Abstract
Economic and social transitions in the era of globalization warrant a fresh look at the neurological risks associated with environmental change. These are driven by industrial expansion, transfer and mobility of goods, climate change and population growth. In these contexts, risk of both infectious and non-infectious diseases are shared across geographical boundaries. In low- and middle-income countries, the risk of environmentally mediated brain disease is augmented several-fold by lack of infrastructure, poor health and safety regulations, and limited measures for environmental protection. Neurological disorders may occur as a result of direct exposure to chemical and/or non-chemical stressors such as ultrafine particulate matters. Individual susceptibilities to exposure-related diseases are modified by genetic, epigenetic and metagenomic factors. The existence of several uniquely exposed populations, including those in the areas surrounding the Niger Delta or north western Amazon oil operations; those working in poorly regulated environments, such as artisanal mining industries; or those, mostly in sub-Saharan Africa, relying on cassava as a staple food, offers invaluable opportunities to advance the current understanding of brain responses to environmental challenges. Increased awareness of the brain disorders that are prevalent in low- and middle-income countries and investments in capacity for further environmental health-related research are positive steps towards improving human health.
Reports from the World Health Organization (WHO) indicate that the global burden of disease is determined by patterns of disease and disability in low- and middle-income countries (LMICs), which, predictably, have their own environmental signatures (http://www.who.int/healthinfo/global_burden_disease/about/en/). However, the impact of such signatures on both brain health and region or global disability-adjusted life years (DALYs) remains unknown and needs to be added to the agenda of global environmental health research. As for high-income countries, environmental health research programmes in LMICs must primarily focus on elucidating the entire range and source of exposures to define the human ‘exposome’ (the measure of all the exposures of an individual in their lifetime and how these exposures relate to health) in LMICs. The research agenda should include mechanistic and translational research, as well as capacity building to foster a new generation of environmental health scientists.
SCOPE
In this Review, we focus on environmental risk factors for brain diseases and conditions in LMICs (http://data.worldbank.org/about/country-and-lending-groups). An iterative search of the literature was conducted using PubMed to retrieve information related to environmental determinants and mechanisms of brain disease in LMICs. Additional opinion was obtained from interviews with leading environmental scientists and neuroscientists, as well as programme officers at the US National Institutes of Health and US National Institute of Environmental Health Sciences (NIEHS), and Fogarty International Center. This Review integrates the goals and approaches to environmental health research as per the NIEHS 2012–2017 strategic plan (https://www.niehs.nih.gov/about/strategicplan/).
ENVIRONMENTAL EXPOSURE AND BRAIN HEALTH
LMICs are home to around 80–85% of the world’s population1. Of these 5.8 billion people2, 1 billion remain in extreme poverty, living below the US$1.25 per day poverty line3. Around 3 billion people do not have piped drinking water in their home and 173 million people rely on the direct use of surface water. Without proper sanitation, about one billion continue to defecate in gutters, in the open bush or in open water bodies4. Wildfires and deforestation are commonplace and drought and floods, possibly due to climate change, degrade the existing farming systems and create food insecurity5–7. Armed conflicts and population displacements impose an unnecessary toll on human life8. Industrial expansion coexists with an unprecedented rise in artisanal mining and unprotected labour9. In some instances, normal urbanization operations, such as road construction and quarantines (for example during Ebola outbreaks in the Democratic Republic of the Congo) have created conditions that exacerbated the risk of environmental exposure and brain disease10. Flawed regulations compounded by a lack of infrastructure set the stage for environmental degradation and pollution to pose serious threats — of a chemical or non-chemical nature — to human health. The degradation of local ecosystems leads to the creation of ‘microenvironments’ that have a high risk of harmful exposures, often resulting in unique challenges and increased risk of human disease (Fig. 1).
Figure 1.
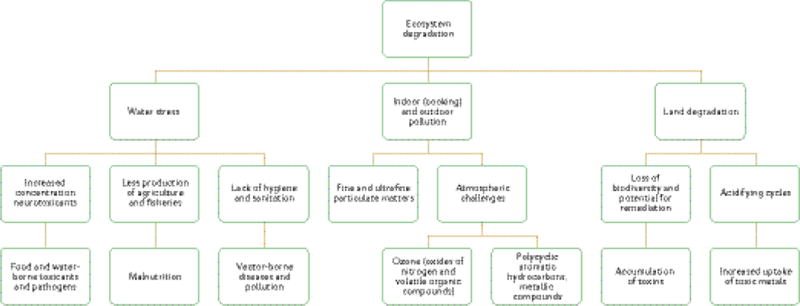
Environmental (chemical and non-chemical) threats to brain health in low- and middle-income countries. Multiple sources of exposure (air, water and food) coexist, and malnutrition and vector-borne diseases, notably infections, compound the risk of brain disease. Co-exposures not shown include heat, psychological stress and a poor physical environment, such as crowding.
