomyocytes were isolated from hearts of C57BL/6J mice by enzymatic dissociation using collagenase type II in a Langerdorff perfusion system, according to a previously described protocol with modification [19]. In brief, heparinized and anesthetized mouse heart were rapidly excised, cannulated, and perfused with Ca2+ free isolation buffer (120 mM NaCl, 15 mM KCl, 0.6 mM KH2PO4, 0.6 mM Na2HPO4, 4.6 mM NaHCO3, 1.2 mM MgSO4, 10 mM HEPES, 30 mM Taurine, 5.5 mM glucose, 10 mM 2,3-Butanedione monoxime (BDM), 10 mM Creatine monohydrate, PH 7.4) for 3 min followed by collagenase type II (worthington) mixed isolation buffer (50 mg in 50 ml isolation buffer, 40 nM CaCl2) until digestion was complete. Myocytes were exposed to stopping buffer (10% FBS and 12.5 μM CaCl2 in isolation buffer) for increasing Ca2+ concentration to 1.4 mM, plated cells in laminin-precoated dishes with plating buffer (2.5% FBS and 1.4 mM CaCl2 in isolation buffer), after 2 h, cells cultured with prewarmed culture medium (0.1% BSA, 10mM HEPES, 4mM NaHCO3, 1% penicillin/streptomycin, 10uM blebbistatin, 0.5% insulin–selenium–transferrin in MEM + earle’s salts + L-glutamine medium).
2.4. In vivo ischemia/reperfusion injury
All animal procedures were performed in accordance with the National Institute of Health (NIH) and University of Rochester institutional guidelines. Ischemia was performed in C57BL/6J male mice by ligating left anterior descending artery (LAD) below the tip of the left auricle, according to a previously described protocol with modification [19]. Briefly, occlusion of LAD was confirmed by the change of color and the elevation of ST segment on electrocardiogram. After 30-min of occlusion, suture was untied for reperfusion, and chest cavity and skin incision were closed. After 24-h of reperfusion, LAD was re-occluded at the same position for ischemia-reperfusion surgery. The heart was perfused with 2% Evans Blue (Sigma-Aldrich) to delineate the risk area, and heart tissue slices were stained with 1% 2,3,5-Triphenyltetrazolium chloride (TTC) for the infarct areas. The area at risk (unable to perfuse by Evans blue dye, red plus white areas) and the myocardial infarct area (white, unstained by TTC) were measured using NIH ImageJ software.
2.5. Ex vivo ischemia/reperfusion injury
C57BL/6J male mice hearts were perfused in the Langendorff mode with oxygenated Krebs-Henseleit buffer (118mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 25 mM NaHCO3, 1.2 mM MgSO4, 7 mM glucose, 0.4 mM sodium oleate, 1% BSA, 10 μU/ml insulin, and 1.4 mM CaCl2), as other group described with modification [20]. For ischemia/reperfusion studies, hearts were stabilized at a flow rate of 4ml/min for 20 min before 30 min of no-flow global ischemia, then 30 min of reperfusion. The extent of myocardial necrosis was evaluated by 2,3,5-triphenyltetrazolium chloride (TTC) staining of viable tissue.
2.6. Western blot analysis
Western blot analysis was performed as described previously in Ref. [21]. In brief, lysates were prepared by adding ice cold modified RIPA buffer containing the following: 50 mM Tris-HCl pH 7.4, 1% NP-40, 150mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM NaF, and 1 μ/ml each of aprotinin, leupeptin, and pepstatin. The cell lysates were loaded on SDS-polyacrylamide gel electrophoresis, electrophoresed, and transferred onto the polyvinylidenedi fluoride membranes. The membranes were blocked in 5% nonfat dry milk in 0.01% Tween/PBS, incubated in primary antibody overnight at 4°C, then incubated in horseradish peroxidase conjugated secondary antibodies and developed by using Enhanced Chemiluminescence plus detection reagent. The primary antibodies used in this study were: cleaved and total caspase 3 and 9; p-AKT and total AKT (Cell Signaling Technology); and GAPDH (Santa Cruz Biotechnology).
2.7. Trypan blue viability assay
The trypan blue staining method was used to measure cell viability. AMVMs were seeded in 6-well plates. After the treatments, the cells were incubated with Trypan blue solution (Sigma-Aldrich, final concentration of 0.04%) for 5 min, and then were counted under a phase-contrast inverted microscope, 30 fields in every dish was randomly selected for quantification. The dead cells were expressed as percentage of the total counted cells.
2.8. TUNEL staining in AMVMs and heart tissues
TUNEL (The terminal deoxynucleotidyl transferase UTP-mediated nicked end labeling) staining was used to determine apoptosis in adult mouse ventricular myocytes (AMVMs) as well myocardial apoptosis in heart tissues. For isolated cardiomyocytes, adhered cells were fixed with 4% paraformaldehyde for 1 h at room temperature, then permeabilized with 0.5% triton X-100 at room temperature for another 1 h, outline area of interest with PAP pen. Add 50 μl TUNEL reaction mixture (In Situ Cell Death Detection Kit, Fluorescein, Roche) to each dish, Incubate at 37°C for 1 h, 1:5000 DAPI in PBS incubated for 5 min at room temperature to staining nuclear. The ratio of cell apoptosis was expressed as the percentage of TUNEL staining positive cells versus DAPI staining positive cells. For heart tissues, the procedure was carried out in the paraffin-embedded heart sections following the manufacturer’s instructions (Roche, Nutley, NJ, USA). Slides were examined under a microscope at 400 magnifications and imaged by a high-resolution digital camera (Olympus, Tokyo, Japan). Ten random fields in the ischemic area from each heart sample were selected and quantitated. The number of TUNEL-positive cells (with purple blue nuclei) is presented as a percentage of the total cells.
