For more than three decades, policy discourse in the United States has debated the extent and consequences of low-value surgical care. Such debates frequently invoke the concept of “appropriateness” and the potential for different policy strategies to reduce “inappropriate” surgery. However, largely absent from such debates is an explicit consideration of what is meant by appropriateness, from whose perspective this concept is defined, or what implications alternate approaches to defining appropriateness might have on influencing efforts to improve the utilization of surgical resources. We propose that broadening the concept of appropriateness to include high-quality, patient-centered, shared decision-making will result in higher value procedural care. As our population ages and healthcare costs continue to increase, anesthesiologists and surgeons should play key roles in promoting decision-making paradigms to ensure that procedural care is conducted in a manner that adds value from the patient perspective.
Historical Evolution of Surgical Appropriateness
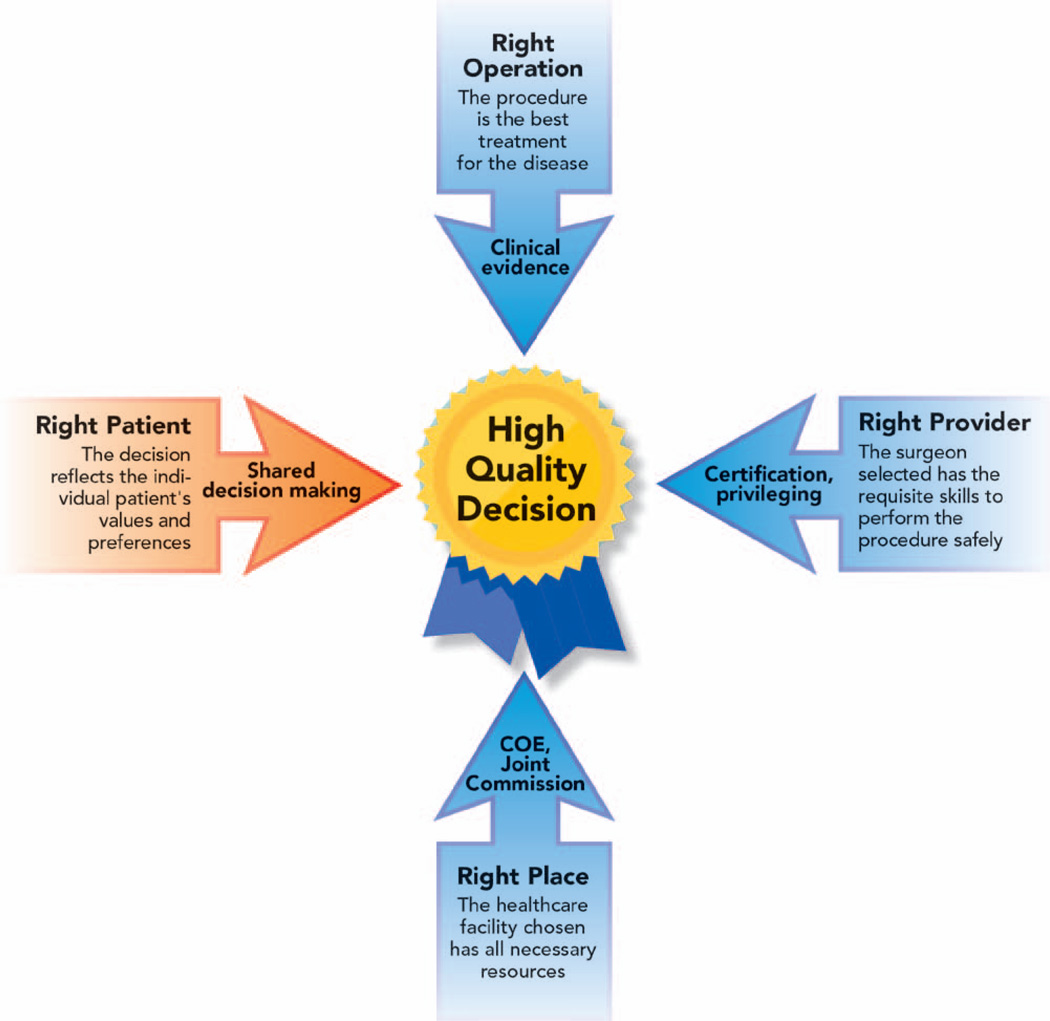
Lavis and Anderson1 described appropriate care as that which does more good than harm for a patient given a certain set of clinical indications. Risk–benefit evaluation for a given patient generally includes meeting a minimum set of clinical criteria defining an illness that has evidence-based treatment. The other perspective that overlays any individual patient’s risk–benefit ratio is the consideration of cost. In high-value procedures, overall benefits outweigh the risks with sufficient margin to make the procedure worth doing despite the costs for a particular patient and society. Of equal importance, the patient should demonstrate a full understanding of risks, benefits, and alternatives, and there should be concordance between patient preferences and values and expected clinical outcomes (fig. 1).