HIGH-RISK POPULATIONS AND MICROENVIRONMENTS
Risk of exposure-related brain disease is determined by age, gender and microenvironments created by natural disasters in which economic, social and cultural determinants of health often have important roles. One example of a profit-mediated environmental risk is that caused by the oil industry through accidental spills or mismanagement of oil operations. For instance, crude oil operations have polluted large areas of rainforests, including streams and rivers in Ecuador, Peru and Colombia11. The population of Nigeria has faced similar challenges owing to reoccurring oil spills as a result of ageing, ill-maintained or sabotaged pipelines in the Niger Delta. The impact of such man-made and preventable natural disasters on human health has yet to be determined. Effects on human health will depend on the type and composition of the spilled oils, which often contain a mixture of polycyclic hydrocarbons that are known to be toxic to the nervous system11. Oil spills arise owing to reasons, such as a lack of vigilance, neglect of necessary health and safety checks, or sometimes even promotion of commercial interests at the expense of communities. Symptoms of acute exposure to raw oil include consistent episodes of headache, nausea, dizziness and fatigue. Chronic effects include psychological disorders, endocrine abnormalities and genotoxic effects12.
Microenvironments in which the population has a higher susceptibility to exposure-related diseases have also been created by extreme poverty and natural disasters, including drought and flooding that can degrade soils, plants and farming operations. The burden of conventional neurodevelopmental stressors (for example, lead) on children is exacerbated by unique environmental challenges, including malnutrition and enteric infections13–16 and, possibly, a diet of neurotoxicant-containing plants such as cassava (Manihot esculenta; also known as tapioca), the grass pea Lathyrus sativus or the seeds from the cycad plants, which are all known to be associated with a high-burden of neurodisabilities at a population level17–22. Populations with unique exposures and risks include those living in the tropical cassava belt of Angola, the Central African Republic, Cameroon, Democratic Republic of the Congo, Tanzania, Uganda, Nigeria and Mozambique23–30; those reliant on L. sativus as a staple food in Ethiopia, Eritrea, India and Bangladesh20,31–33; and the people of the Pacific island Guam or the Japanese Kii Peninsula where the rates of environmentally linked syndromes such as amyotrophic lateral sclerosis-parkinsonism-dementia complex (ALS/PDC) have been declining for reasons that have yet to be uncovered34,35.
The impact of early childhood diseases that lead to a vicious cycle of enteric infections and malnutrition has been underestimated and neglected, especially in areas that lack acceptable levels of hygiene and sanitation and that have reduced accessibility to vaccines and antimicrobials. This has caused clinically silent, chronic-illness-related effects, which jeopardize the child’s full cognitive development13,15. This vicious cycle establishes what is called environmental enteropathy, a mostly subclinical condition (even without diarrhoea) caused by various degrees of intestinal barrier dysfunction, luminal-to-blood intestinal bacterial translocation, low-grade local and systemic inflammation, and poor innate intestinal immune responses that may affect growth36 and cognition37 and possibly lead to neurodegeneration as well as liver, and metabolic diseases later in life38,39.
Adolescents in LMICs experience a higher burden of exposures (in contrast with those in high-income countries), primarily because of the childhood labour crisis. Although there are regulations and international agreements restricting child labour, often there are exceptions for certain industries, notably the growing agricultural industry, one of the most hazardous industries worldwide40,41. In this context, adolescent workers are at risk of exposure to agrochemicals such as pesticides42,43. Other work-related threats include exposure to organic solvents in work that involves painting and manufacture, to toxic metals and fine particulate matters in artisanal mining, and to heat and ambient air pollution while working long hours and outside41. Exposure to industrial solvents such as n-hexane, for example, may occur because of poor safety regulations or recreational glue sniffing. This may result in headache, acute encephalopathy or sensorimotor neuropathies that are reversible on cessation44.
Adults in LMICs may be at a particularly high risk of environmental exposure and related brain diseases compared with those in high-income countries. In general, adults experience a higher burden of disease owing to a lifetime of cumulative exposures and co-morbidities that are highly prevalent in LMICs. The latter include malaria, nutritional deficiencies and neurotropic infections such as those caused by human T-cell lymphotropic virus (HTLV). For example, it was reported that endemic foci of HTLV-I-associated myelopathy coexist with outbreaks of konzo, which causes spastic paraparesis and is linked to the toxicity of cassava cyanogens within some areas of the Democratic Republic of the Congo45,46. In certain circumstances, women of childbearing age are particularly vulnerable because they are more susceptible to the toxicity of cassava cyanogens for reasons that have not been elucidated, although may be linked to hormonal influences and poor nutrition47.
PATHWAYS TO BRAIN DISEASE
Exposure-related brain damage may result from chemical and/or non-chemical stressors. Damage to the nervous system often leads to a range of bilateral and symmetrical motor and/or sensory symptoms. Behavioural problems, cognition deficits and psychiatric illness may also occur. Non-chemical stressors include, but are not limited to, psychological stress, heat, noise, fine and ultrafine particulate matter (FUPM), and waterborne, airborne or foodborne pathogens that may occur under the conceptual framework shown in Fig. 1. Chemicals with neurotoxic potential that people are commonly exposed to are listed in Tables 1–3. Mixed exposure, for example chemical-covered FUPM from industrial emissions; co-exposure to chemical and non-chemical stressors; and repeated and multiple exposure can occur, creating a complex human environmental exposome.
Table 1.
