Abstract
Over the past five decades, palliative care has evolved from serving patients at the end of life into a highly specialized discipline focused on delivering supportive care to patients with life-limiting illnesses throughout the disease trajectory. A growing body of evidence is now available to inform the key domains in the practice of palliative care, including symptom management, psychosocial care, communication, decision-making, and end-of-life care. Findings from multiple studies indicate that integrating palliative care early in the disease trajectory can result in improvements in quality of life, symptom control, patient and caregiver satisfaction, quality of end-of-life care, survival, and costs of care. In this narrative Review, we discuss various strategies to integrate oncology and palliative care by optimizing clinical infrastructures, processes, education, and research. The goal of integration is to maximize patient access to palliative care and, ultimately, to improve patient outcomes. We provide a conceptual model for the integration of supportive and/or palliative care with primary and oncological care. We end by discussing how health-care systems and institutions need to tailor integration based on their resources, size, and the level of primary palliative care available.
Introduction
Since the 1960s, when Dame Cicely Saunders established the modern hospice movement in the UK, palliative care has evolved from a philosophy aimed at improving care for patients at the end of life to a professional specialty that provides comprehensive care for patients with life-limiting illnesses, throughout the disease trajectory.1 In the 1970s, Dr Balfour Mount coined the term ‘palliative care’ to label his hospital-based service.2 Since the late 1980s, the term ‘supportive care’ has been used to describe a variety of patient-care services that offer essentially every care intervention other than disease-directed therapies, across settings ranging from survivorship to bereavement.3 Nowadays, hospice care is mostly used to describe community-based palliative care for patients in the last months of life. Considerable overlaps in the aims and approaches of ‘supportive care’, ‘palliative care’, and ‘hospice care’ have been recognized (Figure 1), and we previously identified the similarities and differences between these terms through a systematic review of the literature.4
Figure 1.
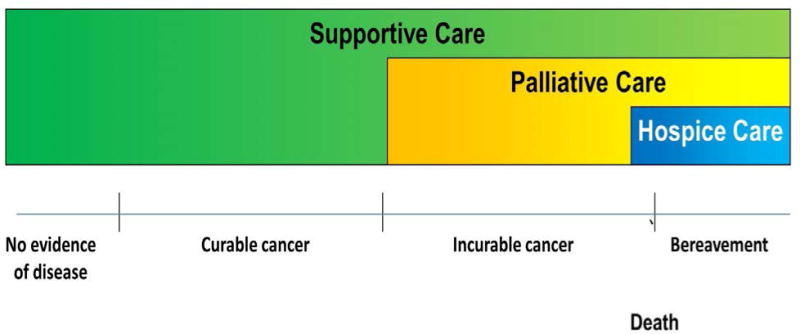
Conceptual framework for supportive care, palliative care and hospice care, based on a systematic literature review.4 Supportive care is defined as “the provision of the necessary services for those living with or affected by cancer to meet their informational, emotional, spiritual, social or physical need during their diagnostic treatment or follow-up phases encompassing issues of health promotion and prevention, survivorship, palliation and bereavement.”120 Palliative care is supportive care for patients with advanced-stage disease, and includes interventional programmes used in both acute-care hospitals and the community. Hospice care is a form of community-based palliative care predominantly serving patients and their loved-ones at the end of life. Under this conceptual framework, hospice care is a branch of palliative care, and palliative care is, in turn, a branch of supportive care. Supportive care also include many other services such as teams that manage cancer treatment related toxicities and cancer related complications (e.g. dermatologists who treat epidermal growth factor related rash, and pulmonologists who provide endoscopy and airway stenting), wound care teams, psychosocial oncology, social workers and chaplains. Bereavement care refers to support provided to family caregivers after the death of the patient, and may last for months to a year or longer.
In 2013–2014, the World Health Organization (WHO), and Worldwide Hospice Palliative Care Alliance (WHPCA) published a report that categorized the global level of palliative-care development, using a range from no known hospice palliative-care activity (level 1), capacity-building activity (level 2), isolated palliative-care provision (level 3a), generalized palliative-care provision (level 3b), preliminary integration into mainstream service provision (level 4a), to advanced integration into mainstream service provision (level 4b).5 ‘Advanced integration’ was defined based on the availability of a critical mass of palliative-care activism, comprehensive provision of all types of palliative care, broad awareness of palliative care, unrestricted access to opioids, the effect of palliative care on public health policy, development of education centres, academic links with universities, and the existence of a national palliative-care association.5 On the basis of the WHO–WHPCA classification, the nations that have achieved advanced integration of palliative care into their health-care systems were mostly developed countries located in North America, Western Europe, Australia, and Asia (specifically, Japan).5 The European Association for Palliative Care (EAPC) and the International Association for Hospice and Palliative Care (IAHPC) have also published an atlas of palliative care development in Europe and Latin America, respectively.6, 7 Even in countries recognized to have ‘advanced integration’, however, substantial regional variation in palliative-care provision has been reported. For example, a survey of cancer centres in the USA showed that inpatient consultation teams, outpatient palliative-care clinics, acute palliative-care units and institution-run hospices were present in 92%, 59%, 26%, and 31% of National Cancer Institute (NCI)-designated cancer centres, and 56%, 22%, 20%, and 42% of non-NCI-designated cancer centres, respectively.8 Furthermore, fewer than half of the cancer centres had palliative-care fellowship training and research programmes in place.8
Over the past decade, a growing body of literature has emerged, supporting the role of palliative care in improving patient and caregiver outcomes.9–11 With this evidence comes a heightened interest among professional organizations globally in integrating palliative care and oncology. But what exactly does integration entail and how can it be assessed? A systematic review, published in 2015,12 has examined the studies reported to date, and summarized the 38 different aspects (indicators) of integration identified (Figure 2), which goes some way towards defining how integration can be assessed. The objective of this Review, therefore, is to address the question of what integration involves; specifically, we discuss the various strategies to integrate oncology and palliative care through optimization of clinical infrastructures, processes, education, and research.
Figure 2.
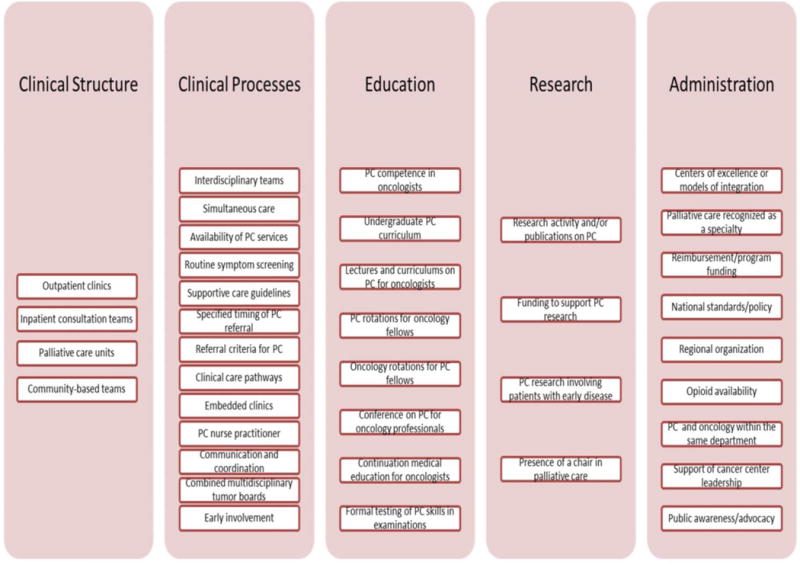
Indicators of successful integration of palliative care into oncology practice. Previously, we reviewed the literature to identify factors relevant to different aspects that are associated with efforts to integrate palliative care into clinical practice in oncology; we identified four indicators related to clinical structure, 13 indicators related to clinical processes, eight related to education, four related to research, and nine related to administration.12, 82 Subsequently, a Delphi study of international experts identified 13 major (>90% consensus) indicators and 30 minor (70–90% consensus) indicators of integration. Major indicators were defined as the most relevant and important indicators related to integration. Eight of the major indicators are highlighted with an asterisk and 5 represented new additions (i.e. routine documentation of advance care plans in patients with advanced cancer; proportion of outpatients with pain assessed on either of the last two visits before death; proportion of patients with 2 or more emergency room visits in last 30 days of life; place of death consistent with patient’s preference; combined educational activities for palliative care and oncology fellows/trainees). These indicators represent strategies to improve efforts to combine these disciplines of care. Furthermore, these indicators may be used to assess the level of integration, which can in turn allow patients and clinicians to identify cancer centers that offer high level of palliative care access, policy makers and administrators to conduct program evaluation and allocate appropriate resources to improve care, educators to develop curriculum, and researchers to assess how integration can improve outcomes.