Fig. 1.
Components of high-quality surgical decision making. COE = center of excellence.
A brief review of previously developed methodologies to prevent overuse in surgeries suggests that they fall short in completely fulfilling all of these criteria. Institutions and programs exist to address the appropriateness of provider and place, including board certifications, hospital privileging procedures, certification, and “center of excellence” designations, addressing the “right provider” and “right site” issues. However, these frameworks do not consider the “right patient,” with a notable absence of variables related to alignment of patient and provider goals, the extent of patient engagement in decision-making, and decisional quality.
Previous Appropriateness Methodologies: Peer Review Organizations and Internal
Peer review organizations (also known as Professional Standards Review Organizations) were introduced in the 1970s and were largely made up of clinicians or administrators outside of clinical practice. Their task was determining whether care was “necessary, of acceptable quality, and delivered in the most economical setting possible.”2 Over time these organizations began offering widely variable guidelines that were proprietary and not necessarily clinically validated. Overall concerns included that these organizations were focused primarily with cost and efficiency, threatened the primacy of the doctor–patient relationship, and functioned like regulatory agencies evaluating clinical practitioners.3,4 Physicians and patients had little faith in this method, demonstrating that buy-in and satisfaction with the system is critical if cost savings are to be achieved.5
To encourage a greater focus on appropriateness, peer review efforts shifted to internal peer review within departments/ institutions. Internal peer review is associated with reduced procedure-related morbidity, mortality, and hospital costs.6 Significant limitations in peer review are variability among clinicians directing review processes, focus on unusual adverse events, and improving surgical technique/practice.7 Its ability to improve appropriateness is questionable.
Previous Appropriateness Methodologies: Indications Review
Indications review is a form of internal peer review involving classification of patient eligibility based on the standard surgical indications. Examples include a weekly conference to prioritize cardiac surgical cases using standardized criteria of coronary anatomy/symptoms and electronic decision support systems based on the standard guidelines for the appropriate use of computed tomographic procedures.8,9 An important benefit is reducing variation in physician style and emphasizing objectivity.
Previous Appropriateness Methodologies: Utilization Review
In response to escalating costs, external oversight by independent entities such as the government and third-party payers scrutinized clinical decisions and imposed more rigid criteria than previously existed regarding preauthorization of surgical procedures.10 The concern is emphasis on cost over quality and ultimately denying patient’s access to needed services.11 Evidence suggests that utilization review will approve inappropriate procedures in some situations and that there is generally a wide variability for preprocedure review criteria that can differ significantly from identified practice guidelines.12
Previous Appropriateness Methodologies: Rand Corporation–University of California at Los Angeles Appropriateness Method
In the 1980s, a group at Rand Corporation and University of California at Los Angeles developed a system based on literature review and expert consensus (Rand Corporation–University of California at Los Angeles Appropriateness Method).13 An expert panel rated clinical scenarios for procedures, based on the available literature, least (1) to most (9) appropriate. Although it showed promise as a mechanism to reduce inappropriate care, and the regional variation demonstrated in the Dartmouth Atlas of Healthcare,14 this methodology did allow the incorporation of patient factors that could influence the appropriateness of the procedure.15 An example that Barnato and Garber present is evaluating outcomes that are hard to measure and where individual preferences matter. Coronary artery bypass graft surgery reduces angina and mortality but may cause stroke or cognitive impairment. Outcome measurement and individual patient values cannot be generalized easily in this situation.15
Previous Appropriateness Methodologies: Payment Incentives
A more recent strategy to motivate clinicians is the use of payment incentives, which have shown mixed results. Referral patterns may reflect cost considerations rather than provider or facility quality. For example, Halm et al.16 noted that patients were referred to centers for carotid endarterectomy that had negotiated more favorable financial contracts rather than referring patients to the highest quality providers and facilities. Bundled payment systems aim to reduce inappropriate care with a single payment for all services within a surgical episode.