2.9. Flow cytometry analysis
For Annexin V-FITC/propidium iodide (PI) staining and flow cytometry, NRVMs were collected, washed with PBS. Cells were stained with Annexin V/PI detection kit (BD Biosciences) according to manufacturer’s instructions. Both 5 μl Annexin V-FITC and 10 μl PI were added to 2 × 105 of suspended cells, and incubated for 30 min at 37 °C in the dark prior to analysis using flow cytometer. Cells that were Annexin V-positive only and both Annexin V- and PI-positive were considered early and late apoptosis cells, respectively. All experiments were performed in triplicate.
2.10. Caspase-3 activity in AMVMs
After different treatment of AMVMs measurement of caspase-3 activity was carried out by the manufacturer’s instruction (Biovision, USA). Briefly, 30 μl of cell lysates was mixed with 80 μl of reaction solution containing caspase-3 substrate (Ac-DEVD-pNA) and incubated for 120 min at 37 °C. The absorbance was measured by a microplate reader at 405 nm. The enzyme activity was expressed as the absorbance normalized by protein concentration in each group. The results were shown as the fold compared with the control group.
2.11. Statistics
Data are expressed as mean ± SEM. Comparisons between two groups were evaluated using student’s t-test. One-way ANOVA followed Bonferroni/Dunn post-hoc test was used for multiple comparisons. P-values <0.05 were considered statistically significant.
3. Results
3.1. Higenamine protects against I/R-induced myocardial infarction in mice
To confirm the protective effect of higenamine in vivo, we performed I/R injury on C57BL/6J WT mice by ligation of the LAD (ischemia) for 30min followed by reperfusion for 24h treatment with vehicle (saline) or higenamine (10mg/kg body weight) 2 h prior to the surgery via intraperitoneal injection. We found that myocardial infarct areas (MI) in mice treated with higenamine were much lower than those with saline (19% vs. 51%) when areas at risk (AAR) were similar in both groups (Fig. 1A–C). In addition, we assessed apoptotic cells with TUNEL staining in the infarct zones. Consistently, we found that the apoptotic cells are drastically induced in I/R hearts, which is largely reduced in higenamine treated I/R hearts (Fig. 1D and E). These results demonstrate the cardiac protective effects of higenamine in vivo through increased myocyte survival.
Fig. 1.
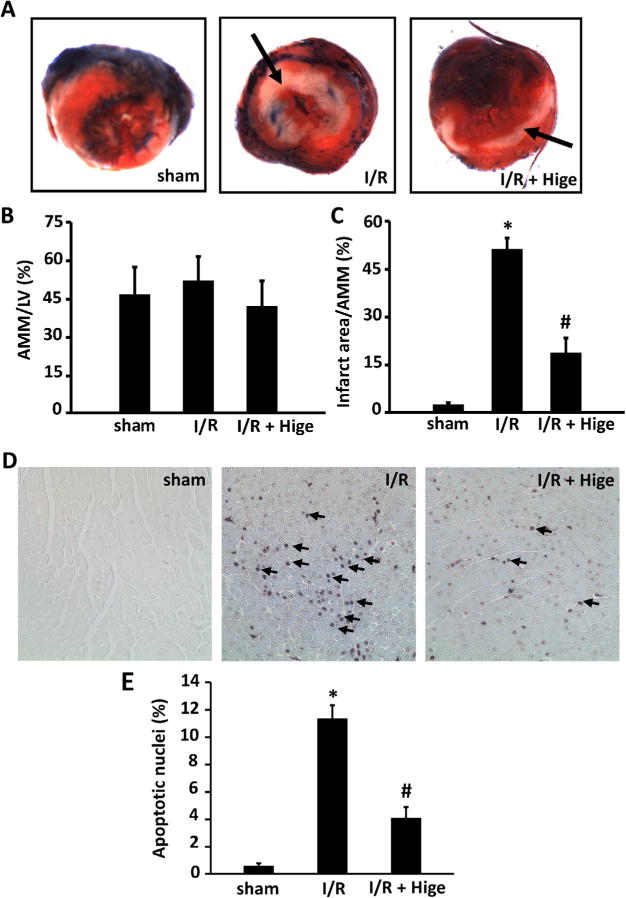