Evidence to support integrated care
Several randomized controlled trials (RCTs) have provided level I evidence that supports early involvement of palliative care for patients with advanced-stage cancer. For example, Temel et al.13, 14 published the landmark trial that enrolled 151 patients within 8 weeks of diagnosis of metastatic non-small-cell lung cancer (NSCLC) and randomly assigned them to receive routine oncological care either alone or combined with early specialist palliative care. The early provision of palliative care was associated with better quality of life (QoL), fewer depressive symptoms, less aggressive care at the end of life, improved longitudinal prognostic awareness, and longer survival.13, 14 More recently, in a large cluster randomization trial that compared specialist palliative care to routine oncological follow up in 461 patients with metastatic solid tumours and a prognosis of 6–24 months, use of palliative care was associated with improved QoL, symptom burden, and patient satisfaction over time, whereas the group assigned to standard oncological care experienced a deterioration in these domains.15 A trend favouring the palliative-care arm for the primary outcome of QoL, as assessed by Functional Assessment of Chronic Illness Therapy—Spiritual Well-Being (FACIT-Sp) questionnaire, was observed at 3 months (mean change score 1.60 versus −2.00; P = 0.07), which became statistically significant at 4 months (mean change score +2.46 versus −3.95; P = 0.006).15
In contrast to involvement of an interdisciplinary palliative-care team in oncological care, as investigated in the aforementioned trials, Bakitas et al.16 examined the effect of combining nurse-led palliative-care intervention and standard care in the Project ENABLE II trial. QoL and mood were found to be significantly better in the palliative-care arm of this trial (mean treatment effect of 4.6 for QoL (P = 0.02) and −1.8 for depressed mood (P = 0.02), although symptom burden, quality of end-of-life care and survival were similar.16 In the subsequent ENABLE III trial,17 the investigators examined early nurse-led palliative care, introduced within 30–60 days of a diagnosis of advanced-stage cancer, in comparison to delayed palliative care initiated 3 months later in a wait-list-design study. Unlike in the previous study, QoL, symptom burden, and mood in ENABLE III did not differ between the two groups at 3 months, 6 months or 12 months after enrolment.17 Interpretation of these findings is complicated by the facts that half of the delayed-care group received a consultation by a palliative-care team ahead of the nursing intervention, and that only 207 of the 360 patients planned were enrolled in this study.17 Despite the lack of benefit demonstrated by these outcomes, the 1-year survival rate was improved in the early palliative care group (63% versus 48%; P = 0.04), although the difference in overall survival between the cohorts did not reach statistical significance (median survival 18.3 months versus 11.8 months, P = 0.18).17
The results of these clinical trials and those of a large number of prospective cohort and retrospective studies.11, 18–24 provide important insights into the potential benefits associated with integration of palliative care into the oncological care of patients with advanced-stage cancer, and raise some questions on how we can improve care. Firstly, outpatient access to palliative care is clearly important; because oncological care is predominantly ambulatory in nature, outpatient palliative care facilitates early access and longitudinal monitoring throughout the disease trajectory.
Secondly, the interdisciplinary nature of palliative care enables the multidimensional care needs of patients to be addressed. The two nurse-led palliative-care RCTs had mixed findings,16,17 and two nonrandomized prospective studies designed to evaluate palliative care delivered by advanced-practice nurses also reported limited benefits of this approach.25, 26 By contrast, the findings of studies incorporating interdisciplinary involvement were more consistent.13, 14,15 Of note, however, the heterogeneity in study designs precludes any direct comparisons between the interdisciplinary versus unidisciplinary approaches; RCTs of these interventions are needed before we can draw any definitive conclusions.
Thirdly, when and who should be referred for early palliative care remains a topic of debate. In the four aforementioned RCTs, palliative-care referral was based on diagnosis and prognosis, instead of symptom burden. This method of referral is in contrast to that used in contemporary practice, in which most clinicians refer patients based on care needs. The timing of referral will be discussed further in a later section of this Review.
Fourthly, a survival benefit has been associated with early palliative care for patients with advanced-stage cancer.17 This result was, however, a secondary outcome, and not consistently observed in all studies. Could palliative care improve survival by optimizing symptom control; enhancing the patient’s eligibility for and tolerance of cancer treatments; maximizing psychosocial support; preventing premature death from overly aggressive cancer care; minimizing other aggressive interventions, such as intubation at the end of life; or a combination of these factors? Further studies are needed to address this question.
Fifthly, oncologists have an important role in delivering primary palliative care. Primary palliative care refers to the delivery of supportive care by health-care professionals who are not palliative-care specialists, such as oncologists and primary-care clinicians.27, 28 By contrast, secondary palliative care involves palliative-care specialists acting as consultants, and in tertiary palliative care, palliative-care specialists assume responsibility as the attending team for patients with complex issues—for example, in the acute palliative-care unit. Oncologists attend to patients in the front lines of care and are, therefore, essential in providing proper symptom assessments and prompt treatments. Integration of oncology and palliative care necessitates collaboration and communication regarding the roles and responsibilities between primary, secondary, and tertiary palliative care, such that the different teams can function in unison.
Sixthly, the role of general practitioners (or primary-care providers) in the provision of primary palliative care remains undefined. All of the four RCT we discussed were conducted in North America, with no defined primary-care involvement. In many countries and continents, however, family physicians are extremely important to the delivery of palliative care. This subject is an area of further research.29
Finally, access to secondary and tertiary palliative care remains limited. Palliative-care referral was permissible in the usual-care arms of the aforementioned RCTs, but few patients in these cohorts were referred to receive specialist palliative care.14–17 A study has revealed that even in well-integrated oncology–palliative-care programmes, only approximately 50% of patients receive palliative care before death.30 In multiple other studies, investigators have also demonstrated that palliative-care access is limited and often delayed.31, 32 Numerous barriers to early palliative-care referral have been recognized, including oncologist-related, patient-related, palliative-care-related, and system-related challenges.8, 33–35
Conceptual framework for cancer care
Examining the care needs of a typical cancer patient is important to understanding how oncology and palliative-care teams can better integrate to improve patient outcomes. The key requirements include cancer management, attending to the patients’ personal care needs, and addressing comorbidities (Figure 3a). Traditionally, the oncology team, palliative-care team, and primary care and other subspecialties work in silos (that is, in isolation). Integration of oncology and palliative care involves communication, collaboration, and sharing of resources and expertise among these teams to more-comprehensively address the care needs of patients.36
Figure 3.
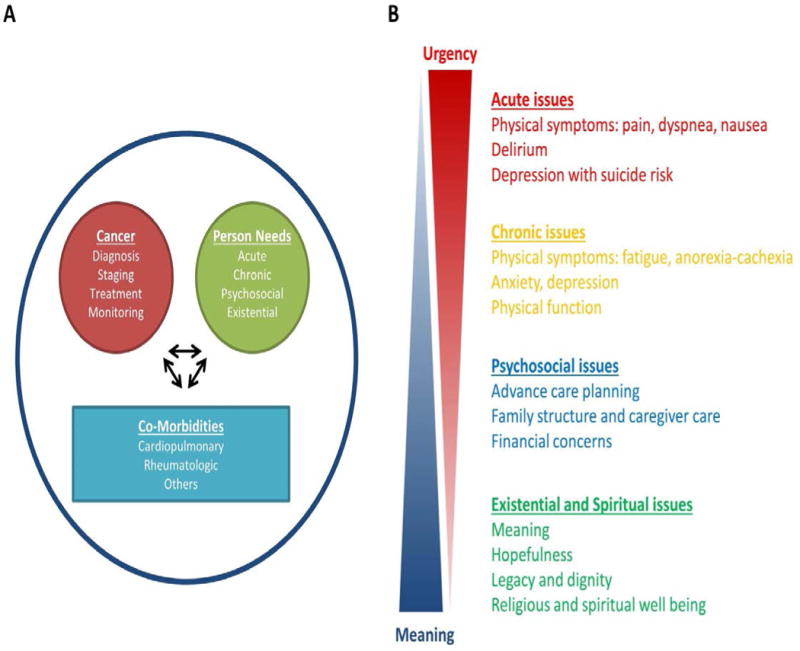
Care needs of patients with advanced-stage cancer. a the care needs of a patient with cancer can be classified under three domains: cancer management; symptom management and personal care needs; and the management of comorbidities. Considerable interactions exist between these domains and, therefore, interventions relating to one domain of care can influence the needs pertaining to another (arrows), which necessitates dynamic monitoring of the patient and modification of their care. For instance, chemotherapeutic agents can cause renal failure, which requires the initiation of different medical interventions and can also affect the ability of the patient to proceed with oncological investigations and treatments. Disease progression might affect the emotional state of the patient, which might, in turn, affect her adherence to treatment. To optimize patient outcomes, the oncology team, palliative-care team, primary-care team, and other subspecialists need to collaborate closely and communicate often. b Personal care needs can be further subdivided into acute issues, chronic issues, psychosocial issues, and existential and spiritual issues. Relevant expertise, close collaboration and interdisciplinary teamwork, and adequate resources are important requirements to comprehensively address these supportive-care issues, longitudinally.
Personal care needs can be subdivided into four categories: acute issues; chronic issues; psychosocial issues; and existential and/or spiritual issues (Figure 3b). This model provides some insights into the operation of the oncology–palliative-care interface. First, addressing the most-acute needs before dealing with the more-chronic and time-consuming issues is important. For instance, focusing on managing the acute-pain crisis in a patient before discussing their financial distress and spirituality is a logical approach. Once their pain is under control and trust has been built, the patient will be more prepared to discuss deeper emotional issues. Second, oncology and primary-care teams should provide as much supportive care as possible, and the palliative-care team can then build on their efforts to complete the spectrum of care. For instance, if a patient has already been treated for peripheral neuropathy and chemotherapy-induced nausea and vomiting by the oncology team, the palliative care team can focus on addressing fatigue, early satiety, anxiety, and discussions regarding advance care planning. Third, interdisciplinary palliative care teams are needed to appropriately address the multidimensional and complex psychosocial needs of patients and their caregivers. Finally, a single visit cannot fully address all of the dynamic care needs of a patient; thus, longitudinal follow-up consultations are needed in order to fully support the patient and their family over time.
Oncology teams, psychosocial teams, and palliative-care teams often address the complex personal needs of patients with advanced-stage cancer to variable extents, owing to their focus on different aspects of care (Figure 4). Oncologists have a critical role in providing supportive care in the front lines; however, expecting these professionals to fully address all the patient’s personal care needs is unrealistic because of time constraints, limited experience with or access to psychosocial resources, variable interest in this aspect of care, and the need for specialized training. Indeed, patients have been reported to prefer their oncologists to be optimistic about treatment options and to avoid pessimistic discussions about end-of-life care,37 suggesting the focus of oncologists on cancer treatment and physiological symptom relief, rather than psychosocial or existential issues, may be desired. Consistent with this observation, several studies have shown that patients with cancer often prefer to discuss advance care planning with a physician who they have never met, rather than their own clinical oncologist.38, 39 The type of health professional that is best suited to engage patients and families in end-of-life discussions remains to be defined and needs to be tailored to the needs of each individual. Psychosocial teams consist of professionals from different disciplines (for example, psychiatrists, psychologists, child-life counsellors, social workers, and chaplains), each with a unique focus for care. Because of the narrow focus of each discipline, patients who are exposed to only one psychosocial service might not have all their emotional, social, and spiritual needs addressed adequately. Patients with access to multiple psychosocial services might encounter some redundancy in their care. Palliative-care teams, because of their interdisciplinary nature and communication among team members, can address the patients’ personal needs efficiently and comprehensively.
