Abstract
The physiological importance of the intestinal plasma membrane calcium pump, isoform 1, (Pmca1, Atp2b1), in calcium absorption and homeostasis has not been previously demonstrated in vivo. Since global germ-line deletion of the Pmca1 in mice is associated with embryonic lethality, we selectively deleted the Pmca1 in intestinal absorptive cells. Mice with loxP sites flanking exon 2 of the Pmca1 gene (Pmca1fl/fl) were crossed with mice expressing Cre recombinase in the intestine under control of the villin promoter to give mice in which the Pmca1 had been deleted in the intestine (Pmca1EKO mice). Pmca1EKO mice were born at a reduced frequency and were small at the time of birth when compared to wild-type (Wt) litter mates. At two months of age, Pmca1EKO mice fed a 0.81% calcium, 0.34% phosphorus, normal vitamin D diet had reduced whole body bone mineral density (P <0.037), and reduced femoral bone mineral density (P <0.015). There was a trend towards lower serum calcium and higher serum parathyroid hormone (PTH) and 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) concentrations in Pmca1EKO mice compared to Wt mice but the changes were not statistically significant. The urinary phosphorus/creatinine ratio was increased in Pmca1EKO mice (P <0.004). Following the administration of 200 ng of 1α,25(OH)2D3 intraperitoneally to Wt mice, active intestinal calcium transport increased ∼2-fold, whereas Pmca1EKO mice administered an equal amount of 1α,25(OH)2D3 failed to show an increase in active calcium transport. Deletion of the Pmca1 in the intestine is associated with reduced growth and bone mineralization, and a failure to up-regulate calcium absorption in response to 1α,25(OH)2D3.
Keywords: Plasma membrane calcium pump; Pmca1; Atp2b1; intestine; bone density; 1α,25-dihydroxyvitamin D3; calcium transport
Introduction
Absorption of dietary calcium (Ca) by the intestine is essential for the maintenance of normal Ca homeostasis [1]. The efficiency of Ca absorption increases or decreases inversely with the amount of dietary Ca, and adaptations to changes in Ca intake are dependent upon vitamin D and its active metabolite, 1α,25(OH)2D3 [1,2]. Ca transport in the intestine occurs by transcellular and para-cellular routes [1,3,4]. Passive, para-cellular Ca transport occurs at higher Ca concentrations and increases with increasing concentrations of luminal Ca [3,4]. In a Ca-transporting cell such as the enterocyte of the duodenum, apically situated, TRPV 5/6 cation channels mediate the increase in Ca uptake from the lumen into the cell [5]; intracellular Ca binding proteins such as calbindin D9K and D28K facilitate the movement of Ca across the cell [4,6]; and the basal-lateral plasma membrane Ca pump (PMCA) [7,8,9] and the Na-Ca exchanger (NCX) [10] assist in the extrusion of Ca from within the cell into the ECF. The Na gradient for the activity of the NCX is maintained by the Na-K ATPase. Intestinal transcellular Ca transport is regulated by vitamin D through its active metabolite, 1α,25(OH)2D, which increases the expression of TRPV 6 channels [11], the intra-cellular concentrations of calbindin D9K and D28K [6,12,13,14], and the expression of the PMCA pump isoform 1 [15,16].
There is a paucity of information regarding the requirement of various intestinal Ca transporter proteins in transcellular Ca transport in vivo. Deletions of TrpV6 and calbindin D9K genes are not associated with alterations in intestinal Ca transport in vivo in the basal state and following the administration of 1α,25(OH)2D3 [17,18]. One report suggests that basal Ca transport on an adequate Ca diet is normal in TrpV6 knockout mice but adaptations to a low Ca diet are impaired [19]. The role of PMCA 1, the major Ca pump in the intestine, has been difficult to establish in vivo because appropriate animal models with tissue-specific deletions have not been available. Germ line deletion of the predominant Pmca1 gene that is expressed in the intestine and kidney, is associated with early embryonic death, thus making it impossible to investigate the role of this protein in vivo [20]. Liu et al. previously demonstrated a possible role for the enterocyte Pmca1b in Ca homeostasis [21]. These authors showed that murine knockout of enterocyte protein 4.1R which associates with PMCA1b and reduces its expression, results in significantly impaired small intestinal calcium absorption and secondary hyperparathyroidism. However, experiments in which the Pmca1 has been deleted in the enterocyte have not been performed.