Heavy metals and exposure-related outcomes
| Heavy metal | Source of exposure | Susceptibility window | Neurological outcomes | Proposed mechanisms |
|---|---|---|---|---|
| Lead76–78 | Lead-contaminated dust, lead-based paint, soil, drinking water, air, leaded gasoline, toys and lead-contaminated sweets | Lifelong | Visual and verbal memory decline, intellectual deficits, decline in executive functioning (fine motor function, hand-eye coordination and reaction time) and hyperactivity in children | Disruption of neurotransmitter release and function, and prenatal disruption of neuronal migration and differentiation. Aggravating factors include poor nutrition (deficiency in iron, zinc and calcium) and younger age. |
| Mercury79,80 | Mining industry, power plants, crematoria, charcoal industry, and contaminated food (mostly sea food) and water | From neural development to neurulation, and adolescence | Ataxia in adults and language, attention, and visuospatial performance deficits in children | Oxidative stress or impairment of intracellular calcium and glutamate homeostasis |
| Arsenic80,81 | Contaminated food and drinking water, air and arsenic-based treatments | 5–15 years | Impaired selective and focused attention and long-term memory in children, and sensorimotor polyneuropathy | Oxidative stress or disruption of metabolism of neurotransmitters |
| Copper82 | Contaminated drinking water and food, uncoated copper cookware and infant formula containing copper | Children Those over 65 |
Alzheimer’s disease, OCD, ADHD, antisocial behaviour and anxiety in children | Oxidative stress, microglia cell activation or promotion of α-synuclein and fibril formation |
| Cobalt82,83 | Contaminated drinking water and food, inhalation of dust containing cobalt particles in various industries | Prenatal, young children and the elderly | Optic, auditory and peripheral neuropathy, motor deficits and verbal memory loss | Alteration of mitochondrial oxidative phosphorylation or depletion of neurotransmitters |
| Cadmium83 | Fumes or dust, cigarette smoke, and contaminated food and water | Prenatal, young children and the elderly | Antisocial behaviour and attention impairment in children, parkinsonism and peripheral neuropathy | Oxidative damage and neurotransmitter disruption |
| Manganese76,79 | Airborne as fumes, aerosols or suspended particulate matter and contaminated water | Childhood and the elderly | Reduced IQ, impaired verbal learning and working and immediate memory in children, and Parkinson-like symptoms | Disruption of mitochondrial respiratory chain reaction. Aggravating factors include iron deficiency and impaired biliary excretion (liver injury or disease). |
| Aluminium84 | Contaminated air, water and food, cosmetics (such as antiperspirants), metal industries and pharmaceuticals | Lifelong | Alzheimer’s pathology in the form of neurofibrillary tangles | Disruption of mitochondrial respiratory chain reaction or inflammation. Zinc deficiency acknowledged as an aggravating factor. |
ADHD, attention-deficit hyperactivity disorder; OCD, obsessive compulsive disorder.
Table 3.
Complex exposures and neurological outcomes
| Exposure | Source of exposure | Susceptibility window | Neurological outcomes | Proposed mechanisms |
|---|---|---|---|---|
| Coal/charcoal burning99,100 | Charcoal/coal combustion, gas grilling, wood smoke, or coal mine dust or ash | Lifelong | Neurological signs of exposure to arsenic | Oxidative stress |
| Car emissions101,102 | Contaminated air | Lifelong | Learning disability and motor impairment | Oxidative stress or neurotransmitter disruption |
| Fine and ultrafine particulate matters103 | Air pollution from car or construction equipment exhausts, wood burning, heating oil or coal, forest fires, volcanic eruptions, tobacco smoke and cooking | Lifelong | Behavioural and decreased IQ, impaired fluid cognition, memory and executive functions, and possibly autism | Oxidative stress, neurotransmission disruption or neuroinflammation |
Brain damage linked to chemical exposure may result from chemicals interfering with neurotransmission through molecular mimicry or reacting with crucial biomolecules and causing incorrect function (for example, protein or DNA adduction and/or crosslinking). For both chemical and non-chemical exposures, the mechanisms of brain damage may include injury to the vascular system (for example, fine particulate-matter-induced vascular pathology), systemic dyshomeostasis (for example, cadmium-induced kidney disease) and hormonal imbalance (for example, through endocrine disruption; Table 1).
The susceptibility to exposure-related disease is, however, determined by mechanisms of functional genetics, epigenetics and metagenomics at the interface between risk factors and neurological outcomes (Fig. 2).
Figure 2.
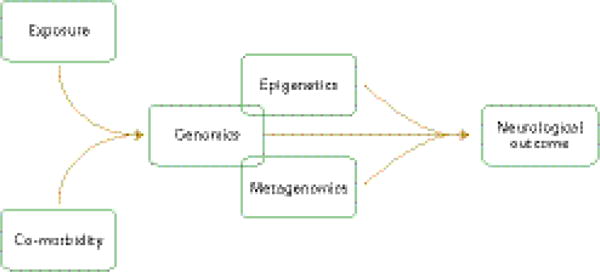
Environmental framework and pathways to environmentally induced neurological disease in low- and middle-income countries. Susceptibility to neurological disease is determined at the interface between a particular exposure, epigenetic and metagenetic make up, and the presence of co-morbidities.