Figure 4.
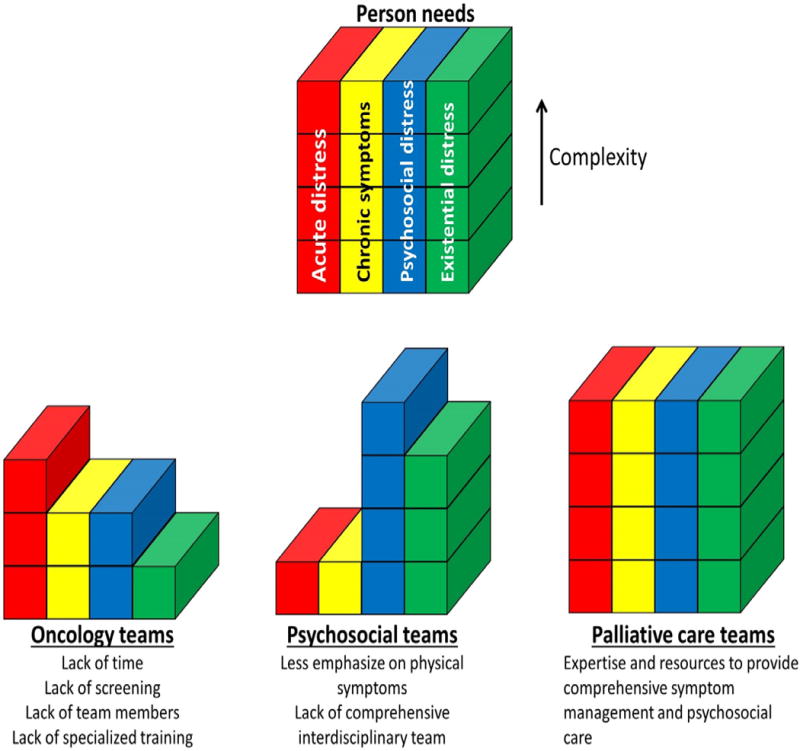
Management of personal care needs. The diagrams depict schematically how different health-care teams can address the personal needs of patients with cancer, including the acute issues, chronic issues, psychosocial issues, and existential issues. a Oncology teams have an important role providing primary palliative care; however, addressing all personal care needs comprehensively might be difficult for these teams, owing to time constraints, a lack of routine screening, unavailability of interdisciplinary team members, and limited advanced expertise in supportive-care issues. b Psychosocial teams often have a narrow focus for palliative care (for example, spirituality for chaplains, anxiety and depression for psychologists and psychiatrists, family and financial issues for social workers, child care for child-life specialists), and rarely address physical symptoms fully. c Palliative-care teams, because of their interdisciplinary nature and expertise, can comprehensively manage the personal care needs of patients longitudinally.
Structures to support integrated care
Outpatient clinics, inpatient consultation teams, palliative-care units, and home-based palliative-care services are all important branches of palliative care, each focusing on delivering care to patients along different stages of the disease trajectory, and in different settings (Table 1). As mentioned earlier, outpatient clinics are a hallmark of oncology–palliative-care integration because they provide patients with access to specialist palliative-care early in the disease course and can accommodate large numbers of patient visits over time.
Table 1.
Palliative care in different clinical settings
| Characteristic | Outpatient clinics | Inpatient consultation teams | General or acute palliative-care units | Home-based palliative-care services, home hospices, and inpatient hospices |
|---|---|---|---|---|
| Care setting | Ambulatory clinic | Inpatient | Inpatient | Community-based |
| ECOG performance status of patients | 0–3, sometimes 4 | 2–4 | 3–4 | 3–4 |
| Prognosis of patient population | Weeks to years | Days to months | Days to weeks | Days to weeks |
| Areas of focus for care | Symptom management Counselling End-of-life discussion Advance care planning |
Acute-symptom management Discharge planning End-of-life care |
Intensive symptom management Interdisciplinary psychosocial, spiritual and caregiver care Discharge planning End-of-life care |
Symptom management End-of-life care |
Abbreviation: ECOG, Eastern Cooperative Oncology Group
Currently, these palliative-care clinics are available in less than half of all palliative-care programmes.40, 41 Only a few groups, predominantly located in North America and Europe, have published their experience related to the processes and outcomes of such programmes.42–48 In a study published in 2013,49 investigators documented the practice of 20 palliative-care clinics in the USA, and identified wide variations in the availability, staffing, and patient volume of each clinic. These clinics provide patients with early pain and symptom management to prevent crisis,50 longitudinal psychosocial care to improve their wellbeing, repeated discussions to enhance their understanding of the disease,51 and advance care planning to minimize aggressive end-of-life care.22 Importantly, a single visit to even a specialist palliative-care unit has been shown to be inadequate to address the complex symptom burden in patients with advanced-stage cancer.52, 53 In a cohort study, investigators found that when patients were first referred to palliative care as outpatients they hadimproved outcomes at the end of life compared to those first referred as inpatients to the same interdisciplinary palliative-care team, such as a lower rate of in-hospital death (18% versus 34%; P = 0.001), and lower rates of intensive-care-unit admission (4% versus 14%; P = 0.001), prolonged hospitalization of 2 weeks or longer (8% versus 20%; P = 0.002), and emergency-room visits (48% versus 68%; P <0.001) in the last 30 days of life.18 Outpatient referrals occurred significantly earlier than inpatient referrals (median duration between referral and death 3.7 months versus 0.7 months, P <0.001), which allowed for more visits and opportunities for timely interventions.
Processes to support integrated care
Embedded versus stand-alone clinics
Embedding a palliative-care consultant or team geographically within the oncology clinic has the potential advantages of improving the volume and timeliness of referral and reinforcing communication between the oncology and palliative-care teams, while also maximizing convenience for patients. At the same time, the embedded approach mandates allotment of clinical space and adequate resources for a fully functional palliative-care team within the oncology clinic. In large cancer centres, in which each oncology clinic is organized by treatment modality and tumour site (for example, thoracic medical oncology or breast radiation oncology), the palliative-care service might be at risk of fragmentation, because comprehensive palliative care necessitates a sophisticated interdisciplinary team and adequate clinic space that are only available in standalone clinics, where the resources can be pooled. Furthermore, the principles and practice of palliative care, involving symptom management and psychosocial care, are common regardless of disease site; thus, tumour-site-specific approaches to palliative care might be unwarranted.54 In smaller general oncology clinics, the embedded clinic model of palliative care might not be cost-effective owing to low referrals. Only a limited number of studies have reported on the practices and related outcomes of embedded clinics,55, 56 therefore, current evidence is insufficient to determine if they are superior to nonembedded clinics in regard to palliative-care access, patient outcomes, and costs. Further research is needed to examine the true value of embedded clinics in various oncology settings.
Physician-driven versus patient-based referral
The questions of who, when, and how to refer patients to palliative care are complex, and the answers might depend on national and/or regional health-care policies, local resources, comprehensiveness of the palliative-care teams, patient characteristics, and the level of palliative care provided by oncology and/or primary-care teams. Currently, the two main approaches to specialist palliative-care referral are oncologist-driven referral, and automatic referral (Figure 5).
Figure 5.
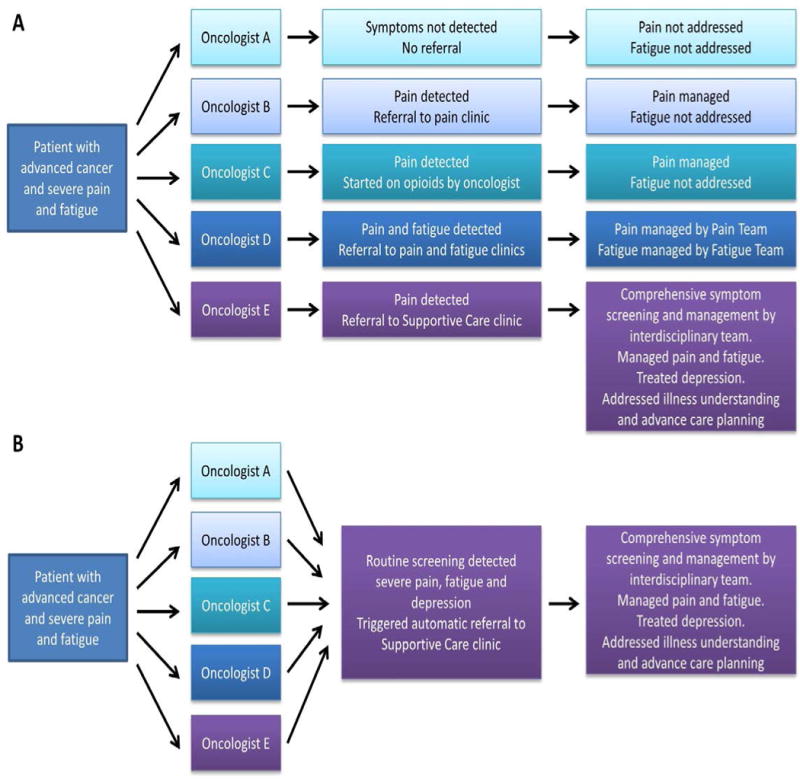
Palliative care referral patterns. a In an oncologist-based referral model, the patient might be directed to different services and, thus, receive variable care, depending on the palliative-care expertise and referral preferences of their oncologist, as well as local supportive care resource availability. b Automatic referral models streamline access to palliative care and help to standardized care through the use of routine screening and the implementation of automatic triggers for referral. The criteria for referral need to be tailored to achieve a balance between local palliative care resource availability and patient care needs.