We now report the intestine-specific deletion of the Pmca1 gene in mice. We show that these mice are viable and have a phenotype suggesting altered bone and mineral homeostasis.
Methods and Materials
Animal care
All protocols were approved by the University of Manchester and Mayo Clinic institutional animal care committees. Mice were fed a 0.81% Ca, 0.34 percent phosphorus (non-phytate) diet containing 2.3 I.U. of vitamin D3 per gram (PicoLab Rodent Diet 20).
Generation of floxed Pmca1 mice
The targeting strategy to create mice with a floxed Pmca1 gene is shown in Fig. 1. The Pmca1 gene on mouse chromosome 10 contains 21 exons spread over more than 100 kb of DNA. Exon 2 contains the ATG initiation codon and was therefore targeted for insertion of the flanking LoxP sites: deletion of this exon prevents the production of a functional Pmca1 protein because of the absence of an appropriate downstream initiation codon in the proper reading frame.
Figure 1.
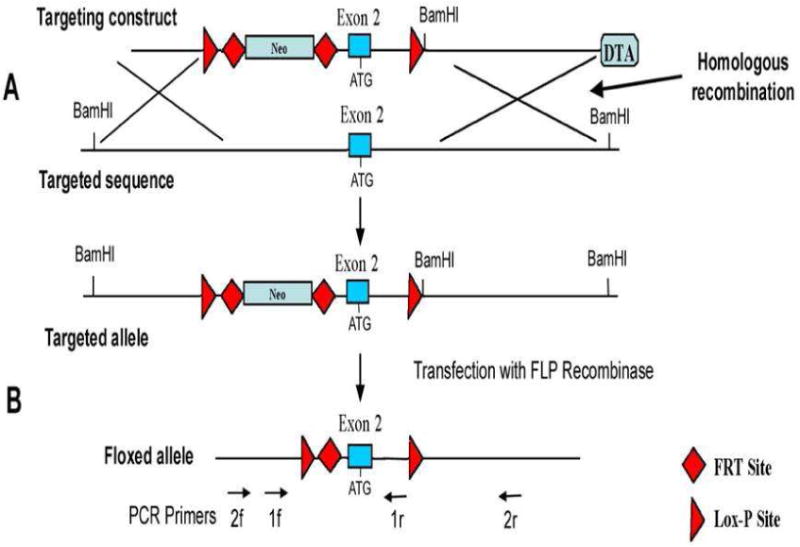
Generation of Pmca1fl/fl mice. The targeting construct consists of two LoxP sites flanking exon 2 of the Pmca1 (Atp2b1) gene, a short 5′ arm homology of 1.7 kb, a neomycin resistance gene (Neo) flanked by FRT sites, a long (5.7 kb) 3′ homology arm, a diphtheria toxin A (DTA) expression cassette and an exogenous BamHI restriction site. The construct underwent homologous recombination in mouse embryonic stem (ES) cells to produce the targeted allele (A). Targeted ES cells were transfected with FLP recombinase to remove the neo cassette and generate the floxed Pmca1 allele (B). Targeted ES cells were injected into C57Bl/6 blastocysts and resultant chimeric mice were mated to test for germ line transmission of the floxed allele. Heterozygotes were crossed to give homozygous Pmca1fl/fl mice. The location of PCR primers for genotyping (1f, 1r) and analysis of deletion of the floxed exon 2 (2f, 2r) are shown on the bottom.
Generation of mice with small intestine-specific knockout of Pmca1
We used villin-Cre transgenic mice [22] from Jackson Labs (B6.SJL-Tg(Vil-cre)997Gum/J) to obtain mice with intestinal enterocyte-specific deletion of the Pmca1 gene (abbreviated as Pmca1EKO mice). The villin gene promoter drives Cre recombinase expression in the small intestine starting in late embryogenesis and throughout adulthood [22] and has been successfully used to knock out specific genes in the absorptive epithelium of the duodenum, jejunum and ileum [23,24]. Of note, the 12.4-kilobase region of the mouse villin gene promoter used in these mice has been shown to drive high level expression of two different reporter genes (LacZ and Cre recombinase) within the entire intestinal epithelium, whereas no expression is noted in the embryonic kidney [22]. We crossed villin-Cre transgenic male mice with female Pmca1fl/fl mice to generate villin-Cre-Pmca1+/fl(+/-) offspring, which were then back-crossed to Pmca1fl/fl mice to create the desired villin-Cre-Pmca1fl/fl (-/-) genotype (henceforth abbreviated as Pmca1EKO mice) (Fig. 2A, boxed). The following primers were used for genotyping: Primers 1f and 1r (Fig. 1) for WT Pmca1 (754 bp) and Pmca1fl/fl (898 bp): 1f – 5′-CTG GCC TCA CCT AGT TTG CTA AAC C-3′; 1r – 5′-CTG TGG AGT ACA TGC TTC GTT CTG C-3′. Primers for vil-cre (1100 bp): Forward - 5′-GTG TGG GAC AGA GAA CAA ACC-3′; Reverse - 5′-ACA TCT TCA GGT TCT GCG GG-3′.