Higenamine protected against ischemia/reperfusion injury in C57 BL/6J mice in vivo. (A) Representative photographs of post-I/R mouse heart slices perfused with 2% Evans Blue and stained with 1% triphenyltetrazoliumchloride (TTC). Mice were subjected to myocardial ischemia for 30 min and reperfusion for 24 h through ligation of the left anterior descending artery (n=8). Hearts were then perfused with 2% Evans Blue to delineate the area at risk (AAR), transversely cut into 4–5 slices, and stained with TTC to access the myocardial infarct area (MI). AAR was the unstained area by Evans blue dye (red and white) and MI area was the unstained area by TTC (white, pointed by arrows). (B and C) Quantification of AAR/left ventricular area, MI/AAR, *P< 0.05, compared with sham group, #P< 0.05, compared with I/R group. (D) Representative images showing myocardial apoptosis detected by TUNEL staining (n=6–8). Apoptotic nuclei are stained with purple blue (pointed by arrow). (E) Quantitative results of TUNEL staining, *P<0.05 vs sham group, #P<0.05 vs I/R group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.2. Higenamine inhibits H2O2-induced cardiomyocyte apoptosis
Because myocyte apoptosis is one of the processes of cell death in I/R injury, we first examined the effects of higenamine on H2O2-induced apoptosis in neonatal rat ventricular myocytes (NRVMs). We found higenamine dose dependently attenuated H2O2-stimulated early and late apoptosis of NRVMs analyzed by annexin V FITC/propidium iodide (PI) assay with flow cytometry (Fig. 2 and Fig. S1). We also determined the anti-cardiac apoptosis function of higenamine by examining the protein levels of apoptosis marker cleaved-caspase 3 (C-caspase-3) and its upstream regulator cleaved-caspase 9 (C-caspase-9). We found that higenamine can inhibit H2O2-induced increase of C-caspase-9 and C-caspase-3 in a dose dependent manner in NRVMs (Fig. 3A).
Fig. 2.
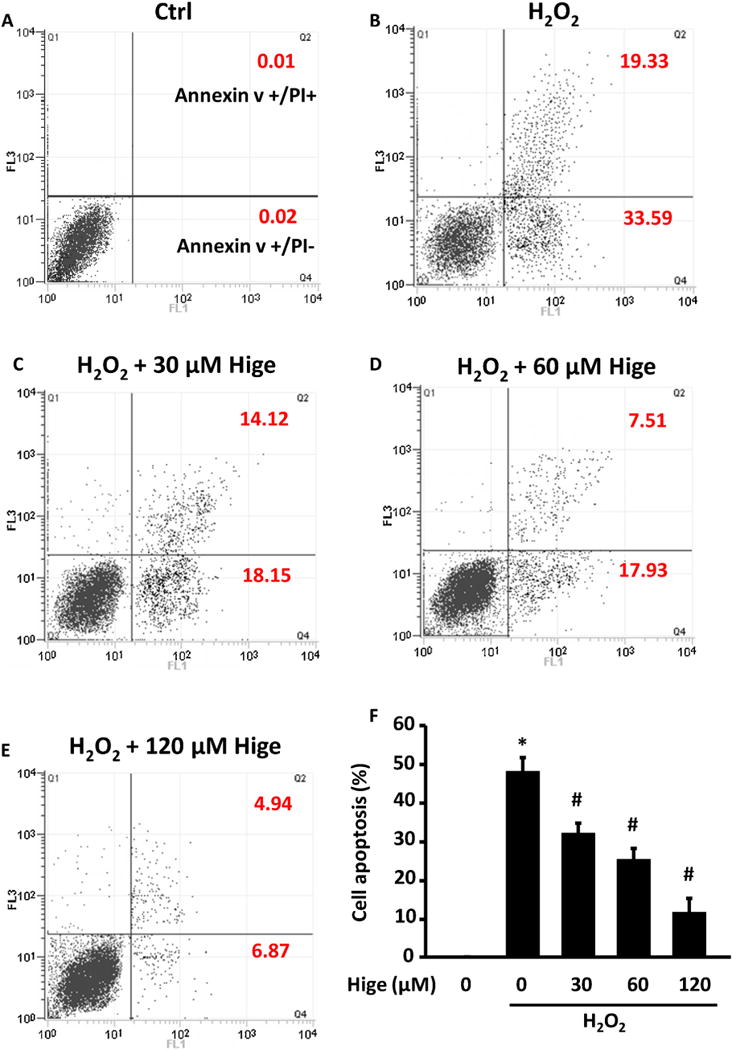
Higenamine dose-dependently reversed H2O2 induced cell death and apoptosis in neonatal rat ventricular myocytes (NRVMs). (A–E) Represent flow cytometry plots showing NRVMS apoptotic cells stained with Annexin V-FITC/propidium iodide (PI). NRVMs were stimulated with 250 μM H2O2 for 24 h to induce apoptosis indicate in the presence of indicated doses of higenamine (Hige). Annexin V positive but PI negative population is early apoptotic, while Annexin V positive and PI negative population is late apoptotic. (F) Quantification of apoptotic cells analyzed by FACS (including both early and late apoptotic cells), Data are mean ± SD of 3 independent experiments. *P<0.05, compared with control cells, #P<0.05, compared with H2O2 alone treated cells.
Fig. 3.