At present, oncologist-driven referral of patients with advanced-stage cancer to palliative-care services is the norm, worldwide. This approach requires oncologists to identify patients who have specific symptoms and supportive-care needs, and to initiate a referral. Access to palliative care is currently inequitable, owing to variable detection of such patients and differential thresholds for referral,33, 57 and involvement of specialists in palliative care is often delayed (Table 2, Figure 5). Routine symptom screening in the oncology setting has the potential to increase awareness of supportive-care needs and enhance patient referral to specialist palliative care, although this remains to be confirmed.58, 59
Table 2.
Models for referral of patients to specialist palliative care
| Characteristic | Oncologist-driven referral | Automatic referral | |
|---|---|---|---|
| Universal | Selective | ||
| Mechanism |
|
|
|
| Referral criteria |
|
|
|
| Advantages |
|
|
|
| Disadvantages |
|
|
|
| Level of evidence | Retrospective and prospective case series64, 66–68 | ||
Abbreviation: RCT, randomized controlled trial.
The alternative approach, automatic referral, involves the use of predefined criteria relating to the patients’ diagnoses, prognoses, and/or needs to routinely trigger a palliative-care consultation. The four RCT discussed earlier all used diagnosis-based and/or prognosis-based criteria to trigger a palliative-care referral.14–17 Indeed, of the potential approaches to patient referral, this approach offers the greatest extent of palliative-care access and has the highest level of supportive evidence. This referral paradigm remains, however, more a vision than a reality, as no hospital has a large enough palliative-care team with adequate infrastructure and resources to provide care to most patients with cancer.
Automatic referral applying more-selective criteria, based on a combination of time from diagnosis, patient prognosis, and personal care needs might help to streamline referral practices. Importantly, these criteria need to be dynamic in order to tailor the patient volume to the available resources of the palliative-care team. In the clinical setting, institution of care pathways for palliative-care referral requires a consensus on appropriate institution-specific criteria, a process to routinely screen for patients who meet these criteria, a commitment to ensure adequate resources to support a sustainable programme that can provide timely longitudinal care, and the implementation of systems to provide improvements in quality. Carlson et al.60, 61 conducted a RCT to compare three types of distress screening: minimal screening, full screening, and full screening coupled with the option for patient referral to the psychosocial team (the ‘triage’ group). The primary end point of this trial, distress at 3 months, did not reach statistical significance; however, the triage group had improved distress scores and a greater number of referrals to the psychosocial team at follow up, compared with the minimal screening group.55, 56 Referral to the psychosocial team was in turn associated with greater reductions in anxiety and depression.55, 56 Findings of this study highlight the need to link screening with referral.
Several investigators have proposed automatic triggers for palliative-care referral (or clinical-care pathways).62, 63 Weissman et al.64 from the Center to Advance Palliative Care, a national member-based organization aimed at improving palliative care throughout the USA,65 proposed a consensus list of criteria for palliative-care assessment at the time of patient admission. Subsequently, researchers at the Mount Sinai Hospital, New York, NY, USA, conducted a pilot quality-improvement study to examine the implementation of a set of standardized criteria that partially overlapped with the CAPC criteria for patient referral to inpatient palliative-care consultation over a 3-month period.66 Specifically, patients were automatically referred to a palliative-care team if they met any of the following criteria: stage IV cancer; stage III lung or pancreatic cancer; prior hospitalization within 30 day (excluding routine chemotherapy); hospitalization for longer than 7 days; or uncontrolled symptoms, including pain, nausea, vomiting, dyspnoea, delirium, and psychosocial distress.66 In a before–after comparison, a significant increase in frequency of palliative-care referral (82% versus 41%; P <0.0001), a reduction in the 30-day readmission rate (17% versus 36%; P = 0.02), and a nonsignificant increase in hospice-utilization rate (25% versus 14%; P = 0.15) were reported for patients who met these criteria.66
The National Comprehensive Cancer Network (NCCN) has also proposed a list of consensus criteria for screening of patients care needs and subsequent referral of patients to receive palliative care.67 In a retrospective cohort study of patients admitted to the Gastrointestinal Oncology Service (GIOS) at a Memorial Sloan Kettering Cancer Center, New York, NY, USA, Glare et al.68 examined the use of six NCCN criteria to screen for palliative care needs and stratify patients for a palliative-care referral; automatic triggering of referral was based on the presence of any one of the 6 NCCN screening criteria. The automatic-trigger group was associated with greater number of consultations (47 among 113 patients versus 15 among 124 patients; P <0.0001), and a lower average baseline symptom scores among the patients who underwent consultations (1.6 versus 2.4; P = 0.005).68 The timing of consultation was also earlier in the automatic-trigger group (22 months versus 15 months after diagnosis), although this difference was not statistically significant.68
Glare et al.69 also developed an abbreviated screening tool for identifying patients with unmet palliative-care needs who would benefit from an outpatient consultation. The criteria for referral to an outpatient consultation include: the presence of metastatic or locally advanced cancer; decreased Eastern Cooperative Oncology Group (ECOG) performance status; the presence of one or more serious complication of advanced-stage cancer that is associated with a prognosis of less than 12 months; the presence of one or more serious comorbid diseases that are also associated with poor prognosis; and the presence of palliative-care problems such as uncontrolled symptoms. Using the final criteria list, the patient is assigned a score on a scale from 0 to 13, with a higher score indicating a greater palliative-care need. A score of 4 was found to have the best sensitivity and specificity for identifying the patients who met a ‘broad’ definition of the need for palliative care (metastatic or locally advanced cancer, a limited prognosis, or an active source of suffering).69 This tool was subsequently revised to include prolonged length of stay as an extra item (taking the total to 11 items and adding an extra point to the range of possible scores), and examined for its content, construct, and criteria validity.70 A cut-off of 5 out of 14 points was chosen as the optimal threshold for triggering referral to an inpatient palliative-care consultation, with a positive predictive value of 80% and negative predictive value of 44% using the NCCN referral criteria as a standard.70 Further research is needed to examine the optimal criteria for outpatient palliative-care referral tailored to the local health-care system and resource availability.
‘Supportive care’ versus ‘palliative care’
Palliative care is, by definition, supportive care that focuses on patients with advanced-stage diseases (Figure 1). Oncologists are the gatekeepers of palliative-care referral and, therefore, the prevailing misconception that ‘palliative care’ is associated with death and dying can be a barrier to palliative-care access.71 A survey revealed that the term ‘palliative care’ is more likely to be perceived as a barrier to referral by medical oncologists and mid-level health-care providers, decreases hope and causes distress in patients and families, and is interpreted as being synonymous with hospice care, compared to the term ‘supportive care’.72 As a result of these findings, the palliative-care programme at our institution changed its name to ‘supportive care’. In a before–after comparison, our group found that the number of referrals significantly increased (by 41%; P <0.001) and outpatients were referred earlier (median time from hospital registration to palliative-care consultation of 9.2 months versus 13.2 months; P <0.001) after this name change.73 A repeat survey found that both hematological and solid tumour specialists were markedly more receptive to the term ‘supportive care’ when considering early referral.74 In a survey of Canadian oncology-society members, the investigators reported that approximately one-third of oncologists indicated that they would be more likely to refer patients earlier if ‘palliative care’ was renamed ‘supportive care’.75 In addition, Maciasz et al.76 found that patients perceived the term ‘supportive care’ more favourably than ‘palliative care’. Certain aspects of supportive care, such as management of neutropenic risk, are predominantly under the direction of oncologists; however, many domains, such as management of fatigue and peripheral neuropathy, require close communication and collaboration between the oncology and palliative-care teams to deliver optimal supportive care.77
Combined patient care rounds
Combined tumour boards represent another opportunity to integrate palliative care and oncology, through enhanced communication. Traditionally, multidisciplinary tumour boards bring together medical, radiation, and surgical oncologists, as well as pathologists and radiologists, to discuss cancer treatment, with the aim of improving decision-making. Inclusion of a member of the palliative-care team in these meetings could potentially broaden the patient-care discussion, trigger palliative-care consultations, and open more options for disease management.78 Alternatively, routine involvement of oncologists in palliative care team meetings might also strengthen this partnership to enhance care.79,80 Combined tumour boards require a concerted effort from both oncology and palliative-care teams, however, and in larger centres with multiple tumour boards, it might not be possible to include a palliative-care specialist in all discussions.
Education to support integration
Education is one of the most important routes to enhancing integration of oncological and palliative care.81 Through an international Delphi survey completed in 2015,82 four major indicators of oncology–palliative-care integration related to education were identified: a didactic palliative-care curriculum for oncology fellows, provided by palliative-care teams; continuing medical education in palliative care for attending oncologists; combined palliative care and oncology educational activities for fellows and trainees; and a routine rotation in palliative care for oncology fellows.
Continuing medical education for oncologists
Similar to the scenario in other branches of oncology, the practice of supportive and/or palliative care is rapidly evolving, with new developments in symptom-management strategies, novel communication techniques, and shifting paradigms on palliative-care referral and topics related to end-of-life care. Survey studies have revealed that a vast majority of oncologists felt that they should be providing palliative care and that they should have a high level of proficiency in palliative care, but they had mixed opinions about their own training in this area.83, 84 Recognizing this knowledge gap, several organizations have developed continuing education curriculums for oncology professionals. We discuss some examples in the following paragraphs.