Figure 2.
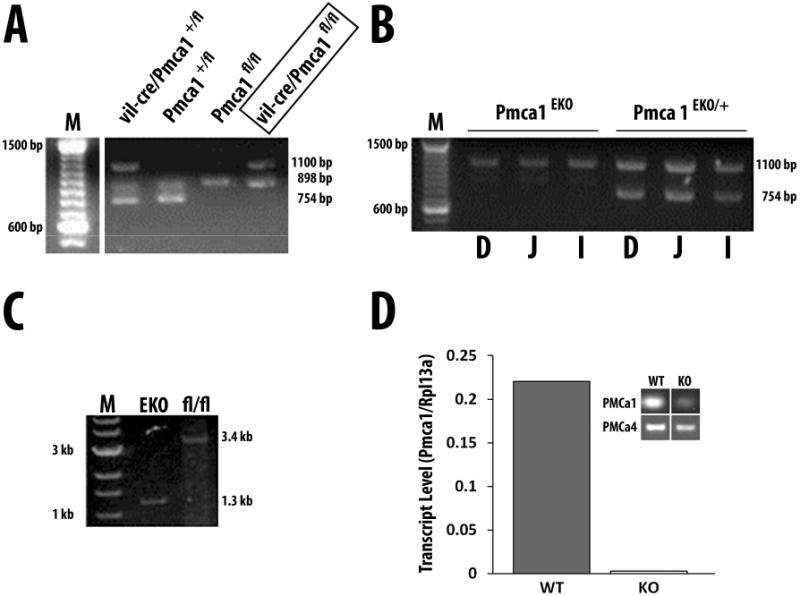
Genotyping of Pmca1EKO mice and confirmation of Atp2b1 exon 2 deletion in the small intestine. A. Genotyping of offspring of a vil-cre/Pmca1+/fl × Pmca1fl/fl pairing reveals vil-cre/Pmca1+/fl, Pmca1+/fl, Pmca1fl/fl, and vil-cre/Pmca1fl/fl individuals as indicated by the presence of PCR fragments of 754 bp for the wild-type Atp2b1 allele, 898 bp for the floxed Atp2b1 allele, and 1100 bp in villin-Cre hemizygous mice. The single vil-cre/Pmca1fl/fl (= Pmca1EKO) genotype is boxed. M, 100 bp DNA ladder. B and C. PCR confirming enterocyte-specific deletion of the Pmca1 exon 2 in Pmca1EKO mice. In B, primer pair 1f/1r (Fig. 1) was used to amplify genomic DNA from mucosal cell scrapings of the duodenum (D), jejunum (J) and ileum (I) of a Pmca1EKO and a heterozygote Pmca1EKO/+ mouse as indicated. The presence of the villin-cre gene was confirmed by separate primers resulting in the expected 1100 bp band. Note the absence of a Pmca1-specific band in the Pmca1EKO tissues, while the expected 754 bp Atp2b1 band is observed in the heterozygote. M, DNA size marker ladder. In C, primer pair 2f/2r (Fig. 1) was used for PCR on genomic DNA isolated from the small intestine of a Pmca1EKO and a control Pmca1fl/fl mouse. Removal of the floxed exon 2 region results in a 1.3 kb fragment in the Pmca1EKO tissue, whereas the expected fragment in the Pmca1fl/fl control is 3.4 kb as indicated. M, DNA size marker lane. D, Pmca1 mRNA expression was assessed by quantitative RT-PCR in the intestinal epithelium isolated by collagenase treatment of duodenal loops of Pmca1EKO mice. Inset shows agarose gel of Pmca1 and Pmca4 from Pmca1EKO (KO) and Pmca1fl/fl (WT) epithelium isolated by sequential collagenase treatment of everted gut sacs and assessed by semi-quantitative RT-PCR (residual amount of Pmca1 is derived from non-epithelial cells).