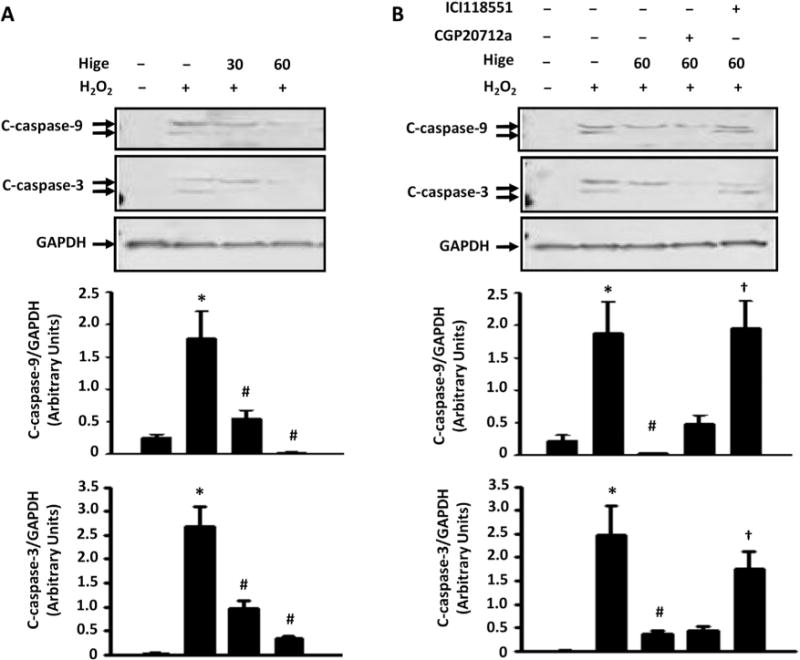
The inhibitory effects of Higenamine on apoptotic protein expression were reversed by β2-AR but not β1-AR antagonism. (A) Higenamine (100 μM) dose-dependently decreased cleaved-caspase 9 and 3 proteins stimulated by H2O2 (250 μM for 24 h) in NRVMs. (B) The effects of higenamine were significantly blocked by β2-AR specific antagonist ICI118551 (0.5 μM), but not β1-AR specific antagonist CGP20712a (1 μM). *P<0.05 vs. control; #P<0.05 vs. H2O2 alone; †P<0.05 vs. H2O2 and higenamine. Results were derived from 3 independent experiments.
3.3. β2-AR and PI3K/AKT activation is critical for the anti-apoptotic effect of higenamine in cardiomyocytes
It has been previously reported that higenamine can stimulate bronchial relaxation as a β2-AR agonists [14]. We first examined the effects of β2-AR antagonist ICI118551 and β1-AR antagonist CGP20712a on higenamine-mediated regulation of cleaved caspases in NRVMs. As shown in Fig. 3B, we observed that the inhibitory effects of higenamine on H2O2-induced C-caspase-9 and C-caspase-3 were abolished in the presence of β2-AR antagonist but not β1-AR antagonist (Fig. 3B).
We also confirmed the role of β2-AR in higenamine-mediated protective effects in adult mouse ventricular myocytes (AMVMs). As shown in Fig. 4A and B, H2O2 induced AMVM death assessed by round/rod cell ratio, which was significantly attenuated by higenamine. Interestingly, the protective effect of higenamine was blocked by β2-AR but not β1-AR antagonism. AMVM apoptosis measured by TUNEL staining further confirmed the role of β2-AR in mediating the protective effect of higenamine on AMVM apoptosis (Fig. 4C and D). In cardiomyocytes, β2-AR stimulation through PI3K/AKT activation illustrates an anti-apoptotic effect [10,22,23]. Therefore, we examined the involvement of PI3K and found that PI3K inhibitor wortamanin blocked the anti-apoptotic function of higenamine (Fig. 4A and B). Furthermore, higenamine stimulated AKT phosphorylation at Ser 473 (activation), which was blocked by β2-AR antagonist or PI3K inhibitor but not changed by β1-AR antagonist (Fig. 4E).
Fig. 4.