The Education in Palliative and End-of-life Care for Oncology (EPEC-Onocology®) project is a programme focused on oncologists, with educational information delivered online, via CD, or on paper.85 This educational programme has 15 modules (i.e. comprehensive assessment; cancer-related pain management; symptoms; loss, grief and bereavement; survivorship; last hours of living; communicating effectively; clarifying diagnosis and prognosis; negotiating goals of care; clinical trials; withdrawing nutrition and/or hydration; conflict resolution; advance care planning; physician-assisted suicide; cancer doctors and burnout) and 3 plenary sessions (i.e. gaps in oncology, models of comprehensive care, and charting the future).85 Its ‘train-the-trainer’ courses—whereby a small number of individuals receive formal training in the relevant topic, as well as how to teach others the information they learn—facilitate wide dissemination of the curriculum, with at least one of the modules reaching approximately 120,000 professionals in 2 years, via only 184 ‘Certified EPEC® Trainers’.86 A process evaluation of the programme among 195 participants suggested that the EPEC® modules were clinically relevant and that the material presented was clear and easy to understand, and most participants felt that their practice behaviour would probably change as a result of having attended the course.87, 88
The End-of-Life-Nursing Education Curriculum (ELNEC) programme was developed by nurses for nurses, and is administered by the American Association of Colleges of Nursing (AACN).89 This curriculum consists of eight modules: nursing care at the end of life; pain management; symptom management; ethical and legal issues; cultural considerations in end-of-life care; communication; loss, grief, and bereavement; and preparation for and care at the time of death.83, 84 An oncology-specific programme is also available. Similar to EPEC, train-the-trainer courses are available.90, 91 The ELNEC curriculum has been translated and implemented in over 70 countries, with approximately 20,000 nurses and other health care professionals participating in the courses.89
The Palliative care Emphasis program on symptom management and Assessment for Continuous medical Education (PEACE) is a Japanese programme in which nine training modules are delivered in-person over 2-days.92 Over 37,000 physicians have completed the programme.93 In a before–after comparison, investigators noted statistically significant improvements in palliative-care knowledge (measured using the palliative-care knowledge questionnaire for PEACE [PEACE-Q] at baseline, then immediately after training, and again 2 months later; P <0.0001) and self-reported competencies in palliative-care delivery (according to the Palliative Care self-reported Practice Scale [PCPS] and the Palliative Care Difficulties Scale [PCDS]; P <0.0001 by both measures) among 85 physicians who participated in the programme.94
Other educational curricula in palliative care include the Pallium Canada’s Learning Essential Approaches to Palliative and End-of-Life Care (LEAP) 95 and the Virtual Learning Collaborative (VLC). LEAP is a Canadian programme that consists of 16 online training modules on various palliative care topics over 2 days. A one-day course focusing on oncology specific issues (LEAP Mini Onco) is available.97 At the time of writing, the VLC, a web-based education module, is under development by ASCO and American Academy of Hospice and Palliative Medicine (AAHPM).
Palliative-oncology conferences, such as the ASCO Palliative Care Symposium98 and Multinational Society for Supportive Care in Cancer (MASCC)99, provide further opportunities to learn about the contemporary approaches to patient care. Some institutions also regularly hold palliative-care workshops and review courses, such as the MD Anderson Cancer Center Updates in Hospice and Palliative Medicine and Intensive Board Review100 and various training opportunities through the Harvard Medical School Center for Palliative Care 101. The International Association for Hospice and Palliative Care (IAHPC) maintains a list of educational programmes that provide certificates and diplomas in palliative care from different organizations worldwide.102 In Japan, Morita et al.103 developed a regional educational programme for clinicians, to improve communication, collaboration, and palliative-care referral. Finally, organizations, such as the NCCN and European Society for Medical Oncology (ESMO), have published clinical-practice guidelines on various issues related to palliative care.104–107
Palliative-care rotation for oncology fellows
The second edition of the Global Core Curriculum in Medical Oncology, which was published in 2010, is endorsed by both ASCO and ESMO, and outlines specific competencies for oncologists related to supportive and palliative care (Table 3).108, 109 In the aforementioned Delphi survey,82 international experts reached a consensus that oncologists should ideally undertake a palliative-care rotation of at least 1 month in duration. Currently, approximately 25% of oncology fellows in the USA participate in a palliative-care rotation.8, 110 A clinical rotation is essential for integration of these disciplines because it could help oncology fellows to acquire knowledge of the basic principles of symptom management and communication, understand when referral is appropriate, and build a working relationship with the palliative-care team. A rotation could also help to de-stigmatize palliative care, and might result in increased interest among oncologists for subspecialization in palliative oncology.
Table 3.
Supportive-care and palliative-care competencies* stipulated in the Global Core Curriculum in Medical Oncology93
| Complications of treatment | Palliative care and end-of-life care | Supportive measures |
|---|---|---|
|
|
|
Key challenges to address include standardizing the educational elements of the palliative-care rotation, ensuring that the members of the interdisciplinary palliative-care team have adequate time for teaching, and defining the optimal timeframe for the rotation. Furthermore, commitment from National Accrediting Agencies is needed to mandate palliative-care rotations for oncology fellows. In addition, the relationship between such training and palliative-care referral needs to be defined: on the one hand, increased familiarity with the palliative-care service might promote referral; on the other hand, oncologists who are highly confident with the delivery of palliative care might be less likely to refer patients to units that specialize in this discipline.33 Further studies are required to examine this possible paradox.
Oncology training for palliative-care fellows
Integration of oncology and palliative care is a two-way street. Similar to the way in which patient care might be improved by educating oncologists in palliative care, a rotation in medical and/or radiation oncology for palliative-care fellows, to increase their familiarity with the natural history of cancer, cancer-treatment modalities, and the complex decision-making process surrounding cancer treatment at the end of life, could be of benefit. Such rotations might also help palliative-care specialists and oncologists nurture a mutual understanding, to strengthen their partnerships. In the USA, palliative-care fellowships last only 1 year, which makes inclusion of a mandatory oncology rotation challenging.
Accreditation of palliative care
Accreditation is crucial for the sustainability of palliative care as a profession because it enables patients to be reassured of the standard of care they receive, prospective trainees to be attracted to this new discipline, practicing clinicians to be recognized for their expertise, educators to standardize the fellowship programmes, researchers to evaluate programme outcomes, and administrators and policy makers to allocate resources.111 Palliative medicine achieved specialty/subspecialty status in the UK in 1987, the USA in 2006, Canada in 2016.112, 113 This discipline has also been established as a speciality in multiple countries in the Asia-Pacific region, including Australia, New Zealand, Hong Kong, Japan, Taiwan, Malaysia, Singapore and India.114 Many other countries in Europe, Asia, the Middle East, South America and Africa are actively working toward accreditation.
Dually trained palliative oncologists
‘Palliative oncologists’, who are dually trained in both palliative care and oncology, can further foster communication, collaboration, and ‘cross-pollination’ between the two teams to further catalyse the process of integration.115 Of note, individuals boarded in palliative care might be attractive candidates for oncology training programmes because of their unique specialist experience in an important aspect of oncology. In addition to clinical work in both specialties, palliative oncologists can take an active role in educating oncologists about palliative care, teaching palliative-care teams about oncology, conducting palliative-oncology research, and advocating for increased allocation of resources and greater awareness. For these reasons, many larger comprehensive cancer centres in the USA now include at least one palliative oncologist.
Research to support integration
In the era of evidence-based medicine, results of well-conducted studies designed to support palliative care can have an important effect on clinical practice. For instance, the RCT by Temel and co-workers14 that evaluated early integration of palliative care in oncology captured the attention of oncologists and palliative-care specialists worldwide, and contributed to a shifting paradigm of care; since this trial was published in 2010, the number of publications related to such integration has grown dramatically.12
Despite the expansion in palliative-care research, much room for improvement remains. A sample of the palliative-oncology literature revealed concerns regarding both the quantity and quality of studies.116 For instance, 51% of original articles in palliative oncology were found to be case reports or case series.116 RCTs comprised 6% of the studies identified, and the methodological scores for these trials were generally low.117 Approximately 97% of the palliative-care literature is derived from developed countries, with North American and Europe contributing 41% and 39%, respectively.116 Inadequate funding and a lack of trained researchers are two major barriers to palliative-care research.118, 119
To further promote integration of oncology and palliative care, researchers need to conduct adequately powered, well-designed clinical studies that address fundamental questions related to integration. Such studies could examine the effects of palliative care interventions on clinically meaningful outcomes, such as symptom management, psychosocial and spiritual care, patient–clinician communication, and decision-making needs at different stages of disease trajectory (for example, at diagnosis, during cancer treatments, and in last months and days of life). Collaboration with oncologists would be helpful to ensure the research questions are relevant to their practice.
Conclusions
In only half a century, palliative care has evolved from a philosophy of care to a professional discipline of medicine in many countries, with a defined body of supporting knowledge. The question is no longer whether palliative care should be provided to patients with cancer, but when and how it should be delivered to optimize patient outcomes. As highlighted in this Review, concerted efforts to grow the clinical infrastructure, optimize the clinical processes, standardize the education curricula, and conduct ground-breaking research are needed to promote integration of oncological and palliative care.
Exactly how integration should occur needs to be tailored to the characteristics of the health-care system, to the hospital setting, and to the local resource availability. A Delphi study of international experts identified 13 major and 30 minor indicators of integration of specialty palliative care and oncology programmes in hospitals with ≥100 beds (Figure 2),82 which might be used to identify centres with a high level of integration, and facilitate benchmarking, quality improvement and research. The model for integration of palliative care and oncology in small community hospitals might differ dramatically compared with large academic cancer treatment centres. Furthermore, the interface between primary and secondary and/or tertiary palliative care remains to be defined. Oncologists, palliative-care specialists, educators, researchers, hospital administrators, funding bodies, accreditation agencies, professional organizations, and governments all have a role in this important movement to optimize patient care.
Key points.