It is increasingly acknowledged that genetic and epigenetic factors, including the effect of maternal stress on brain function, influence the effect of environmental exposure48,49. For example, the E4 allele of the APOE gene that is reportedly associated with higher risk of late-onset Alzheimer’s disease, although not in people from sub-Saharan Africa and with a mild association among Hispanic people, is also associated with protection against early childhood diarrhoea and its related cognitive impairment50–52. One example of gene–environment interactions is the relationship between air pollution components and the gene encoding the MET receptor tyrosine kinase. Several studies have implicated MET as an autism risk gene53–55. Stratification of the risk conferred by a functional promoter variant in this gene (rs1858830) and by local traffic-related air pollution (regional particulate matter less than 10 microns in diameter and nitrogen dioxide exposure) revealed significant multiplicative interaction between the risk genotype and the air pollution exposure56.
Our knowledge of the pathways that lead to late onset of exposure-related neurological disease is still sparse57,58. Many studies suggest that the genetic and environmental causes of late onset diseases act in parallel and share common molecular mechanisms59. A number of studies have supported the concept that early-life exposure to pollutants reprograms global gene expression in old age through epigenetic mechanisms60–63. Variation in exposure response, even among individuals exposed to the same environment could be due not only to early-life exposures, but also to differences in genetic make up64–66. The extent and nature of exposures and related brain diseases in LMICs provide opportunities to explore and overcome the long reach that childhood exposure has into adulthood, as well as provide us with new advances in environmental health sciences67.
Exposure-related neurological deficits in LMICs range from peripheral neuropathies to a large number of acute, subacute or chronic central nervous system diseases. Deficits may occur prenatally, or during childhood or adolescence, and may be carried through to old age. Clinical implications include, but are not limited to, neural tube defects, learning disabilities, behavioural problems, psychiatric disorders, cognitive decline and the occurrence of distinct entities such as neurolathyrism, tropical ataxic neuropathy, ALS/PDC and konzo20,30,35,68,69 (Fig. 3).
Figure 3.

Neurocognition deficits in konzo, a disease linked to eating cyanogenic cassava. a, Spasticity in a 14-year old boy severely affected by konzo. b, Deficits in mental processing are evident from the results of a neuropsychological test.
The human microbiome may be of particular interest to the mechanistic understanding of exposure-related diseases in LMICs because it may influence the burden of heavy metals70, the metabolism of food-borne neurotoxicants such as cassava cyanogens18, and the outcome of enteric diseases in early life, including the child’s neurodevelopmental potential71–73.
RESEARCH AND CAPACITY BUILDING
Recent advances in environmental health sciences have elucidated the myriad risk factors and mechanisms of brain damage that are associated with environmental exposures. The existence of uniquely exposed populations in LMICs offers invaluable opportunities to advance our current understanding of brain responses to environmental threats. In some instances, well-characterized neurotoxicants may be used as chemical probes to dissect the pathophysiology of the nervous system. Challenges at the population level still remain, including setting exposure limits and developing metrics and methodologies to assess the long-term impact of environmental exposures on disease burden in LMICs and, therefore, globally. Climate change and mining of rare elements, which may include radioactive materials, present unpredictable risks, and should be added to the environmental health research agenda. The toll of such exposures on the global burden of disease may be efficiently addressed only through competent partnerships and alliances established on a global scale and focused on key areas and priorities (Box 1). Although there is evidence that some of these are already in place, more research and research capacity is needed to continue this agenda to improve human health, globally.
BOX 1. INTERWOVEN RESEARCH AREAS AND INVESTMENT PRIORITIES IN GLOBAL ENVIRONMENTAL RESEARCH.
Epidemiology and statistical modeling for exposure and risk assessment in co-exposure and co-morbidity scenarios
High-throughput ‘-omic’ methodologies
Bioinformatics and knowledge management
Development of diagnostic and remediation tools — validation and implementation of environmental sensors, detectors and biomonitors of exposure and related outcomes
Development of metrics and methodologies to assess the long-term impact of environmental exposures on neurological disease burden
Understanding the pathways that lead to late onset of exposure-related neurological diseases
Training and capacity building in the areas listed above
ONE HEALTH–GLOBAL HEALTH DIMENSIONS
Environmental degradation and contamination, changes in climate and ecosystems, and vector-born pathogens or neurotoxicants are the primary environmental threats to human life and intellectual performance. Humans, plants and animals adapt to environmental challenges, but some may overcome their adaptive capabilities and create imminent risks for all17,74. Addressing environmental threats and risks for incapacitating diseases will therefore require a serious commitment to trans-disciplinary work, vigilance and capacity building on a global scale75
Table 2.