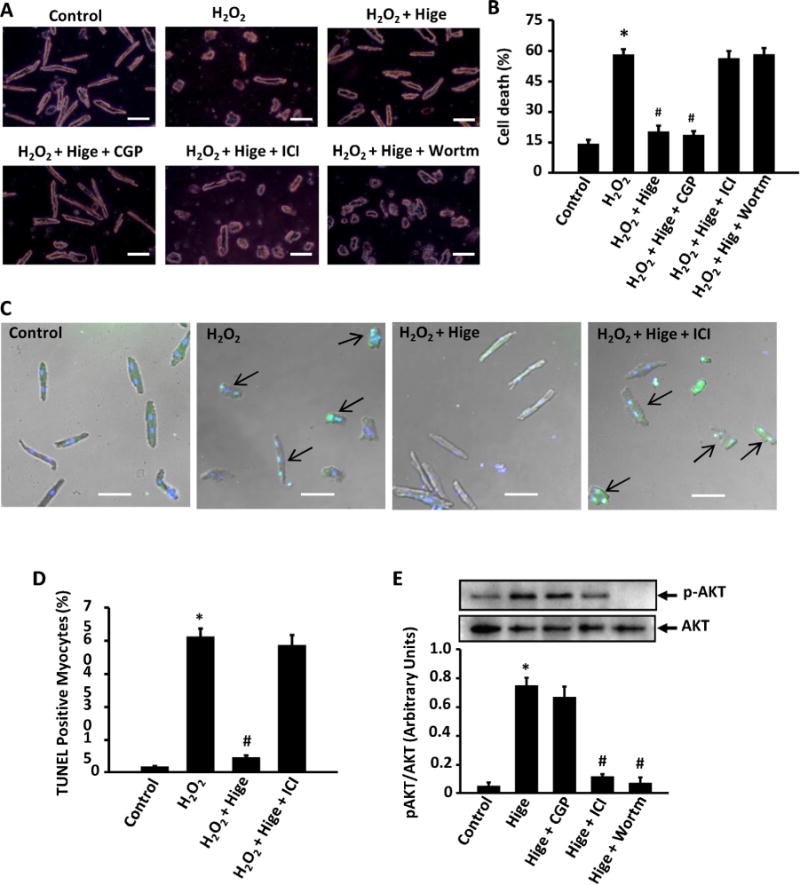
Role of β2-AR/PI3K/AKT pathway in higenamine mediated inhibition of H2O2-induced cell death, apoptosis, and AKT activation in AMVMs. (A) Trypan blue staining showing that the protective effect of Hignamine (100 μM) on H2O2 (20 μM for 24 h) induced cell death in adult mouse ventricular myocytes (AMVMs) were blocked by β2-AR specific inhibitor ICI118551 (0.5 μM) and PI3K inhibitor Wortmannin (10 μM), but not by β1-AR specific inhibitor CGP20712a (1 μM). Scale bar, 20 μm. (B) Quantification of A; (C) TUNEL staining of AMVMs showing that the anti-apoptotic effect of Higenamine (100 μM) on H2O2 (20 μM for 24 h) induced apoptosis was blocked by β2-AR specific inhibitor ICI118551 (0.5 μM). Scale bar, 20 μm. (D) Quantification of C. (E) The increase of AKT phosphorylation (activation) induced by Higenamine was attenuated by β2-AR specific inhibitor ICI118551 (0.5 μM) but not β1-AR specific inhibitor CGP20712a (1 μM). *P<0.05 vs. control cells, #P<0.05 vs. H2O2 treated cells, All results based on 3 independent duplicated experiments. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
To further verify the protective effect of higenamine is through β2-AR signaling, we also tested a different β2-ARspecific antagonist butoxamine and β2-AR specific agonist formoterol hemifumarate. As shown in Supplemental Fig. S2, butoxamine blocked the effect of higenamine on H2O2 stimulated caspase 3 activity and AKT activation. In contrast, a β2-AR specific agonist formoterol hemifumarate reduced caspase 3 activity and increased AKT activation similar to higenamine. Addition of β2-AR formoterol hemifumarate to higenamine did not elicit additive effect. All these results together indicated the β2-AR/PI3K/AKT signaling plays a critical role in mediating the anti-apoptosis effect of higenamine in cardiomyocytes.
3.4. β2-AR antagonism blocks the protective effect of higenamine on I/R-induced myocardial infarction
To confirm the role of β2-AR in intact hearts, we examined if β2-AR antagonism blocked the protective effect of higenamine on myocardial infarction in an ex-vivo perfused ischemia/reperfusion (I/R) model, with 30 min globe no flow mimic ischemia and follow-up 30 min reperfusion. We found that hearts perfused with higenamine had significantly decreased myocardial infarction area compared to vehicle (11.6% vs. 42.7%) (Fig. 5A and B). The cleaved caspase-3 was also reduced with higenamine treatment and the reduction was abolished in the presence of β2-AR antagonist (Fig. 6A and B). In contrast, AKT phosphorylation was increased by higenamine and the increase was abolished by β2-AR antagonism (Fig. 6A and C). These together strongly suggest that higenamine protects myocardial injury through β2-AR/PI3K/AKT mediated anti-apoptosis (Fig. 7).
Fig. 5.
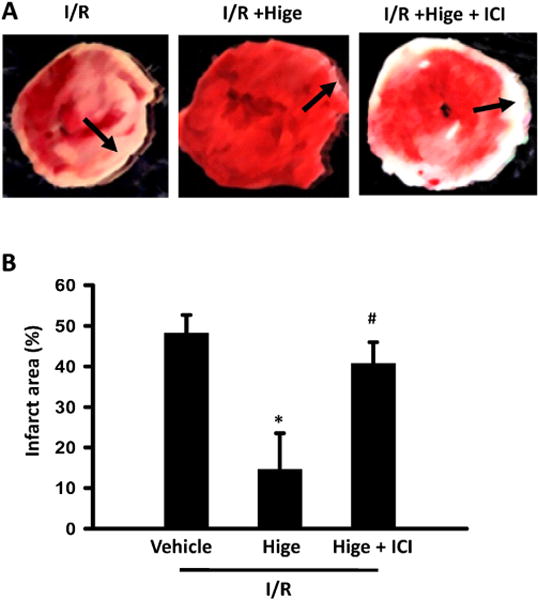
Higenamine protected against I/R injury of perfused mice heart through β2-AR/PI3K/AKT pathway (A) Image of TTC staining slides in different groups, in ex vivo I/R experiment, heart was perfused with oxygenated Krebs–Henseleit buffer in Langendorff system for 30 min, then 30 min no-flow ischemia, followed by 40 min reperfusion, in I/R+ Hige group, 100 μM Higenamine was added to working buffer, in I/R+ Hige +ICI group, 0.5 μM ICI118551 and 100 μM Higenamine were added in working buffer. Heart slices were stained by 1% 2, 3, 5-Triphenyltetrazolium chloride (TTC) forthe infarcted area. Non infarct area is red and infarct area is white. (B) Quantification of infarct area (n=5 in each group), *P<0.05 vs I/R group, #P<0.05 vs I/R + Higenamine group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 6.