Patient referral to specialist palliative care is associated with improved quality of life, symptom control, patient and caregiver satisfaction, end-of-life care, costs of care, and, potentially, survival
Oncologists and general practitioners have an important role in the delivery of primary palliative care, and in facilitating timely referral of patients to specialist palliative care teams
The approach to integration of oncological and palliative care needs to be tailored to the demands of the individual health-care system and hospital setting, according to the resources available locally
Outpatient palliative-care clinics are a hallmark of integration, providing patients with access to specialist palliative care early in the disease trajectory and can accommodate multiple repeat visits over time
Routine symptom screening with automatic referral criteria, combined (multidisciplinary) patient-care rounds, a name change for palliative care to ‘supportive care’, and embedded oncology–palliative-care clinics represent potential strategies to encourage integration of care
Education initiatives include a mandatory palliative-care rotation for oncology fellows, combined palliative care and oncology educational activities for trainees, and continuing medical education in palliative care for practicing oncologists
Acknowledgments
The work of D.H. is supported in part by a Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-148-01–CCE) from the American Cancer Society, and a National Institutes of Health grant (R21CA186000-01A1). The work of E.B. is supported in part by National Institutes of Health grants R01NR010162-01A1, R01CA122292-01, and R01CA124481-01.
Biographies
David Hui, MD, is an Assistant Professor in the Department of Palliative Care and Rehabilitation Medicine, with a joint appointment to the Department of General Oncology, at The University of Texas MD Anderson Cancer Center, Houston, TX, USA. He completed his medical oncology training at the British Columbia Cancer Agency, BC, Canada, and did a palliative oncology fellowship at the University of Texas MD Anderson Cancer Center. His research interests include integration of supportive/palliative care into oncology practice, clinical trials investigating interventions for symptom control, and prognostication. Under the mentorship of Dr Eduardo Bruera, he has authored or coauthored more than 150 articles in peer-review journals.
Eduardo Bruera, MD, MSc, received his medical degree in 1979 and trained in medical oncology in Argentina. In 1984, he joined the Cross Cancer Institute and the University of Alberta in Edmonton, AB, Canada, where he helped develop the regional palliative-care programme. In 1999, he relocated to the University of Texas MD Anderson Cancer Center, where he currently holds the F.T. McGraw Chair in the Treatment of Cancer, and is Chair of the Department of Palliative Care and Rehabilitation Medicine. His research interests include cancer pain, anorexia–cachexia, fatigue, delirium, communication, decision-making, and outcomes research. He has a strong interest in the global development of palliative care, and has collaborated for many years with the World Health Organization. In addition to having published more than 800 articles, he has trained hundreds of physicians who are currently practicing palliative care around the world.
Footnotes
Author contributions
Both authors researched data for the article, contributed to discussion of content, and wrote and reviewed/edited the manuscript before submission.
Competing interests
The authors declare no competing interests.
References
- 1.Saunders C. In: The Management of Terminal Malignant Disease. Saunders C, Sykes N, editors. Hodder and Stoughton; London, Great Britain: 1993. pp. 1–14. [Google Scholar]
- 2.Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. CMAJ. 1976;115:119–121. [PMC free article] [PubMed] [Google Scholar]
- 3.Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372–9. doi: 10.1097/CCO.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui D, et al. Concepts and definitions for “supportive care,” “best supportive care,” “palliative care,” and “hospice care” in the published literature, dictionaries, and textbooks. Supportive Care in Cancer. 2013;21:659–685. doi: 10.1007/s00520-012-1564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch T, Connor S, Clark D. Mapping levels of palliative care development: a global update. J Pain Symptom Manage. 2013;45:1094–106. doi: 10.1016/j.jpainsymman.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Pastrana T, Eisenchlas J, Centeno C, De Lima L. Status of palliative care in Latin America: looking through the Latin America Atlas of Palliative Care. Curr Opin Support Palliat Care. 2013;7:411–6. doi: 10.1097/SPC.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 7.Centeno C, Lynch T, Donea O, Rocafort J, Clark D. EAPC Atlas of Palliative Care in Europe 2013 - Full Edition. European Association for Palliative Care; Milano: 2013. http://dadun.unav.edu/handle/10171/29291?locale=en Last accessed: 2/12/2015. [Google Scholar]
- 8.Hui D, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–61. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hearn J, Higginson IJ. Do specialist palliative care teams improve outcomes for cancer patients? A systematic literature review. Palliative Medicine. 1998;12:317–332. doi: 10.1191/026921698676226729. [DOI] [PubMed] [Google Scholar]
- 10.Higginson IJ, et al. Do hospital-based palliative teams improve care for patients or families at the end of life? J Pain Symptom Manage. 2002;23:96–106. doi: 10.1016/s0885-3924(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA. 2008;299:1698–709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]
- 12.Hui D, et al. Integration of Oncology and Palliative Care: A Systematic Review. Oncologist. 2015;20:77–83. doi: 10.1634/theoncologist.2014-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temel JS, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. Journal of Clinical Oncology. 2011;29:2319–26. doi: 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 14.Temel JS, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New England Journal of Medicine. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann C, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–30. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 16.Bakitas M, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–9. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakitas M, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol. 2015;33:1438–45. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui D, et al. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer. 2014;120:1743–9. doi: 10.1002/cncr.28628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higginson IJ, et al. Is there evidence that palliative care teams alter end-of-life experiences of patients and their caregivers? J Pain Symptom Manage. 2003;25:150–168. doi: 10.1016/s0885-3924(02)00599-7. [DOI] [PubMed] [Google Scholar]
- 20.Higginson IJ, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med. 2014;2:979–87. doi: 10.1016/S2213-2600(14)70226-7. [DOI] [PubMed] [Google Scholar]
- 21.Dudgeon DJ, et al. Palliative Care Integration Project (PCIP) quality improvement strategy evaluation. J Pain Symptom Manage. 2008;35:573–582. doi: 10.1016/j.jpainsymman.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright AA, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. Journal of Clinical Oncology. 2010;28:1203–8. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison RS, et al. Cost savings associated with US hospital palliative care consultation programs. Archives of Internal Medicine. 2008;168:1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 25.Dyar S, Lesperance M, Shannon R, Sloan J, Colon-Otero G. A nurse practitioner directed intervention improves the quality of life of patients with metastatic cancer: Results of a randomized pilot study. Journal of Palliative Medicine. 2012;15:890–895. doi: 10.1089/jpm.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prince-Paul M, Burant CJ, Saltzman JN, Teston LJ, Matthews CR. The effects of integrating an advanced practice palliative care nurse in a community oncology center: A pilot study. Journal of Supportive Oncology. 2010;8:21–27. [PubMed] [Google Scholar]
- 27.Payne R. The integration of palliative care and oncology: the evidence. Oncology (Williston Park) 2011;25:1266. [PubMed] [Google Scholar]
- 28.Rangachari D, Smith TJ. Integrating palliative care in oncology: The oncologist as a primary palliative care provider. Cancer Journal (United States) 2013;19:373–378. doi: 10.1097/PPO.0b013e3182a76b9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKenzie MA. The interface of palliative care, oncology and family practice: a view from a family practitioner. CMAJ. 1998;158:1705–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Hui D, et al. Access to palliative care among patients treated at a comprehensive cancer center. Oncologist. 2012;17:1574–80. doi: 10.1634/theoncologist.2012-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beccaro M, Costantini M, Merlo DF, Group, I.S Inequity in the provision of and access to palliative care for cancer patients. Results from the Italian survey of the dying of cancer (ISDOC) BMC Public Health. 2007;7:66. doi: 10.1186/1471-2458-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita T, Miyashita M, Tsuneto S, Sato K, Shima Y. Late referrals to palliative care units in Japan: nationwide follow-up survey and effects of palliative care team involvement after the Cancer Control Act. J Pain Symptom Manage. 2009;38:191–196. doi: 10.1016/j.jpainsymman.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Schenker Y, et al. Oncologist Factors That Influence Referrals to Subspecialty Palliative Care Clinics. J Oncol Pract. 2013;10:e37–44. doi: 10.1200/JOP.2013.001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis MP, Bruera E, Morganstern D. Early integration of palliative and supportive care in the cancer continuum: challenges and opportunities. Am Soc Clin Oncol Educ Book. 2013:144–50. doi: 10.14694/EdBook_AM.2013.33.144. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez KL, Barnato AE, Arnold RM. Perceptions and utilization of palliative care services in acute care hospitals. J Palliat Med. 2007;10:99–110. doi: 10.1089/jpm.2006.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruera E, Hui D. Integrating supportive and palliative care in the trajectory of cancer: Establishing goals and models of care. Journal of Clinical Oncology. 2010;28:4013–4017. doi: 10.1200/JCO.2010.29.5618. [DOI] [PubMed] [Google Scholar]