Organic compounds and exposure-related outcomes
| Organic compound | Source of exposure | Susceptibility window | Neurological outcomes | Proposed mechanisms |
|---|---|---|---|---|
| Bisphenol A85,86 | Food from cans with linings that contain BPA, and contaminated food and water | Prenatal and childhood | Anxious behaviour, hyperactivity and depressive behaviour, and learning impairment in children | Unclear but females seem to be more susceptible |
| Phthalates86–88 | Food or drink that has been in contact with containers or products containing phthalates, and air and dust containing phthalates | Prenatal and childhood | Depressive and conduct-related behaviours (ODD, attention problems, rule-breaking and aggressive behaviour in children) | Oxidative stress |
| Organophosphates89,90 | Contaminated food and water, polluted air and professional dermal contact | Lifelong | Neurodevelopmental deficits, impaired attention and working memory, impaired speed and executive functions, and delayed peripheral polyneuropathy | Inhibition of acetyl-cholinesterase |
| Organochlorinated compounds (DDT/PCBs)91 | Contaminated food, drinking water and air | Prenatal and lifelong | Impaired intellectual ability, ADHD- like behaviours and locomotor deficits | Disruption of neurotransmitter function, oxidative stress or derangement of calcium homeostasis. Children seem to be more susceptible. |
| Organobromide compounds (PBDEs)92,93 | Contaminated food, water and air | Lifelong | IQ deficits, impaired attention, fine motor coordination and cognition functioning in children | Impairment of thyroid hormone homeostasis |
| Organic solvents94,95 | Air and professional dermal contact and glue sniffing | Adolescents and adults | Headache, memory deficits, and central and peripheral neuropathy | Protein adduction or oxidative stress misfolding |
| Food-born neurotoxicants (cassava cyanogenic glucosides and BOAA in Lathyrus sativus)96–98 or contaminants (fungal toxins) | Oral ingestion | Lifelong | Spastic paraparesis, cognition deficits and epilepsy | Oxidative stress, excitotoxicity and protein carbamylation for cassava cyanogens. Children and females seem to be more susceptible. Malnutrition is acknowledged as an aggravating factor |
ADHD, attention-deficit hyperactivity disorder; BOAA, beta-(N)-oxalyl-amino-L-alanine acid; BPA, bisphenol A; DDT/PCBs, dichlorodiphenyltrichloroethane/polychlorinated biphenols; ODD, oppositional defiant disorder.
Acknowledgments
All the authors are thankful to NIEHS and Fogarty International Centre for research grant support and the scientific expertise of their respective programme officers A. Kirshner and K. Michels and staff members. The intellectual contribution of R. Kalaria of Newcastle University, UK, is very much appreciated.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests. Financial support for publication has been provided by the Fogarty International Center.
References
- 1.Sumner A. Global poverty and the new bottom billion: what if three-quarters of the world’s poor live in middle-income countries? IDS; 2010. pp. 1–43. [Google Scholar]
- 2.World Bank. Data. Low and middle income countries. World Bank; 2015. http://data.worldbank.org/income-level/LMY. [Google Scholar]
- 3.World Bank. Global Monitoring Report 2014/2015: ending poverty and sharing prosperity. World Bank; 2015. [Google Scholar]
- 4.Lang V, Lingnau H. Defining and measuring poverty and inequality post-2015. J Int Develop. 2015;27:399–414. [Google Scholar]
- 5.Haile M. Weather patterns, food security and humanitarian response in sub-Saharan Africa. Phil Trans R Soc Lond B. 2005;360:2169–2182. doi: 10.1098/rstb.2005.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorklund G. Workshop 4 (synthesis): securing food production under climate variability — exploring the options. Water Sci Technol. 2004;49:147–149. [PubMed] [Google Scholar]
- 7.Kim KH, Kabir E, Ara Jahan S. A review of the consequences of global climate change on human health. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2014;32:299–318. doi: 10.1080/10590501.2014.941279. [DOI] [PubMed] [Google Scholar]
- 8.Rieder M, Choonara I. Armed conflict and child health. Arch Dis Child. 2012;97:59–62. doi: 10.1136/adc.2009.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seccatore J, et al. An estimation of the artisanal small-scale production of gold in the world. Sci Total Environ. 2014;496:662–667. doi: 10.1016/j.scitotenv.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Banea M, Tylleskar T, Rosling H. Konzo and ebola in Bandundu region of Zaire. Lancet. 1997;349:621. doi: 10.1016/S0140-6736(05)61569-3. [DOI] [PubMed] [Google Scholar]
- 11.Jernelov A. The threats from oil spills: now, then, and in the future. Ambio. 2010;39:353–366. doi: 10.1007/s13280-010-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy BS, Nassetta WJ. The adverse health effects of oil spills: a review of the literature and a framework for medically evaluating exposed individuals. Int J Occup Environ Health. 2011;17:161–167. doi: 10.1179/107735211799031004. [DOI] [PubMed] [Google Scholar]
- 13.Guerrant RL, et al. The impoverished gut — a triple burden of diarrhoea, stunting and chronic disease. Nature Rev Gastroenterol Hepatol. 2013;10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrant RL, et al. Magnitude and impact of diarrheal diseases. Arch Med Res. 2002;33:351–355. doi: 10.1016/s0188-4409(02)00379-x. [DOI] [PubMed] [Google Scholar]