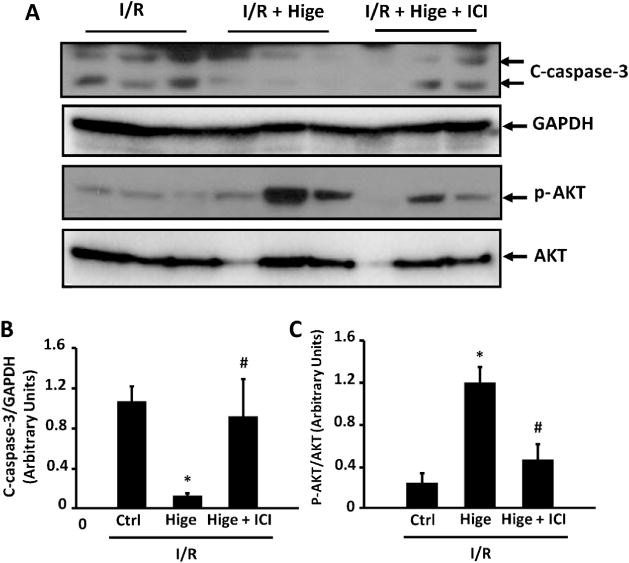
Role of β2-AR signaling in higenamine-mediated attenuation of caspase-3 cleavage and AKT inactivation induced by ex vivo I/R in mouse hearts. (A) Representative Western blots showing the level of cleaved-caspase 3 (C-caspase-3), phosphorylated AKT at S473 (p-AKT) in sham and I/R hearts treated with higenamine (100 μM) and/or β2-AR antagonist ICI118551 (0.5 μM). (B and C) Quantification of A, *P<0.05 vs. I/R group, #P<0.05 vs. I/R + Higenamine group.
Fig. 7.
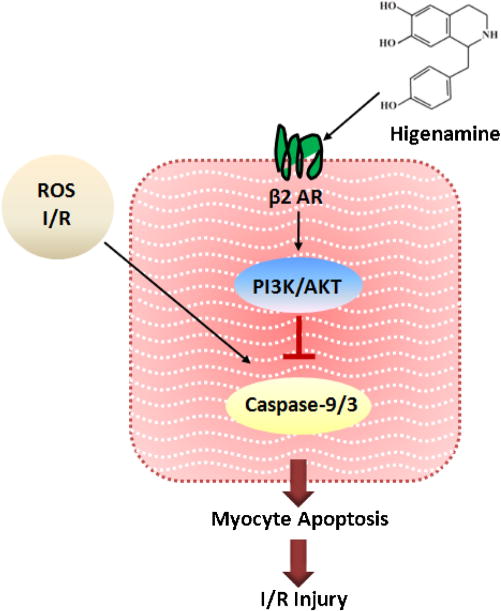
Proposed model. The mechanistic diagram showing β2-AR/PI3K/AKT pathway plays an important role in mediating the protective effect of Higinamine against cardiac I/R injury.
4. Discussion
Higenamine was the main cardiotonic compound purified from aconite root and aconite root has been one of the substances in the Chinese herb medicine prescribed to treat the symptoms of heart failure for thousands of years in the oriental Asian countries. In addition to the positive inotropic and chronotropic action of higenamine in the heart [13,24], recent studies have revealed the anti-apoptotic function of higenamine in rat neonatal cardiomyocytes and rat myocardia [17]. In this study, we provide further evidence demonstrating that higenamine antagonizes cardiomyocyte apoptosis and protective ischemia/reperfusion induced myocardial infarction in vitro using both neonatal and adult cardiomyocytes as well as ex vivo and in vivo with mouse I/R models. The cardiac protective effect of higenamine should be largely contributed by its anti-apoptotic effect because we observed very similar changes of C-caspase-9 and -3, well-established biochemical markers of apoptosis. However, we cannot rule out the beneficial effect from the cardiac vasculature because it has been shown that higenamine also has vasodilatory effects [25,26].