Importance of Patient Engagement
This overview of methodologies provided reflects significant and sincere work aiming to improve the value of surgical care. However, it is not surprising that these efforts consistently exclude patient engagement and the need to ensure that high-quality shared decision-making has occurred (table 1). There is a core tension between “shared decision-making,” occurring at the level of the individual patient, and “appropriateness” criteria, for the larger public; and also between what is ideal and what is achievable. Perioperative clinicians are generally not trained to frame informed discussions within the context of an individual patient’s preferences, goals, and values; nor are current perioperative workflows structured to facilitate these conversations
Table 1.
Methods of Reducing Inappropriate Care
| Definition | Strengths | Weaknesses | |
|---|---|---|---|
| Internal peer review | Utilization review by peer groups within specialty. Born out of concern that if MDs do not self-regulate, “unqualified individuals” will take responsibility for their utilization review. E.g., M&M, second opinions, event audits | Group of expert reviewers with experience and context when reviewing events. Feedback through both formal and informal routes is possible. | Generally is a retrospective investigation into rare events and is often subjective. Questions regarding quality, standardization, and whether assessment of concrete outcomes is possible. |
| Indications review | Utilization review by comparison to a set of indications for each procedure/treatment. E.g., CPOE with decision support | Standardized, quantifiable data that makes it possible to compare between institutions and healthcare systems. | Does not identify solutions, only highlights problems. Underlying assumption is that clinical indications are the only relevant factor to consider. |
| External utilization review | Utilization review by third-party organization. E.g., insurance company review | Theoretically decreases bias as third-party organization is not vested in topic of interest. Demonstrated changes in practice in many settings. | Costly. Difficult to assemble a team of knowledgeable individuals who are truly unbiased and able to objectively review. |
| RAND/UCLA appropriateness method | Multidisciplinary panel using literature review to guide creation of appropriateness criteria. Done in multiple rounds and includes both retrospective review of current practices and prospective creation of clinical decision aids. | Reproducible, practical—expert panels can be brought together virtually or by mail, if not possible in person. Concrete set of appropriateness criteria are formed. True to real-life experience of clinical decision-making, relying on imperfect or incomplete research to guide decision. | Lack of data available from reliable clinical trials for most treatments. Variability in process—composition of panel, role of moderator, individual scenarios that are not generalizable, lack of specificity about what outcomes are being considered. Greater concern that this process simply legitimizes current dogma that may not be evidence based. |
| Payment incentives | Linking processes believed to lead to higher quality care or desirable outcomes to reimbursement. Goal is to reduce cost and improve quality. E.g., episode-based reimbursement | Incentive structure that aligns values of cost–benefit analysis with each clinical decision. Quantifiable data output. Evidence-based processes are used—greatest strength is ultimately measuring outcomes. | Most programs only include process measures and lack clinical outcome measures. Perverse incentives: inadvertently incentivizing overutilization and possible disincentives for providing care. |
CPOE = Computerized Physician Order Entry; M&M = Morbidity and Mortality Rounds; RAND/UCLA = Rand Corporation, University of California at Los Angeles.