Primers 2f and 2r (Fig. 1) for Pmca1 in DNA extracts of the intestinal scrapings (WT allele 3.4 kb, KO allele 1.2 kb): 2f – 5′-AAT GCT CTC TGA GCG TAT GGT CTG G-3′; 2r – 5′-CCA GAG ACC ATT CAT GGC TTC TAC C-3′.
Confirmation of deletion of Pmca1 from intestinal mucosal cells
Enterocytes from the duodenum of wild-type or knockout mice were isolated by treatment of 8 cm duodenal segments with Type 1A collagenase (100 active U/mL) in oxygenated enterocyte isolation buffer (125 mM NaCl, 10 mM D-Fructose, 0.15 mM CaCl2, 30 mM Tris-HCl (pH 7.4)) at 37 °C for 15 minutes with shaking. At 15-minute intervals, duodenal segments were placed in fresh enterocyte isolation buffer, for a total of four cycles. Detached cells were centrifuged, washed in buffer, and RNA was isolated using RNA spin columns (Clontech). Pmca1 and Pmca4 mRNA was identified by RT-PCR using the following primers: Pmca1: 5′ primer: 5′- CGGAAAATACAGGAGAGCTATGG-3; 3′ primer: 5′-CTTTCCAAACACTGCTTCTCTTC-3′ (123 bp PCR product); Pmca4: 5′ primer: 5′-TGTGGGCAGTGAGTGAGAAT-3′; 3′ primer: 5′-TCAGGTTGGGTCATGAAGGT-3′ (110 bp PCR product). Primers for Rpl13a reference gene: 5′ primer: 5′-CCCTCCACCCTATGACAAGA-3′; 3′ primer: 5′-GCCCCAGGTAAGCAAACTT-3′.
Measurement of serum and urinary Ca and P, 1α,25(OH)2D, PTH, and FGF-23
These were measured using methods described previously [25].
Measurement of bone mineral density
Assessment of calcium transport in response to the administration of 1α,25(OH)2D3
Intestinal Ca transport was assessed by the everted gut sac method of Martin and DeLuca following the administration of 50 ng 1α,25(OH)2D3 in 50 μL ethanol intra-peritoneally 16 h prior to measurement [27].
Results
Pmca1EKO mice are viable and breed normally. We confirmed deletion of exon 2 in intestinal epithelium using two independent strategies shown in Fig. 2B and C. Panel 2B shows results obtained using primers 1f and 1r (Fig. 1). Since the binding site for primer 1r lies within the LoxP sites, and is predicted to be deleted as a result of Cre-recombinase activity in the intestine, no DNA product should be generated. This is what is observed in homozygous Pmca1EKO mice; heterozygotes display the appropriate sized 754 bp band. A second approach was used to confirm these results. Primer pair 2f/2r (Fig. 1) was used for PCR on genomic DNA isolated from the small intestine of a Pmca1EKO and a wild type Pmca1fl/fl mouse. Removal of the floxed exon 2 region results in a smaller 1.3 kb fragment in the Pmca1EKO tissue as a result of deletion of exon 2, whereas the expected fragment in the wild type Pmca1fl/fl is 3.4 kb as indicated. We confirmed the lack of expression of the Pmca1 mRNA in intestinal scrapings by performing RT-PCR with appropriate primers. In Pmca1EKO mice, Pmca1 mRNA expression is absent in enterocytes, whereas it is readily detected in Pmca1fl/fl mice (Fig. 2D). These data show that we have generated mice in which the Pmca1 gene has been disabled in enterocytes.
Pmca1EKO mice are born at a frequency lower than the predicted 25% Mendelian frequency. The mice are smaller than wild-type or Pmca1fl/fl mice at birth and into adulthood (Fig. 3). The results of measurements of serum and urinary minerals, serum calciotropic hormones and FGF-23, and bone mineral density are shown in Table 1. Whole body bone mineral density (BMD) and femoral BMD were decreased in Pmca1EKO mice compared to control mice. Spinal BMD tended to be lower but the results were not statistically significant. Serum calcium, phosphorus, parathyroid hormone, FGF-23, and 1α,25(OH)2D was similar in knockout and wild-type mice. Urinary calcium and phosphorus tended to be lower in Pmca1EKO mice compared to wild-type mice (P = 0.15 calcium and P = 0.08 for phosphorus). Following the administration of 1α,25(OH)2D3 intestinal calcium transport increased in wild-type mice but failed to do so in Pmca1EKO knockout mice.