More importantly, in this study we provide experimental evidence showing that β2-AR but not β1-AR antagonism blocked the effect of higenamine in protecting cardiomyocyte apoptosis and myocardial infarction. An early pharmacological screening study with a CHO cell line expressing β2-AR and a GFP reporter gene has shown that higenamine can function as a β2-AR agonist [14]. Thus, we propose that higenamine functions as a β2-AR agonist in mediating the anti-apoptotic effect of higenamine in cardiomyocytes (Fig. 7). In line with the effect of β2-AR activation in trachea, higenamine indeed has been shown to stimulate tracheal relaxation [27] and illustrate a protective effect in an experimental asthma mode [14]. The vasodilatory effect of higenamine in vasculature is also likely mediated by the β2-AR. However, it has been also reported that higenamine might be a β1-AR agonist in mediating the inotropic and chronotropic effect of higenamine [24,28].
Stimulation of β2-AR activates PI3K-AKT cascade, which is critical for myocyte survival. We also confirmed that PI3K-AKT activation is essential for the anti-apoptotic effect of higenamine. The role of PI3K/AKT was also implicated in a previous study showing that the compound containing higenamine protects doxorubicin-induced H9C2 and NRVM apoptosis [16]. In addition, another previous study showed that higenamine inhibits myocyte apoptosis through induction of hemeoxygenase 1 (HO-1) [17]. Higenamine has been shown to stimulate PI3K/AKT/Nrf2 signaling and subsequently induce HO-1 expression in C6 cells, which is critical for higenamine antagonizing C6 apoptosis and hypoxia-induced brain injury [15]. Therefore, these findings together should support that β2-AR/PI3K/AKT cascade is critical in mediating the anti-apoptotic effect of higenamine (Fig. 7).
In summary, we have provided in vitro, ex vivo, and in vivo evidence to demonstrate that higenamine is protective from cardiomyocyte apoptosis and injury induced myocardial damage. Moreover, we have defined that β2-AR is a potential molecular target of higenamine in mediating the protective effect of higenamine in myocardia. Given the therapeutic effects of higenamine in heart failure patients, our findings have great therapeutic and pharmacological significance.
Supplementary Material
Acknowledgments
This research was supported by grants from National Natural Science Foundation of China No. 81473379 (to M. Wu), Shanghai Municipal Bureau of Health Science and technology project No. 2011L032B (to M. Wu), and NIH grants HL111291 and HL088400 (to C. Yan).
Abbreviations
- Higenamine
1-[(4-hydroxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol
- I/R
ischemia/reperfusion
- β-AR
β-adrenergic receptor
- AC
adenylyl cyclase
- cAMP
cyclic adenosine monophosphate
- PKA
protein kinase A
- ECC
excitation-contraction coupling
- PTX
pertussis toxin
- PI3K
phosphatidyl inositol-3 kinase
- HO-1
heme oxygenase-1
- NRVMs
neonatal rat ventricular myocytes
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
fetal bovine serum
- Ara-C
cytosine arabinoside
- ITS
insulin–transferrin–selenium
- AMVMs
adult mouse ventricular myocytes
- BDM
2,3-Butanedione monoxime
- LAD
ligating left anterior descending artery
- TTC
2,3,5-triphenyltetrazolium chloride
- TUNEL
the terminal deoxynucleotidyltransferasedUTP-mediated nicked end labeling
- PI
propidium iodide
- AAR
areas at risk
- LV
left ventricle
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.phrs.2015.12.032.
Footnotes
Conflict of interest
None declared.
Contributor Information
Mei-ping Wu, Email: meipingwu1207@hotmail.com.
Yi-shuai Zhang, Email: Yishuai_zhang@outlook.com.
Qian-mei Zhou, Email: tazhou@163.com.
Jian Xiong, Email: xj0918@163.com.
Yao-rong Dong, Email: 1067@szy.sh.cn.
Chen Yan, Email: chen_yan@urmc.rochester.edu.
References
- 1.Singh K, Xiao L, Remondino A, et al. Adrenergic regulation of cardiac myocyteapoptosis. J Cell Physiol. 2001;189(3):257–265. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- 2.Shizukuda Y, Buttrick PM. Subtype specific roles of beta-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol. 2002;34(7):823–831. doi: 10.1006/jmcc.2002.2020. [DOI] [PubMed] [Google Scholar]
- 3.Singh K, Xiao L, Remondino A, et al. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189(189):257–265. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- 4.Xiao RP, Avdonin P, Zhou YY, et al. Coupling of beta2-adrenoceptortoGi proteins and its physiological relevance in murine cardiac myocytes. Circ Res. 1999;84(1):43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Tepe NM, Liggett SB. Functional receptor coupling to Gi is a mechanism of agonist-promoted desensitization of the beta2-adrenergic receptor. J Recept Signal Transduct Res. 2000:20. doi: 10.3109/10799890009150038. [DOI] [PubMed] [Google Scholar]
- 6.Soto D, De AV, Zhang J, et al. Dynamic protein kinase a activities induced by beta-adrenoceptors dictate signaling propagation for substrate phosphorylation and myocyte contraction. Circ Res. 2009;104(6):770–779. doi: 10.1161/CIRCRESAHA.108.187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lissandron V, Zaccolo M. Compartmentalized cAMP/PKA signalling regulates cardiac excitation-contraction coupling. J Muscle Res Cell Motil. 2006;27(5–7):399–403. doi: 10.1007/s10974-006-9077-2. [DOI] [PubMed] [Google Scholar]