- 37.Tanco K, et al. Patient Perception of Physician Compassion After a More Optimistic vs a Less Optimistic Message: A Randomized Clinical Trial. JAMA Oncol. 2015;1:176–83. doi: 10.1001/jamaoncol.2014.297. [DOI] [PubMed] [Google Scholar]
- 38.Dow LA, et al. Paradoxes in advance care planning: the complex relationship of oncology patients, their physicians, and advance medical directives. J Clin Oncol. 2010;28:299–304. doi: 10.1200/JCO.2009.24.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamont EB, Siegler M. Paradoxes in cancer patients’ advance care planning. J Palliat Med. 2000;3:27–35. doi: 10.1089/jpm.2000.3.27. [DOI] [PubMed] [Google Scholar]
- 40.Davis MP, Strasser F, Cherny N. How well is palliative care integrated into cancer care? A MASCC, ESMO, and EAPC Project. Support Care Cancer. 2015;23:2677–85. doi: 10.1007/s00520-015-2630-z. [DOI] [PubMed] [Google Scholar]
- 41.Davis MP, Strasser F, Cherny N, Levan N. MASCC/ESMO/EAPC survey of palliative programs. Support Care Cancer. 2014;23:1951–68. doi: 10.1007/s00520-014-2543-2. [DOI] [PubMed] [Google Scholar]
- 42.Kloke M, Scheidt H. Pain and symptom control for cancer patients at the University Hospital in Essen: integration of specialists’ knowledge into routine work. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 1996;4:404–407. doi: 10.1007/BF01880636. [DOI] [PubMed] [Google Scholar]
- 43.Strasser F, et al. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27:481–491. doi: 10.1016/j.jpainsymman.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Follwell M, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol. 2009;27:206–13. doi: 10.1200/JCO.2008.17.7568. [DOI] [PubMed] [Google Scholar]
- 45.Bruera E, Hui D. Conceptual models for integrating palliative care at cancer centers. Journal of Palliative Medicine. 2012;15:1261–1269. doi: 10.1089/jpm.2012.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaertner J, et al. Implementing WHO recommendations for palliative care into routine lung cancer therapy: A feasibility project. Journal of Palliative Medicine. 2010;13:727–732. doi: 10.1089/jpm.2009.0399. [DOI] [PubMed] [Google Scholar]
- 47.Hydeman J. Improving the integration of palliative care in a comprehensive oncology center: Increasing primary care referrals to palliative care. Omega (United States) 2013;67:127–134. doi: 10.2190/OM.67.1-2.o. [DOI] [PubMed] [Google Scholar]
- 48.Shamieh O, Hui D. A comprehensive palliative care program at a tertiary cancer center in jordan. Am J Hosp Palliat Care. 2015;32:238–42. doi: 10.1177/1049909113513316. [DOI] [PubMed] [Google Scholar]
- 49.Smith AK, et al. The diverse landscape of palliative care clinics. J Palliat Med. 2013;16:661–8. doi: 10.1089/jpm.2012.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yennurajalingam S, et al. Clinical response to an outpatient palliative care consultation in patients with advanced cancer and cancer pain. Journal of Pain and Symptom Management. 2012;44:340–50. doi: 10.1016/j.jpainsymman.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Yoong J, et al. Early palliative care in advanced lung cancer: A qualitative study. JAMA Internal Medicine. 2013;173:283–290. doi: 10.1001/jamainternmed.2013.1874. [DOI] [PubMed] [Google Scholar]
- 52.Kang JH, Kwon JH, Hui D, Yennurajalingam S, Bruera E. Changes in Symptom Intensity Among Cancer Patients Receiving Outpatient Palliative Care. Journal of Pain and Symptom Management. 2013;46:652–60. doi: 10.1016/j.jpainsymman.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Hui D, et al. Minimal Clinically Important Differences in the Edmonton Symptom Assessment Scale in Cancer Patients: A Prospective Study. Cancer. 2015;121:3027–35. doi: 10.1002/cncr.29437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arthur J, Bruera E. In: Palliative Care in Oncology. Alt-Epping B, Nauck F, editors. Springer; Germany: 2015. pp. 1–11. [Google Scholar]
- 55.Muir JC, et al. Integrating palliative care into the outpatient, private practice oncology setting. Journal of Pain and Symptom Management. 2010;40:126–135. doi: 10.1016/j.jpainsymman.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Johnston B, Buchanan D, Papadopoulou C, Sandeman G, Lord H. Integrating palliative care in lung cancer: an early feasibility study. International Journal of Palliative Nursing. 2013;19:433–7. doi: 10.12968/ijpn.2013.19.9.433. [DOI] [PubMed] [Google Scholar]
- 57.Okuyama T, et al. Oncologists’ recognition of supportive care needs and symptoms of their patients in a breast cancer outpatient consultation. Jpn J Clin Oncol. 2011;41:1251–8. doi: 10.1093/jjco/hyr146. [DOI] [PubMed] [Google Scholar]
- 58.Lee SJ, Katona LJ, De Bono SE, Lewis KL. Routine screening for psychological distress on an Australian inpatient haematology and oncology ward: impact on use of psychosocial services. Med J Aust. 2010;193:S74–8. doi: 10.5694/j.1326-5377.2010.tb03933.x. [DOI] [PubMed] [Google Scholar]
- 59.Wagner LI, et al. Bringing PROMIS to practice: brief and precise symptom screening in ambulatory cancer care. Cancer. 2015;121:927–34. doi: 10.1002/cncr.29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carlson LE, Groff SL, Maciejewski O, Bultz BD. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. J Clin Oncol. 2010;28:4884–91. doi: 10.1200/JCO.2009.27.3698. [DOI] [PubMed] [Google Scholar]
- 61.Palmer SC, van Scheppingen C, Coyne JC. Clinical trial did not demonstrate benefits of screening patients with cancer for distress. J Clin Oncol. 29:e277–8. doi: 10.1200/JCO.2010.34.1206. author reply e279–80 (2011) [DOI] [PubMed] [Google Scholar]
- 62.Khatcheressian J, et al. Improving palliative and supportive care in cancer patients. Oncology (Williston Park, N.Y) 2005;19:1365–1376. discussion 1377–1378, 1381–1382, 1384 passim. [PubMed] [Google Scholar]
- 63.Gaertner J, et al. Specifying WHO recommendation: moving toward disease-specific guidelines. Journal of Palliative Medicine. 2010;13:1273–1276. doi: 10.1089/jpm.2010.0016. [DOI] [PubMed] [Google Scholar]
- 64.Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. Journal of Palliative Medicine. 2011;14:17–23. doi: 10.1089/jpm.2010.0347. [DOI] [PubMed] [Google Scholar]
- 65.Center to Advance Palliative Care. Center to Advance Palliative Care; New York: 2015. https://www.capc.org/Last Last accessed: October 6, 2015. [Google Scholar]
- 66.Adelson K, Paris J, Smith CB, Horton J, Morrison SR. Standardized criteria for required palliative care consultation on the solid tumor oncology service. American Society of Clinical Oncology Quality Care Symposium. 2013 Abstact 37 (J Clin Oncol, San Diego) [Google Scholar]
- 67.Levy MH, et al. Palliative care. J Natl Compr Canc Netw. 2012;10:1284–309. doi: 10.6004/jnccn.2012.0132. [DOI] [PubMed] [Google Scholar]
- 68.Glare P, et al. Study using the NCCN guidelines for palliative care to screen patients for palliative care needs and referral to palliative care specialists. J Natl Compr Canc Netw. 2013;11:1087–96. doi: 10.6004/jnccn.2013.0130. [DOI] [PubMed] [Google Scholar]
- 69.Glare PA, Semple D, Stabler SM, Saltz LB. Palliative care in the outpatient oncology setting: evaluation of a practical set of referral criteria. J Oncol Pract. 2011;7:366–70. doi: 10.1200/JOP.2011.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glare PA, Chow K. Validation of a Simple Screening Tool for Identifying Unmet Palliative Care Needs in Patients With Cancer. J Oncol Pract. 2015;11:e81–86. doi: 10.1200/JOP.2014.001487. [DOI] [PubMed] [Google Scholar]
- 71.Cherny NI. Stigma associated with “palliative care”: getting around it or getting over it. Cancer. 2009;115:1808–12. doi: 10.1002/cncr.24212. [DOI] [PubMed] [Google Scholar]
- 72.Fadul N, et al. Supportive versus palliative care: what’s in a name?: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer. 2009;115:2013–2021. doi: 10.1002/cncr.24206. [DOI] [PubMed] [Google Scholar]
- 73.Dalal S, et al. Association between a name change from palliative to supportive care and the timing of patient referrals at a comprehensive cancer center. The Oncologist. 2011;16:105–11. doi: 10.1634/theoncologist.2010-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hui D, et al. Attitudes and Beliefs toward Supportive and Palliative Care Referral among Hematologic and Solid Tumor Oncology Specialists. Oncologist. 2015 doi: 10.1634/theoncologist.2015-0240. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wentlandt K, et al. Referral practices of oncologists to specialized palliative care. J Clin Oncol. 2012;30:4380–6. doi: 10.1200/JCO.2012.44.0248. [DOI] [PubMed] [Google Scholar]
- 76.Maciasz RM, et al. Does it matter what you call it? A randomized trial of language used to describe palliative care services. Support Care Cancer. 2013;21:3411–9. doi: 10.1007/s00520-013-1919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aapro MS. Supportive care and palliative care: a time for unity in diversity. Ann Oncol. 2012;23:1932–4. doi: 10.1093/annonc/mds239. [DOI] [PubMed] [Google Scholar]
- 78.Vergo MT, Cullinan AM. Joining together to improve outcomes: Integrating specialty palliative care into the care of patients with cancer. JNCCN Journal of the National Comprehensive Cancer Network. 2013;11:S38–S46. doi: 10.6004/jnccn.2013.0220. [DOI] [PubMed] [Google Scholar]