Figure 3.
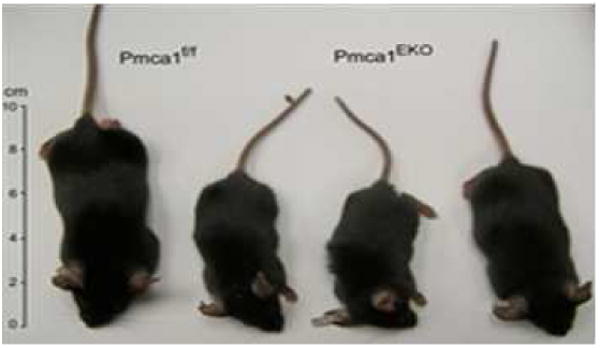
Pmca1EKO mice are stunted. Five week-old littermates of three Pmca1EKO animals and a Pmca1fl/lf control. Note the reduced size of the Pmca1EKO animals.
Table 1.
Bone mineral density, biochemical parameters and intestinal calcium transport in everted gut sacs of wild-type (Pmca1fl/fl) and knockout (Pmca1EKO) mice.
| WT (Pmca1fl/fl) | KO (Pmca1EKO) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SE | n | Mean | SE | n | P | |
| BMD Body (g/cm2) | 0.051 | 0.001 | 13 | 0.046 | 0.002 | 10 | 0.037 |
| BMD Spine (g/cm2) | 0.049 | 0.001 | 13 | 0.046 | 0.002 | 10 | 0.21 |
| BMD Femur (g/cm2) | 0.059 | 0.002 | 13 | 0.051 | 0.002 | 10 | 0.015 |
| PTH (pg/mL) | 120.4 | 26.3 | 7 | 135.2 | 12.1 | 7 | 0.62 |
| FGF-23 (pg/mL) | 103.6 | 17.6 | 8 | 131.8 | 15.4 | 8 | 0.25 |
| 1α,25(OH)2D (pg/mL) | 30.96 | 5.5 | 8 | 38.4 | 5.9 | 9 | 0.38 |
| Serum Ca (mM) | 2.14 | 0.14 | 7 | 2.07 | 0.1 | 9 | 0.69 |
| Serum phosphorus (P) (mM) | 2.8 | 0.1 | 8 | 2.9 | 0.1 | 9 | 0.46 |
| Urine volume (μL/24 h) | 788 | 129 | 8 | 1267 | 196 | 9 | 0.07 |
| Urine Ca (mg/24 h) | 0.026 | 0.003 | 8 | 0.040 | 0.01 | 8 | 0.15 |
| Urine P (mg/24 h) | 1.498 | 0.326 | 8 | 2.211 | 0.217 | 9 | 0.08 |
| Urine Ca/creatinine | 0.605 | 0.091 | 8 | 0.720 | 0.147 | 8 | 0.52 |
| Urine P/creatinine | 37.256 | 2.818 | 8 | 53.282 | 2.213 | 9 | 0.0004 |
| Intestinal 45Ca Transport (Inside/Outside) | 1.97 | 0.06 | 2 | 1.08 | 0.01 | 2 | 0.005 |
Discussion
The plasma membrane calcium pump is a widely distributed protein that plays an important role in cellular calcium homeostasis [7,28]. Four genes (Pmca1-4/Atp2b1-4 in mice) encode mammalian PMCA isoforms [29,30]. The mouse Pmca1 is ubiquitously expressed starting early in embryo development (day 9.5 post coitum), although its levels vary in different tissues and cell types [29,31]. By contrast, the other PMCA isoforms appear later in development and show a more restricted expression pattern [31,32]. PMCA2 and PMCA3 are most abundant in muscle and brain; PMCA2 is also highly expressed in secretory epithelial tissues such as lactating mammary glands [33]. Expression of PMCA4 is widespread, and recent evidence suggests that its major role may be in localized Ca signaling rather than in bulk Ca extrusion [34,35].