Perioperative clinicians must learn to engage patients in shared decision-making, and decisional quality should be measured as a metric of high-value patient-centered care. Currently, signed informed consent can exist without the assurance that high-quality shared decision-making has occurred. Decision quality is an important indicator of patient-centered care yet it is rarely evaluated; studies that have evaluated decisional quality show significant deficits in a variety of elements. In our previous work, we have attempted to conceptualize the domains involved in surgical decisions; each domain can reflect specific types of deficits in decisional quality.17 The first domain is that of structure (knowledge of procedure and risks/benefits). This domain comprises the minimal elements necessary for the formal process of signed informed consent. The second domain focuses on the process of decision-making and captures the patient’s need for more time or discussion to process choice. The final domain reflects outcomes: a decision and treatment aligned with the patient’s personal values, goals, and priorities.
To be patient centered, appropriateness criteria must address variability in patient values, goals, and preferences, as well as the social, compliance, and financial issues that may prevent patients from attaining optimal benefit from procedures, even when procedures are indicated and cost-effective. Unfortunately, even highly successful surgical and preoperative assessment workflows with multiple safety and quality checks lack standardized ways to address these issues. The literature demonstrates that significant numbers of patients do not fulfill even minimum standards for informed decision-making, and even after signed consent, recall on surgical risks and benefits is poor and that the format in which benefits are present is relevant.18,19 Our own work in a general surgical population shows that after signed informed consent, 13% of patients show significant deficits in the elements of informed consent and over 33% exhibited other types of deficits, related to not having addressed patients’ preferences, values, and goals.17 These results are in line with other studies, suggesting that recall is poor for information presented during a preoperative conversation, especially verbally presented medical information.20,21 Eleven percent of patients in this study expressed some doubt as to whether they wanted to undergo the procedure at all.17 Patient engagement is central to this task, collaborating with patients to help them appreciate the relevance of their own values and preferences.22 The ideal timing and method to use these concepts is yet to be determined; the literature supports integrating advance care planning preoperatively and specifically using decision aids.23
Conclusion
It is our belief that encouraging patient engagement and ensuring high-quality shared surgical decision-making will result in fewer inappropriate procedures. There is evidence that patients who engage in high-quality decision-making are not only more likely to play a more active role in behaviors that will ensure a positive surgical outcome but are also less likely to choose surgery that will not benefit them. These patients may elect to choose alternative treatments or nonsurgical therapies.24
Work on measuring the quality of surgical decisional making is in its infancy. Validated tools to identify individuals at risk for low-quality surgical decisions and decisional conflict are lacking, as are validated interventions to use during the preoperative workflow to reduce these deficits. We hope that the current national emphasis on appropriate, patient-centered care will help this important area of work to flourish. Innovations that foster high-quality shared surgical decision-making, in combination with the successful elements of the other appropriateness methodologies, will reduce nonbeneficial procedures and variations in surgical care and increase the appropriateness of the care that our patients receive.
Acknowledgments
Support was provided solely from institutional and/or departmental sources.