- 15.Guerrant RL, et al. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petri WA, Jr, et al. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, et al. Cassava genome from a wild ancestor to cultivated varieties. Nature Commun. 2014;5:5110. doi: 10.1038/ncomms6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tshala-Katumbay D, et al. Cassava food toxins, konzo disease, and neurodegeneration in sub-Sahara Africans. Neurology. 2013;80:949–951. doi: 10.1212/WNL.0b013e3182840b81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarmento A, et al. Valorization of traditional foods: nutritional and bioactive properties of Cicer arietinum L. and Lathyrus sativus L. pulses. J Sci Food Agric. 2015;95:179–185. doi: 10.1002/jsfa.6702. [DOI] [PubMed] [Google Scholar]
- 20.Spencer PS, Schaumburg HH. Lathyrism: a neurotoxic disease. Neurobehav Toxicol Teratol. 1983;5:625–629. [PubMed] [Google Scholar]
- 21.Marler TE, Lindstrom AJ. Free sugar profile in cycads. Front Plant Sci. 2014;5:526. doi: 10.3389/fpls.2014.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisby GE, Spencer PS. Is neurodegenerative disease a long-latency response to early-life genotoxin exposure? Int J Environ Res Public Health. 2011;8:3889–3921. doi: 10.3390/ijerph8103889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banea JP, et al. Effectiveness of wetting method for control of konzo and reduction of cyanide poisoning by removal of cyanogens from cassava flour. Food Nutr Bull. 2014;35:28–32. doi: 10.1177/156482651403500104. [DOI] [PubMed] [Google Scholar]
- 24.Tylleskar T, et al. Konzo in the Central African Republic. Neurology. 1994;44:959–961. doi: 10.1212/wnl.44.5.959. [DOI] [PubMed] [Google Scholar]
- 25.Ciglenecki I, et al. Konzo outbreak among refugees from Central African Republic in Eastern region, Cameroon. Food Chem Toxicol. 2011;49:579–582. doi: 10.1016/j.fct.2010.05.081. [DOI] [PubMed] [Google Scholar]
- 26.Nzwalo H, Cliff J. Konzo: from poverty, cassava, and cyanogen intake to toxico-nutritional neurological disease. PLoS Negl Trop Dis. 2011;5:e1051. doi: 10.1371/journal.pntd.0001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mlingi NL, et al. Recurrence of konzo in southern Tanzania: rehabilitation and prevention using the wetting method. Food Chem Toxicol. 2011;49:673–677. doi: 10.1016/j.fct.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Cliff J, et al. Konzo associated with war in Mozambique. Trop Med Int Health. 1997;2:1068–1074. doi: 10.1046/j.1365-3156.1997.d01-178.x. [DOI] [PubMed] [Google Scholar]
- 29.Okitundu Luwa EAD, et al. Persistence of konzo epidemics in Kahemba, Democratic Republic of Congo: phenomenological and socio-economic aspects. Pan Afr Med J. 2014;18:213. doi: 10.11604/pamj.2014.18.213.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oluwole OS, et al. Persistence of tropical ataxic neuropathy in a Nigerian community. J Neurol Neurosurg Psych. 2000;69:96–101. doi: 10.1136/jnnp.69.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tekle-Haimanot R, et al. Clinical and electroencephalographic characteristics of epilepsy in rural Ethiopia: a community-based study. Epilepsy Res. 1990;7:230–239. doi: 10.1016/0920-1211(90)90020-v. [DOI] [PubMed] [Google Scholar]
- 32.Ludolph AC, et al. Studies on the aetiology and pathogenesis of motor neuron diseases. 1 Lathyrism: clinical findings in established cases. Brain. 1987;110:149–165. doi: 10.1093/brain/110.1.149. [DOI] [PubMed] [Google Scholar]
- 33.Ngudi DD, et al. Research on motor neuron diseases konzo and neurolathyrism: trends from 1990 to 2010. PLoS Negl Trop Dis. 2012;6:e1759. doi: 10.1371/journal.pntd.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SE. Guam dementia syndrome revisited in 2011. Curr Opin Neurol. 2011;24:517–524. doi: 10.1097/WCO.0b013e32834cd50a. [DOI] [PubMed] [Google Scholar]
- 35.Kaji R, et al. ALS-parkinsonism-dementia complex of Kii and other related diseases in Japan. Parkinsonism Relat Disord. 2012;18(Suppl 1):S190–S191. doi: 10.1016/S1353-8020(11)70059-1. [DOI] [PubMed] [Google Scholar]
- 36.Prendergast AJ, et al. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS ONE. 2014;9:e86928. doi: 10.1371/journal.pone.0086928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patrick PD, et al. Limitations in verbal fluency following heavy burdens of early childhood diarrhea in Brazilian shantytown children. Child Neuropsychol. 2005;11:233–244. doi: 10.1080/092970490911252. [DOI] [PubMed] [Google Scholar]
- 38.Korpe PS, Petri WA., Jr Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petri WA, Naylor C, Haque R. Environmental enteropathy and malnutrition: do we know enough to intervene? BMC Med. 2014;12:187. doi: 10.1186/s12916-014-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515:518–522. doi: 10.1038/nature13959. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson KT, et al. The physical environment and child development: an international review. Int J Psychol. 2013;48:437–468. doi: 10.1080/00207594.2013.804190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crane AL, et al. Longitudinal assessment of chlorpyrifos exposure and effect biomarkers in adolescent Egyptian agricultural workers. J Expo Sci Environ Epidemiol. 2013;23:356–362. doi: 10.1038/jes.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohlman DS, et al. Characterizing exposures and neurobehavioral performance in Egyptian adolescent pesticide applicators. Metab Brain Dis. 2014;29:845–855. doi: 10.1007/s11011-014-9565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spencer PS, et al. The enlarging view of hexacarbon neurotoxicity. Crit Rev Toxicol. 1980;7:279–356. doi: 10.3109/10408448009037489. [DOI] [PubMed] [Google Scholar]