There is limited information concerning the physiological role of the various plasma membrane calcium pumps in vivo. Germ line deletion of Pmca1 in mice results in embryonic lethality [20,36]. Mice that are null for Pmca2 appear normal at birth [36,37,38,39,40,41]. By 10 days of age they have difficulty in maintaining their balance. Deafness becomes apparent, and it has been suggested that this isoforms of the PMCA maintains extracellular calcium concentrations around the hair bundles of the outer hair cells. Milk calcium excretion is also reduced in these mice. No data are available concerning the deletion of the Pmca3 in mice. Deletion of the Pmca4 gene results in male infertility possibly as a result of calcium overload and apoptotic cell death [20,36,42].
Given embryonic lethality [20,36] observed in mice with germ line deletion of Pmca1, we attempted to delete the Pmca1 in the intestine, a tissue in which we have previously shown PMCA1 to be present along the baso-lateral membranes of enterocytes of the proximal intestine [9]. In addition, Pmca1 is upregulated in the intestine in response to treatment with 1α,25(OH)2D3, suggesting an important role of this pump in active calcium transport [15].
We now demonstrate that we have successfully deleted the Pmca1 in the intestine using Cre-lox methods. Pmca1EKO mice are viable but are born at a reduced frequency. The precise reason for this is uncertain at the present time but suggests the presence of embryonic lethality, perhaps due to “leaky” villin-cre expression in very early embryogenesis. Additionally, we observe that Pmca1EKO mice are smaller than their wild-type littermates, once again suggesting an important role for the Pmca1 in fetal development. Further experiments will be necessary to understand the role of this important pump during embryonic development.
Pmca1EKO mice have a reduced bone mineral density compared to wild-type littermates suggesting a failure of absorption of Ca from the intestine resulting in reduced bone mineral deposition. Importantly, we found a lack of response of the intestine in Pmca1EKO mice to exogenously administered 1α,25(OH)2D3. These findings are supported by a tendency towards lower urinary calcium concentrations in Pmca1EKO mice. The mean serum Ca concentration of Pmca1EKO mice was lower than in Pmca1fl/fl (2.07 vs. 2.14 mM) although differences were not statistically significant. In accordance with the lower serum calcium, PTH, and 1α,25(OH)2D concentrations tended to be higher in Pmca1EKO mice compared to wild-type mice. The urinary phosphorus concentration normalized for creatinine was higher in Pmca1EKO mice compared with Pmca1fl/fl mice (P<0.0004). Collectively, these findings are consistent with mild secondary hyperparathyroidism which may have contributed to the reduced BMD in addition to the intestinal calcium malabsorption manifest as a failure to respond to exogenous 1α,25(OH)2D3. The data thus underline the importance of Pmca1 as the major Ca extrusion system in 1α,25(OH)2D3-responsive transcellular Ca absorption. Similar concentrations of serum minerals and regulatory hormones in Pmca1EKO mice are not surprising since the animals were fed a normal calcium diet. When dietary calcium is normal or high, a significant proportion of calcium absorption in the intestine occurs by passive para-cellular mechanisms, and hence would not be expected to change as a result of deletion of the Pmca1.
Our findings are consistent with those of Liu et al, who found that ablation of enterocyte 4.1R protein, a basolateral enterocyte protein which binds to and reduces Pmca1b expression, reduced intestinal calcium transport and BMD and caused secondary hyperparathyroidism [21]. Of note, in 4.1R-/- mice, 4.1R protein expression was additionally absent in the brain, kidney proximal tubules and erythrocytes resulting in neurobehavioral deficits and hemolytic anemia [43,44]. Our findings are consistent with a role of the Pmca1 in intestinal calcium transport and overall calcium homeostasis. Future studies will investigate the importance of the intestinal Pmca1 in calcium homeostasis in conditions of dietary calcium restriction and during aging.
Supplementary Material
Highlights.