- 8.Saucerman JJ, Zhang J, Martin JC, et al. Systems analysis of PKA-Mediated phosphorylation gradients in live cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103(34):12923–12928. doi: 10.1073/pnas.0600137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu WZ. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98(4):1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Communal C. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100(22):2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 11.Kosuge T, Yokota M. Studies on cardiac principle of aconite root. Chem Pharm Bull. 1976;24(1):176–178. doi: 10.1248/cpb.24.176. [DOI] [PubMed] [Google Scholar]
- 12.Pyo MK, Lee DH, Kim DH, et al. Enantioselective synthesis of (R)-(+)- and (S)-(−)-higenamine and their analogues with effects on platelet aggregation and experimental animal model of disseminated intravascular coagulation, Bioorg. Med Chem Lett. 2008;18(14):4110–4114. doi: 10.1016/j.bmcl.2008.05.094. [DOI] [PubMed] [Google Scholar]
- 13.Park CW, Chang KC, Lim JK. Effects of higenamine on isolated heart adrenoceptor of rabbit. Arch Int Pharmacodyn Ther. 1984;267(2):279–288. [PubMed] [Google Scholar]
- 14.Bai G, Yang Y, Shi Q, et al. Identification of higenamine in radix aconiti lateralis preparata as abeta2-adrenergic receptor agonist1. Acta Pharmacol Sin. 2008;29(10):1187–1194. doi: 10.1111/j.1745-7254.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 15.Ha YM, Kim MY, Park MK, et al. Higenamine reduces HMGB1 during hypoxia-induced brain injury by induction of heme oxygenase-1 through PI3K/Akt/Nrf-2 signal pathways. Apoptosis. 2012;17(5):463–474. doi: 10.1007/s10495-011-0688-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen YL, Zhuang XD, Xu ZW, et al. Higenamine combined with [6]-gingerol suppresses doxorubicin-triggered oxidative stress and apoptosis in cardiomyocytes via upregulation of PI3K/AktPathway. Evid Based Complement Altern Med. 2013;2013 doi: 10.1155/2013/970490. (Article ID 970490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, Kang YJ, Kim HJ, et al. Higenamine reduces apoptotic cell death by induction of heme oxygenase-1 in rat myocardial ischemia-reperfusion injury. Apoptosis. 2006;11(7):1091–1100. doi: 10.1007/s10495-006-7110-y. [DOI] [PubMed] [Google Scholar]
- 18.Miller CL, Oikawa M, Cai Y, et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte. hypertrophy Circ Res. 2009;105(2) doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oikawa M, Wu M, Lim S, et al. Cyclic nucleotide phosphodiesterase 3A1 protects the heart against ischemia-reperfusion injury. J Mol Cell Cardiol. 2013;64(5):11–19. doi: 10.1016/j.yjmcc.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Qi D, Cheng H, et al. Urocortin 2 autocrine/paracrine and pharmacologic effects to activate AMP-activated protein kinase in the heart. Proc Natl Acad Sci U S A. 2013;110(40):16133–16138. doi: 10.1073/pnas.1312775110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Y, Knight WE, Guo S, et al. Vinpocetine suppresses pathological vascular remodeling by inhibiting vascular smooth muscle cell proliferation and migration. J Pharmacol Exp Ther. 2012;343(2):479–488. doi: 10.1124/jpet.112.195446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaugg M, Xu W, Lucchinetti E, et al. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102(3):344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 23.Ahmet I, Krawczyk M, Heller P, et al. Beneficial effects of chronic pharmacological manipulation of adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110(9):1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 24.Kimura I, Makino M, Takamura Y, et al. Positive chronotropic and inotropic effects of higenamine and its enhancing action on the aconitine-induced tachyarrhythmia in isolated murine atria. Jpn J Pharmacol. 1994;66(1):75–80. doi: 10.1254/jjp.66.75. [DOI] [PubMed] [Google Scholar]
- 25.Chang KC, Chong WS, Lee IJ. Different pharmacological characteristics of structurally similar benzylisoquinoline analogs, papaverine, higenamine, and GS 389, on isolated rat aorta and heart. Can J Physiol Pharmacol. 1994;72(4):327–334. doi: 10.1139/y94-049. [DOI] [PubMed] [Google Scholar]
- 26.Wong KK, Lo CF, Chen C. Endothelium-dependent higenamine-induced aortic relaxation in isolated rat aorta. Planta Med. 1997;63(2):130–132. doi: 10.1055/s-2006-957628. [DOI] [PubMed] [Google Scholar]
- 27.Tsukiyama M, Ueki T, Yasuda Y, et al. Beta2-adrenoceptor-mediated tracheal relaxation induced by higenamine from Nandina domestica Thunberg. Planta Med. 2009;75(13):1393–1399. doi: 10.1055/s-0029-1185743. [DOI] [PubMed] [Google Scholar]
- 28.Kimura I, Islam MA, Kimura M. Potentiation by higenamine of the aconitine-induced positive chronotropic effect in isolated right atria of mice: the effects of cholera toxin, forskolin and pertussis toxin. Biol Pharm Bull. 1996;19(8):1032–1037. doi: 10.1248/bpb.19.1032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