- 45.Tylleskar T, et al. Konzo, an epidemic spastic paraparesis in Africa, is not associated with antibodies to HTLV-I, HIV, or HIV gag-encoded proteins. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:317–318. doi: 10.1097/00042560-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Jeannel D, et al. The risk of tropical spastic paraparesis differs according to ethnic group among HTLV-I carriers in Inongo, Zaire. J Acquir Immune Defic Syndr. 1993;6:840–844. [PubMed] [Google Scholar]
- 47.Tylleskar T, et al. Dietary determinants of a non-progressive spastic paraparesis (Konzo): a case-referent study in a high incidence area of Zaire. Int J Epidemiol. 1995;24:949–956. doi: 10.1093/ije/24.5.949. [DOI] [PubMed] [Google Scholar]
- 48.Vidal AC, et al. Maternal stress, preterm birth, and DNA methylation at imprint regulatory sequences in humans. Genet Epigenet. 2014;6:37–44. doi: 10.4137/GEG.S18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bale TL. Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues Clin Neurosci. 2014;16:297–305. doi: 10.31887/DCNS.2014.16.3/tbale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maestre G, et al. Apolipoprotein E and Alzheimer’s disease: ethnic variation in genotypic risks. Ann Neurol. 1995;37:254–259. doi: 10.1002/ana.410370217. [DOI] [PubMed] [Google Scholar]
- 51.Oria RB, et al. ApoE polymorphisms and diarrheal outcomes in Brazilian shanty town children. Braz J Med Biol Res. 2010;43:249–256. doi: 10.1590/s0100-879x2010007500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oria RB, et al. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005;57:310–316. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- 53.Jackson PB, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2009;2:232–236. doi: 10.1002/aur.87. [DOI] [PubMed] [Google Scholar]
- 54.Sousa I, et al. MET and autism susceptibility: family and case-control studies. Eur J Hum Genet. 2009;17:749–758. doi: 10.1038/ejhg.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Y, et al. MET receptor tyrosine kinase as an autism genetic risk factor. Int Rev Neurobiol. 2013;113:135–165. doi: 10.1016/B978-0-12-418700-9.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volk HE, et al. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology. 2014;25:44–47. doi: 10.1097/EDE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charleta L, et al. Neurodegenerative diseases and exposure to environmental metals Mn, Pb, and Hg. Coord Chem Rev. 2012;256:2147–2163. [Google Scholar]
- 58.Oteiza PI, Mackenzie GG, Verstraeten SV. Metals in neurodegeneration: involvement of oxidants and oxidant-sensitive transcription factors. Mol Aspects Med. 2004;25:103–115. doi: 10.1016/j.mam.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Ali SF, Binienda ZK, Imam SZ. Molecular aspects of dopaminergic neurodegeneration: gene-environment interaction in parkin dysfunction. Int J Environ Res Public Health. 2011;8:4702–4713. doi: 10.3390/ijerph8124702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dosunmu R, Alashwal H, Zawia NH. Genome-wide expression and methylation profiling in the aged rodent brain due to early-life Pb exposure and its relevance to aging. Mech Ageing Dev. 2012;133:435–443. doi: 10.1016/j.mad.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bihaqi SW, et al. Infantile postnatal exposure to lead (Pb) enhances tau expression in the cerebral cortex of aged mice: relevance to AD. Neurotoxicology. 2014;44:114–120. doi: 10.1016/j.neuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang G, et al. Early life origins of metabolic syndrome: the role of environmental toxicants. Curr Environ Health Rep. 2014;1:78–89. doi: 10.1007/s40572-013-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collotta M, Bertazzi PA, Bollati V. Epigenetics and pesticides. Toxicology. 2013;307:35–41. doi: 10.1016/j.tox.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Singh S, et al. Influence of CYP2C9, GSTM1, GSTT1 and NAT2 genetic polymorphisms on DNA damage in workers occupationally exposed to organophosphate pesticides. Mutat Res. 2012;741:101–108. doi: 10.1016/j.mrgentox.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Morahan JM, et al. Genetic susceptibility to environmental toxicants in ALS. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:885–890. doi: 10.1002/ajmg.b.30543. [DOI] [PubMed] [Google Scholar]
- 66.Goodrich JM, Basu N. Variants of glutathione s-transferase pi 1 exhibit differential enzymatic activity and inhibition by heavy metals. Toxicol In Vitro. 2012;26:630–635. doi: 10.1016/j.tiv.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Currie E, Vogl T. Early-life health and adult circumstance in developing countries. Ann Rev Econom. 2013;5:1–36. [Google Scholar]
- 68.Tshala-Katumbay D, et al. Analysis of motor pathway involvement in konzo using transcranial electrical and magnetic stimulation. Muscle Nerve. 2002;25:230–235. doi: 10.1002/mus.10029. [DOI] [PubMed] [Google Scholar]