The Pmca1 was deleted in the intestine to examine its role in Ca homeostasis in vivo
Mice with intestinal Pmca1 deletion are born with reduced frequency and are stunted at birth
At two months of age mice with intestinal Pmca1 deletion have reduced bone mineral density
1α,25(OH)2D3-induced Ca transport is impaired in mice with intestinal Pmca1 deletion
Acknowledgments
Supported in part by NIH grant DK066013.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tebben PJ, Kumar R. The Hormonal Regulation of Calcium Metabolism. In: Alpern RJ, Moe OW, Caplan M, editors. Seldin and Giebisch's The Kidney, Physiology and Pathophysiology. Academic Press; New York: 2013. pp. 2273–2330. [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 3.Wasserman RH, Corradino RA, Fullmer CS, Taylor AN. Some aspects of vitamin D action; calcium absorption and the vitamin D-dependent calcium-binding protein. Vitamins & Hormones. 1974;32:299–324. doi: 10.1016/s0083-6729(08)60017-5. [DOI] [PubMed] [Google Scholar]
- 4.Wasserman RH, Fullmer CS. Vitamin D and intestinal calcium transport: facts, speculations and hypotheses. J Nutr. 1995;125:1971S–1979S. doi: 10.1093/jn/125.suppl_7.1971S. [DOI] [PubMed] [Google Scholar]
- 5.Dimke H, Hoenderop JG, Bindels RJ. Molecular basis of epithelial Ca2+ and Mg2+ transport: insights from the TRP channel family. The Journal of physiology. 2011;589:1535–1542. doi: 10.1113/jphysiol.2010.199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserman RH, Smith CA, Brindak ME, De Talamoni N, Fullmer CS, Penniston JT, Kumar R. Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992;102:886–894. doi: 10.1016/0016-5085(92)90174-w. [DOI] [PubMed] [Google Scholar]
- 7.Brini M, Cali T, Ottolini D, Carafoli E. The plasma membrane calcium pump in health and disease. The FEBS journal. 2013 doi: 10.1111/febs.12193. [DOI] [PubMed] [Google Scholar]
- 8.Brini M, Carafoli E. Calcium pumps in health and disease. Physiological reviews. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 9.Borke JL, Caride A, Verma AK, Penniston JT, Kumar R. Cellular and segmental distribution of Ca2(+)-pump epitopes in rat intestine. Pflugers Archiv : European journal of physiology. 1990;417:120–122. doi: 10.1007/BF00370781. [DOI] [PubMed] [Google Scholar]
- 10.Philipson KD, Nicoll DA. Sodium-calcium exchange: a molecular perspective. Annual review of physiology. 2000;62:111–133. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- 11.Meyer MB, Zella LA, Nerenz RD, Pike JW. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem. 2007;282:22344–22352. doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AN, Wasserman RH. Vitamin D-induced calcium-binding protein: comparative aspects in kidney and intestine. The American journal of physiology. 1972;223:110–114. doi: 10.1152/ajplegacy.1972.223.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Wasserman RH, Brindak ME, Meyer SA, Fullmer CS. Evidence for multiple effects of vitamin D3 on calcium absorption: response of rachitic chicks, with or without partial vitamin D3 repletion, to 1,25-dihydroxyvitamin D3. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:7939–7943. doi: 10.1073/pnas.79.24.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserman RH, Taylor AN. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966;152:791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- 15.Cai Q, Chandler JS, Wasserman RH, Kumar R, Penniston JT. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1345–1349. doi: 10.1073/pnas.90.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SM, Riley EM, Meyer MB, Benkusky NA, Plum LA, DeLuca HF, Pike JW. 1,25-Dihydroxyvitamin D3 Controls a Cohort of Vitamin D Receptor Target Genes in the Proximal Intestine That Is Enriched for Calcium-regulating Components. The Journal of biological chemistry. 2015;290:18199–18215. doi: 10.1074/jbc.M115.665794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutuzova GD, Sundersingh F, Vaughan J, Tadi BP, Ansay SE, Christakos S, Deluca HF. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19655–19659. doi: 10.1073/pnas.0810761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieben L, Benn BS, Ajibade D, Stockmans I, Moermans K, Hediger MA, Peng JB, Christakos S, Bouillon R, Carmeliet G. Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone. 2010;47:301–308. doi: 10.1016/j.bone.2010.04.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. The Journal of biological chemistry. 2004;279:33742–33750. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Weng H, Chen L, Yang S, Wang H, Debnath G, Guo X, Wu L, Mohandas N, An X. Impaired intestinal calcium absorption in protein 4.1R-deficient mice due to altered expression of plasma membrane calcium ATPase 1b (PMCA1b) The Journal of biological chemistry. 2013;288:11407–11415. doi: 10.1074/jbc.M112.436659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. The Journal of biological chemistry. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 23.Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. The Journal of cell biology. 2006;175:505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battle MA, Bondow BJ, Iverson MA, Adams SJ, Jandacek RJ, Tso P, Duncan SA. GATA4 is essential for jejunal function in mice. Gastroenterology. 2008;135:1676–1686 e1671. doi: 10.1053/j.gastro.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, Westendorf JJ, Singh RJ, Kumar R. Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A. 2013;110:6199–6204. doi: 10.1073/pnas.1221255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGee-Lawrence ME, Bradley EW, Dudakovic A, Carlson SW, Ryan ZC, Kumar R, Dadsetan M, Yaszemski MJ, Chen Q, An KN, Westendorf JJ. Histone deacetylase 3 is required for maintenance of bone mass during aging. Bone. 2013;52:296–307. doi: 10.1016/j.bone.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin DL, DeLuca HF. Influence of sodium on calcium transport by the rat small intestine. The American journal of physiology. 1969;216:1351–1359. doi: 10.1152/ajplegacy.1969.216.6.1351. [DOI] [PubMed] [Google Scholar]
- 28.Lopreiato R, Giacomello M, Carafoli E. The plasma membrane calcium pump: new ways to look at an old enzyme. The Journal of biological chemistry. 2014;289:10261–10268. doi: 10.1074/jbc.O114.555565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiological reviews. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 30.Strehler EE, Caride AJ, Filoteo AG, Xiong Y, Penniston JT, Enyedi A. Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Annals of the New York Academy of Sciences. 2007;1099:226–236. doi: 10.1196/annals.1387.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zacharias DA, Kappen C. Developmental expression of the four plasma membrane calcium ATPase (Pmca) genes in the mouse. Biochimica et biophysica acta. 1999;1428:397–405. doi: 10.1016/s0304-4165(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 32.Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Current molecular medicine. 2004;4:323–335. doi: 10.2174/1566524043360735. [DOI] [PubMed] [Google Scholar]
- 33.Reinhardt TA, Filoteo AG, Penniston JT, Horst RL. Ca(2+)-ATPase protein expression in mammary tissue. American journal of physiology Cell physiology. 2000;279:C1595–1602. doi: 10.1152/ajpcell.2000.279.5.C1595. [DOI] [PubMed] [Google Scholar]
- 34.Strehler EE, Filoteo AG, Penniston JT, Caride AJ. Plasma-membrane Ca(2+) pumps: structural diversity as the basis for functional versatility. Biochemical Society transactions. 2007;35:919–922. doi: 10.1042/BST0350919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oceandy D, Mohamed TM, Cartwright EJ, Neyses L. Local signals with global impacts and clinical implications: lessons from the plasma membrane calcium pump (PMCA4) Biochimica et biophysica acta. 2011;1813:974–978. doi: 10.1016/j.bbamcr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochemical and biophysical research communications. 2004;322:1192–1203. doi: 10.1016/j.bbrc.2004.07.156. [DOI] [PubMed] [Google Scholar]
- 37.Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. The Journal of biological chemistry. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- 38.Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, Lim D, Shull GE, Gasparini P, Brini M, Mammano F, Carafoli E. A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1516–1521. doi: 10.1073/pnas.0609775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K, Kitamura K. A point mutation in a plasma membrane Ca(2+)-ATPase gene causes deafness in Wriggle Mouse Sagami. Biochemical and biophysical research communications. 1999;261:773–778. doi: 10.1006/bbrc.1999.1102. [DOI] [PubMed] [Google Scholar]
- 40.Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nature genetics. 1998;19:390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- 41.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. The Journal of biological chemistry. 2004;279:42369–42373. doi: 10.1074/jbc.M407788200. [DOI] [PubMed] [Google Scholar]
- 42.Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP, Neyses L. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. The Journal of biological chemistry. 2004;279:28220–28226. doi: 10.1074/jbc.M312599200. [DOI] [PubMed] [Google Scholar]
- 43.Shi ZT, Afzal V, Coller B, Patel D, Chasis JA, Parra M, Lee G, Paszty C, Stevens M, Walensky L, Peters LL, Mohandas N, Rubin E, Conboy JG. Protein 4.1R-deficient mice are viable but have erythroid membrane skeleton abnormalities. The Journal of clinical investigation. 1999;103:331–340. doi: 10.1172/JCI3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walensky LD, Shi ZT, Blackshaw S, DeVries AC, Demas GE, Gascard P, Nelson RJ, Conboy JG, Rubin EM, Snyder SH, Mohandas N. Neurobehavioral deficits in mice lacking the erythrocyte membrane cytoskeletal protein 4.1. Current biology : CB. 1998;8:1269–1272. doi: 10.1016/s0960-9822(07)00536-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