- 79.Colombet I, et al. Effect of integrated palliative care on the quality of end-of-life care: retrospective analysis of 521 cancer patients. BMJ Support Palliat Care. 2012;2:239–47. doi: 10.1136/bmjspcare-2011-000157. [DOI] [PubMed] [Google Scholar]
- 80.Porzio G, et al. Integrating oncology and palliative home care in Italy: the experience of the “L’Aquila per la Vita” Home Care Unit. Tumori. 2013;99:225–228. doi: 10.1177/030089161309900217. [DOI] [PubMed] [Google Scholar]
- 81.Abrahm JL. Integrating palliative care into comprehensive cancer care. JNCCN Journal of the National Comprehensive Cancer Network. 2012;10:1192–1198. doi: 10.6004/jnccn.2012.0126. [DOI] [PubMed] [Google Scholar]
- 82.Hui D, et al. Indicators of Integration of Oncology and Palliative Care Programs: An International Consensus. Annals of Oncology. 2015;26:1953–9. doi: 10.1093/annonc/mdv269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cherny NI, Catane R, European Society of Medical Oncology Taskforce on, P. & Supportive, C Attitudes of medical oncologists toward palliative care for patients with advanced and incurable cancer: report on a survery by the European Society of Medical Oncology Taskforce on Palliative and Supportive Care. Cancer. 2003;98:2502–2510. doi: 10.1002/cncr.11815. [DOI] [PubMed] [Google Scholar]
- 84.Ferris FD, et al. Palliative cancer care a decade later: accomplishments, the need, next steps. Journal of Clinical Oncology. 2009;27:3052–3058. doi: 10.1200/JCO.2008.20.1558. [DOI] [PubMed] [Google Scholar]
- 85.Education in Palliative and End-of-life Care. Northwestern University Feinberg School of Medicine; Chicago, Illinois: 2015. http://www.epec.net/ Last accessed: October 6, 2015. [Google Scholar]
- 86.Robinson K, et al. Assessment of the Education for Physicians on End-of-Life Care (EPEC) Project. J Palliat Med. 2004;7:637–45. doi: 10.1089/jpm.2004.7.637. [DOI] [PubMed] [Google Scholar]
- 87.Cole BE. An EPEC weekend. Am J Hosp Palliat Care. 1999;16:467–70. doi: 10.1177/104990919901600209. [DOI] [PubMed] [Google Scholar]
- 88.VanGeest JB. Process evaluation of an educational intervention to improve end-of-life care: the Education for Physicians on End-of-Life Care (EPEC) program. Am J Hosp Palliat Care. 2001;18:233–8. doi: 10.1177/104990910101800407. [DOI] [PubMed] [Google Scholar]
- 89.ELNEC Fact Sheet. American Association of Colleges of Nursing; Washington, DC: 2015. http://www.aacn.nche.edu/elnec/about/fact-sheet Last accessed: October 6, 2015. [Google Scholar]
- 90.Coyne P, et al. Oncology End-of-Life Nursing Education Consortium training program: improving palliative care in cancer. Oncol Nurs Forum. 2007;34:801–7. doi: 10.1188/07.ONF.801-807. [DOI] [PubMed] [Google Scholar]
- 91.Ferrell B, Malloy P, Virani R. The end of life nursing education nursing consortium project. Ann Palliat Med. 2015;4:61–9. doi: 10.3978/j.issn.2224-5820.2015.04.05. [DOI] [PubMed] [Google Scholar]
- 92.Tsuneto S. Past, present, and future of palliative care in Japan. Jpn J Clin Oncol. 2013;43:17–21. doi: 10.1093/jjco/hys188. [DOI] [PubMed] [Google Scholar]
- 93.Palliative care Emphasis program on symptom management and Assessment for Continuous medical Education (PEACE) Japanese Society for Palliative Medicine; Osaka, Japan: 2015. http://www.jspm-peace.jp/ Last accessed: October 6, 2015. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto R, et al. Outcome evaluation of the Palliative care Emphasis program on symptom management and Assessment for Continuous Medical Education: nationwide physician education project for primary palliative care in Japan. J Palliat Med. 2015;18:45–9. doi: 10.1089/jpm.2014.0122. [DOI] [PubMed] [Google Scholar]
- 95.LEAP – Learning Essential Approaches to Palliative and End-of-Life Care Courseware. Pallium Canada; Ottawa, Ontario, Canada: 2015. http://fhs.mcmaster.ca/palliativecare/leap.html Last accessed: October 6, 2015. [Google Scholar]
- 96.ASCO Virtual Learning Collaborative. ASCO Institute for Quality; Alexandria, VA, USA: 2015. http://www.instituteforquality.org/asco-virtual-learning-collaborative Last accessed: October 6, 2015. [Google Scholar]
- 97.LEAP Mini Onco. Pallium Canada; Ottawa, Ontario, Canada: 2015. http://pallium.ca/professional-development/leap-2/leap-mini-onco/ Last accessed: October 6, 2015. [Google Scholar]
- 98.Palliative Care in Oncology Symposium. Conquer Cancer Foundation of the American Society of Clinical Oncology; Alexandria, VA, USA: 2015. http://pallonc.org/ Last accessed: October 6, 2015. [Google Scholar]
- 99.Multinational Association of Supportive Care in Cancer. Multinational Association of Supportive Care in Cancer; Hillerød, Denmark: 2015. http://www.mascc.org/ Last accessed: October 6, 2015. [Google Scholar]
- 100.Updates in Hospice and Palliative Medicine and Intensive Board Review. The University of Texas MD Anderson Cancer Center; Houston, TX, USA: 2015. http://www.mdanderson.org/education-and-research/education-and-training/schools-and-programs/cme-conference-management/conferences/d112623-updates-in-hospice-and-palliative-medicine-and-intensive-board-review.html Last accessed: October 6, 2015. [Google Scholar]
- 101.Center for Palliative Care. Harvard Medical School; Boston, MA, USA: 2015. http://www.hms.harvard.edu/pallcare/ Last accessed: October 6, 2015. [Google Scholar]
- 102.Care, I.A.f.H.a.P. Directory of Educational Programs in Palliative Care. International Association for Hospice and Palliative Care; Houston, TX: 2015. http://www.hospicecare.com/global-palliative-care/global-directory-of-education-programs/results.php?idregion=0&idcountry=0&idlanguage=0 Last accessed: 5-25-2015. [Google Scholar]
- 103.Morita T, et al. Effects of a programme of interventions on regional comprehensive palliative care for patients with cancer: a mixed-methods study. Lancet Oncol. 2013;14:638–46. doi: 10.1016/S1470-2045(13)70127-X. [DOI] [PubMed] [Google Scholar]
- 104.Jost L, Roila F. Management of cancer pain: ESMO clinical recommendations. Ann Oncol. 2009;20(Suppl 4):170–3. doi: 10.1093/annonc/mdp164. [DOI] [PubMed] [Google Scholar]
- 105.Cherny NI. ESMO Clinical Practice Guidelines for the management of refractory symptoms at the end of life and the use of palliative sedation. Ann Oncol. 2014;25(Suppl 3):iii143–52. doi: 10.1093/annonc/mdu238. [DOI] [PubMed] [Google Scholar]
- 106.Levy MH, et al. Palliative care, Version 1.2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2014;12:1379–88. doi: 10.6004/jnccn.2014.0136. [DOI] [PubMed] [Google Scholar]
- 107.Schrijvers D, Cherny NI. ESMO Clinical Practice Guidelines on palliative care: advanced care planning. Ann Oncol. 2014;25(Suppl 3):iii138–42. doi: 10.1093/annonc/mdu241. [DOI] [PubMed] [Google Scholar]
- 108.Fabrice A, et al. ESMO/ASCO Recommendations for a Global Curriculum in Medical Oncology: 2010 Update. (ESMO/ASCO, 2010) doi: 10.1136/esmoopen-2016-000097. http://www.esmo.org/Career-Development/Global-Curriculum-in-Medical-Oncology Last accessed: 5-25-2015. [DOI] [PMC free article] [PubMed]
- 109.Hansen HH, et al. Recommendations for a global core curriculum in medical oncology. J Clin Oncol. 2004;22:4616–25. doi: 10.1200/JCO.2004.08.134. [DOI] [PubMed] [Google Scholar]
- 110.Buss MK, et al. Hematology/oncology fellows’ training in palliative care: results of a national survey. Cancer. 2011;117:4304–11. doi: 10.1002/cncr.25952. [DOI] [PubMed] [Google Scholar]
- 111.Legrand SB, Heintz JB. Palliative medicine fellowship: a study of resident choices. J Pain Symptom Manage. 2012;43:558–68. doi: 10.1016/j.jpainsymman.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 112.von Gunten CF, Lupu D. Development of a medical subspecialty in palliative medicine: progress report. J Palliat Med. 2004;7:209–19. doi: 10.1089/109662104773709332. [DOI] [PubMed] [Google Scholar]
- 113.von Gunten CF, Sloan PA, Portenoy RK, Schonwetter RS. Physician board certification in hospice and palliative medicine. J Palliat Med. 2000;3:441–7. doi: 10.1089/jpm.2000.3.4.441. [DOI] [PubMed] [Google Scholar]
- 114.Yamaguchi T, et al. Palliative care development in the Asia-Pacific region: an international survey from the Asia Pacific Hospice Palliative Care Network (APHN) BMJ Support Palliat Care. 2014 doi: 10.1136/bmjspcare-2013-000588. [DOI] [PubMed] [Google Scholar]
- 115.Hui D, Finlay E, Buss MK, Prommer E, Bruera E. Palliative Oncologists: Specialists in the Science and Art of Patient Care. Journal of Clinical Oncology. 2015;33:2314–7. doi: 10.1200/JCO.2014.60.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hui D, et al. Quantity, Design, and Scope of the Palliative Oncology Literature. The Oncologist. 2011;16:694–703. doi: 10.1634/theoncologist.2010-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hui D, Arthur J, Dalal S, Bruera E. Quality of the supportive and palliative oncology literature: a focused analysis on randomized controlled trials. Support Care Cancer. 2012;20:1779–85. doi: 10.1007/s00520-011-1275-9. [DOI] [PubMed] [Google Scholar]
- 118.Hui D, Reddy A, Parsons HA, Bruera E. Reporting of Funding Sources and Conflict of Interest in the Supportive and Palliative Oncology Literature. Journal of Pain and Symptom Management. 2012;44:421–30. doi: 10.1016/j.jpainsymman.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Curtis JR, Morrison RS. The future of funding for palliative care research: suggestions for our field. Journal of Palliative Medicine. 2009;12:26–8. doi: 10.1089/jpm.2009.9691. [DOI] [PubMed] [Google Scholar]
- 120.Page B. What is Supportive Care? Canadian Oncology Nursing Journal. 1994;4:62–63. [Google Scholar]
- 121.Casarett D, Johnson M, Smith D, Richardson D. The optimal delivery of palliative care: a national comparison of the outcomes of consultation teams vs inpatient units. Archives of Internal Medicine. 2011;171:649–55. doi: 10.1001/archinternmed.2011.87. [DOI] [PubMed] [Google Scholar]