- 69.Boivin MJ, et al. Neuropsychological effects of konzo: a neuromotor disease associated with poorly processed cassava. Pediatrics. 2013;131:e1231–e1239. doi: 10.1542/peds.2012-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breton J, et al. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol Lett. 2013;222:132–138. doi: 10.1016/j.toxlet.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 71.Alonso C, et al. Intestinal barrier function and the brain-gut axis. Adv Exp Med Biol. 2014;817:73–113. doi: 10.1007/978-1-4939-0897-4_4. [DOI] [PubMed] [Google Scholar]
- 72.Sommer F, Backhed F. The gut microbiota — masters of host development and physiology. Nature Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 73.Arrieta MC, et al. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gleadow RM, et al. Growth and nutritive value of cassava (Manihot esculenta Cranz) are reduced when grown in elevated CO. Plant Biol. 2009;11(Suppl 1):76–82. doi: 10.1111/j.1438-8677.2009.00238.x. [DOI] [PubMed] [Google Scholar]
- 75.Erisman JW, et al. Put people at the centre of global risk management. Nature. 2015;519:151–153. doi: 10.1038/519151a. [DOI] [PubMed] [Google Scholar]
- 76.Nean A, Guillarte T. Mechanisms of heavy metal neurotoxicity: lead and manganese. Toxicol Res. 2013;2:99–114. doi: 10.1039/C2TX20064C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int. 2014;2014:840547. doi: 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanders T, et al. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health. 2009;24:15–45. doi: 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farina M, Rocha JB, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011;89:555–563. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 81.Florea AM, Busselberg D. Occurrence, use and potential toxic effects of metals and metal compounds. Biometals. 2006;19:419–427. doi: 10.1007/s10534-005-4451-x. [DOI] [PubMed] [Google Scholar]
- 82.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 83.Catalani S, et al. Neurotoxicity of cobalt. Hum Exp Toxicol. 2012;31:421–437. doi: 10.1177/0960327111414280. [DOI] [PubMed] [Google Scholar]
- 84.Kumar V, Gill KD. Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol. 2009;83:965–978. doi: 10.1007/s00204-009-0455-6. [DOI] [PubMed] [Google Scholar]
- 85.Harley KG, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yolton K, et al. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33:558–566. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Engel SM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miodovnik A, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jamal GA, et al. A clinical neurological, neurophysiological, and neuropsychological study of sheep farmers and dippers exposed to organophosphate pesticides. Occup Environ Med. 2002;59:434–441. doi: 10.1136/oem.59.7.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blanc-Lapierre A, et al. Cognitive disorders and occupational exposure to organophosphates: results from the PHYTONER study. Am J Epidemiol. 2013;177:1086–1096. doi: 10.1093/aje/kws346. [DOI] [PubMed] [Google Scholar]
- 91.Fonnum F, Mariussen E. Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. J Neurochem. 2009;111:1327–1347. doi: 10.1111/j.1471-4159.2009.06427.x. [DOI] [PubMed] [Google Scholar]
- 92.Costa LG, et al. A mechanistic view of polybrominated diphenyl esters developmental neurotoxicity. Toxicol Lett. 2014;15:282–294. doi: 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Linares V, Belles M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol. 2015;89:335–356. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- 94.Viaene M. Overview of the neurotoxicants effects in solvent-exposed workers. Arch Public Health. 2002;60:217–232. [Google Scholar]
- 95.Tshala-Katumbay D, et al. New insights into mechanisms of gamma-diketone-induced axonopathy. Neurochem Res. 2009;34:1919–1923. doi: 10.1007/s11064-009-9977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kassa RM, et al. On the biomarkers and mechanisms of konzo, a distinct upper motor neuron disease associated with food (cassava) cyanogenic exposure. Food Chem Toxicol. 2011;49:571–578. doi: 10.1016/j.fct.2010.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spencer PS. Food toxins, ampa receptors, and motor neuron diseases. Drug Metab Rev. 1999;31:561–587. doi: 10.1081/dmr-100101936. [DOI] [PubMed] [Google Scholar]
- 98.Makila-Mabe BG, et al. Serum 8,12-iso-iPF2alpha-VI isoprostane marker of oxidative damage and cognition deficits in children with konzo. PLoS ONE. 2014;9:e107191. doi: 10.1371/journal.pone.0107191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang Y, et al. Arsenic in Chinese coals: distribution, modes of occurrence, and environmental effects. Sci Total Environ. 2011:412–413. doi: 10.1016/j.scitotenv.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 100.Liu J, et al. Chronic arsenic poisoning from burning high-arsenic-containing coal in Guizhou, China. Environ Health Perspect. 2002;110:119–122. doi: 10.1289/ehp.02110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Block ML, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 102.Kilburn KH. Effects of diesel exhaust on neurobehavioral and pulmonary functions. Arch Environ Health. 2000;55:11–17. doi: 10.1080/00039890009603379. [DOI] [PubMed] [Google Scholar]
- 103.Costa LG, et al. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int. 2014;2014:736385. doi: 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]