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Lavis JN, Anderson GM. Appropriateness in health care delivery: Definitions, measurement and policy implications. CMAJ. 1996;154:321–328. [PMC free article] [PubMed] [Google Scholar]
- 2.Dale MG. PSRO: A primer. JAMA. 1974;229:157–158. [PubMed] [Google Scholar]
- 3.Wennberg JE. Unwanted variations in the rules of practice. JAMA. 1991;265:1306–1307. [PubMed] [Google Scholar]
- 4.Halm EA, Causino N, Blumenthal D. Is gatekeeping better than traditional care? A survey of physicians’ attitudes. JAMA. 1997;278:1677–1681. [PubMed] [Google Scholar]
- 5.Hogg W, Baskerville N, Lemelin J. Cost savings associated with improving appropriate and reducing inappropriate preventive care: Cost-consequences analysis. BMC Health Serv Res. 2005;5:20. doi: 10.1186/1472-6963-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olcott C, IV, Mitchell RS, Steinberg GK, Zarins CK. Institutional peer review can reduce the risk and cost of carotid endarterectomy. Arch Surg. 2000;135:939–942. doi: 10.1001/archsurg.135.8.939. [DOI] [PubMed] [Google Scholar]
- 8.Ray AA, Buth KJ, Sullivan JA, Johnstone DE, Hirsch GM. Waiting for cardiac surgery: Results of a risk-stratified queuing process. Circulation. 2001;104(suppl I):I-92–I-98. [PubMed] [Google Scholar]
- 9.Brenner DJ. Medical imaging in the 21st century—Getting the best bang for the rad. N Engl J Med. 2010;362:943–945. doi: 10.1056/NEJMe1000802. [DOI] [PubMed] [Google Scholar]
- 10.Lohr KN, Schroeder SA. A strategy for quality assurance in Medicare. N Engl J Med. 1990;322:707–712. doi: 10.1056/nejm199003083221031. [DOI] [PubMed] [Google Scholar]
- 11.Wickizer TM. The effect of utilization review on hospital use and expenditures: A review of the literature and an update on recent findings. Med Care Rev. 1990;47:327–363. doi: 10.1177/107755879004700303. [DOI] [PubMed] [Google Scholar]
- 12.Kellie SE, Kelly JT. Medicare Peer Review Organization preprocedure review criteria. An analysis of criteria for three procedures. JAMA. 1991;265:1265–1270. [PubMed] [Google Scholar]
- 13.Barnato AE, Garber AM. Performance of the RAND appropriateness criteria. Med Decis Making. 2003;23:177–179. doi: 10.1177/0272989X03252312. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed April 9, 2015]; www.dartmouthatlas.org/keyissues/issue.aspx?con=1338. [Google Scholar]
- 15.Hicks NR. Some observations on attempts to measure appropriateness of care. BMJ. 1994;309:730–733. doi: 10.1136/bmj.309.6956.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halm EA, Press MJ, Tuhrim S, Wang J, Rojas M, Chassin MR. Does managed care affect quality? Appropriateness, referral patterns, and outcomes of carotid endarterectomy. Am J Med Qual. 2008;23:448–456. doi: 10.1177/1062860608323926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ankuda CK, Block SD, Cooper Z, Correll DJ, Hepner DL, Lasic M, Gawande AA, Bader AM. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns. 2014;94:328–333. doi: 10.1016/j.pec.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Uzaman MM, Sinya S, Shaygi B, Vitish-Sharma P, Loizides S, Myint F. Evaluation of patients’ understanding and recall of the consent process after open inguinal hernia repairs. Int J Surg. 2012;10:5–10. doi: 10.1016/j.ijsu.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Covey J. A meta-analysis of the effects of presenting treatment benefits in different formats. Med Decis Making. 2007;27:638–654. doi: 10.1177/0272989X07306783. [DOI] [PubMed] [Google Scholar]
- 20.Sandberg EH, Sharma R, Sandberg WS. Deficits in retention for verbally presented medical information. Anesthesiology. 2012;117:772–779. doi: 10.1097/ALN.0b013e31826a4b02. [DOI] [PubMed] [Google Scholar]
- 21.Neuner-Jehle S, Senn O, Wegwarth O, Rosemann T, Steurer J. How do family physicians communicate about cardiovascular risk? Frequencies and determinants of different communication formats. BMC Fam Pract. 2011;12:15. doi: 10.1186/1471-2296-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry MJ, Edgman-Levitan S. Shared decision making—Pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 23.Schuster AL, Aslakson RA, Bridges JF. Creating an advance-care-planning decision aid for high-risk surgery: A qualitative study. BMC Palliat Care. 2014;13:32. doi: 10.1186/1472-684X-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CN, Ko CY. Beyond outcomes—The appropriateness of surgical care. JAMA. 2009;302:1580–1581. doi: 10.1001/jama.2009.1465. [DOI] [PubMed] [Google Scholar]