Abstract
Macrophages are innate immune cells with evolutionarily conserved functions in tissue maintenance and host defense. As such, macrophages are among the first hematopoietic cells that seed developing tissues, and respond to inflammatory insults by in situ proliferation or de novo differentiation from monocytes. Recent studies have revealed that monocyte-derived tumor-induced macrophages represent a major tumor-associated macrophage population, which can further expand following their differentiation in tumors. Compared to tissue-resident tumor-associated macrophages, these newly differentiated cells are phenotypically distinct, and likely play a unique role in tissue dysregulation and immune modulation in cancer. These findings imply that tumor growth elicits a specific innate immune response. In this review, we explore the different routes of macrophage seeding and maintenance in tissues during steady state and inflammation and how these principles underlie the responses observed during tumor development. In addition, we highlight the relationship between the origin and function of macrophages in different settings and how this knowledge may be used to create new opportunities for cancer immunotherapy.
Understanding Macrophage Origins in the Tumor Microenvironment
Macrophages, first identified by Metchnikoff in the late 19th century, are tissue-resident innate immune cells present in most metazoan species. Characterized by their ability to engulf dying cells, noxious substances, and microbes, macrophages control tissue development and homeostasis as well as host defense against pathogens1,2. Over the last few decades, a great deal of research has been directed towards uncovering the distinct identities, origins, and functions of macrophages in steady state tissues and during infection and sterile inflammatory conditions, including cancer. Macrophage populations are present in all solid tumors, however, the details regarding macrophage origin and function in different tumor types are still being defined. This review focuses on what we have learned so far about the basic principles of macrophage ontogeny during homeostasis and inflammation and how this framework may apply to the understanding of macrophage origins during the unique setting of malignancy. Furthermore, we discuss the implications that lineage may have on tumor-associated macrophage function within the tumor microenvironment.
Macrophage Ontogeny
Macrophage origins during steady state: embryonic precursor- and monocyte-derived macrophages
Until recently, tissue macrophages were thought to be derived entirely from the adult hematopoietic system, originating from stem cells in the bone marrow and differentiating via monocytic precursors in the blood. However, recent studies in genetically engineered mouse models have shown that many tissue-resident macrophage populations do not require input from the blood, but rather maintain their populations through proliferation3,4. Interestingly, the need for replenishment via monocytes seems to be tissue specific, as macrophages in the dermis5,6, intestine7,8, mammary gland9, and a subpopulation of the heart10 require blood-borne precursors to maintain their pool, while most other tissues maintain their populations independently from adult monocytes11. While the exact origins of certain tissue macrophages have been controversial, the current consensus is that macrophages can originate from both early embryonic precursors and adult hematopoietic stem cells (HSCs) in a context-dependent manner12,13 (Figure 1).
Figure 1. Tissue-resident macrophage origins.
Macrophages arise from multiple sources during embryonic development. In mice, at embryonic day (E) 7–7.5 primitive hematopoiesis begins in the yolk sac with the emergence of “early” erythro-myeloid progenitors (EMPs) from the yolk sac blood islands. These precursors give rise to yolk sac macrophages, which colonize embryonic tissues including the head after establishment of blood circulation (E8.5). In the brain, yolk sac macrophages differentiate into microglia without a monocytic intermediate. The second hematopoietic wave, transient definitive hematopoiesis, is marked by “late” EMPs arising from the yolk sac hemogenic endothelium (approximately E8.5) and traveling to the fetal liver where they expand and give rise to fetal monocytes. These fetal monocytes begin to enter the circulation at E11.5–12.5 and seed the majority of tissue-resident macrophage populations. The final “definitive” wave of hematopoiesis begins when hematopoietic stem cells (HSCs) and their precursors in the para-aortic splanchnopleura (P-sp) and the aorta, gonads, and mesonephros (AGM) regions emerge and seed the fetal liver (E10.5). At E13.5–14.5, subsequent to the first wave of “late” EMP-derived fetal monocytes entering the circulation, pre-HSCs and HSCs give rise to fetal monocytes (and potentially tissue-resident macrophages, dashed arrow), before migration to the spleen and bone marrow. Here, HSCs give rise to all other lineages of the hematopoietic system, including “adult” monocytes that differentiate into a limited number of tissue-resident macrophage populations.
Mammalian hematopoiesis occurs in several temporally and spatially regulated waves, culminating with the generation of HSCs in the bone marrow14. During murine embryonic development, the first hematopoietic progenitors appear in the extra-embryonic yolk sac at embryonic day (E) 7–7.5, a phase termed “primitive” hematopoiesis. It was recently proposed that multi-lineage erythro-myeloid progenitors (EMPs) arise from the yolk sac blood islands during this period and are termed “early” EMPs to distinguish these cells from EMPs arising later in hematopoiesis15,16. Primitive hematopoiesis is followed by the “transient definitive” stage, characterized by the emergence of EMPs (“late” EMPs15,16) and lympho-myeloid progenitors (LMPs)17–19. Once circulation is established at E8.5, these progenitors seed the fetal liver, expand, and generate fetal monocytes. Subsequently, “definitive” hematopoiesis begins, first in the para-aortic splanchnopleura (P-sp) region and then in the aorta, gonads, and mesonephros (AGM) region18. These pre-HSCs and mature HSCs then colonize the fetal liver around E10.5, establishing this organ as the major site of hematopoiesis prior to migration of HSCs into the spleen and bone marrow14,20,21. In the midst of these successive developmental waves, both yolk sac-derived and P-sp/AGM-derived hematopoietic progenitor cells, and likely precursors from other hematopoietic sites such as the placenta and umbilical cord, colonize the fetal liver18,20, with fetal monocytes of different sources entering the bloodstream from E11.5 to 16.5. This complexity has obscured the origin of fetal monocytes and thus, tissue macrophages, until the recent generation of elegant fate mapping models.
The Runx1MerCreMer fate mapping mouse first showed that a population of tissue-resident macrophages, microglia, were seeded early in embryonic development by yolk sac precursors, rather than circulating monocytes22. This finding was confirmed and extended to most tissue-resident macrophages using the Csf1rMercreMer fate mapping mouse23. Importantly, it was shown that many tissue-resident macrophages are still present in mice lacking Myb23, a factor required for definitive hematopoiesis24. However, the loss of Csf1rMercreMer reporter labeling in late embryonic development15 and in many tissues of adult mice10 hinted that yolk sac precursors may be replaced by another unlabeled precursor, likely arising from a separate hematopoietic wave. In a subsequent study, the origin of these primitive macrophages was proposed to be EMPs derived from the yolk sac, but expanded in the fetal liver25. This origin was further clarified by a study combining in vivo yolk sac macrophage depletion with multiple fate mapping systems, to suggest that most tissue-resident macrophages are derived from “late” EMPs in the yolk sac that first differentiate into fetal monocytes in the liver and then migrate to their respective tissues15. This is in contrast to microglia, which originate from primitive yolk sac macrophages (thought to be derived from “early” EMPs15,16) without progressing through a monocyte intermediate10,15,22,23,25–27. Furthermore, while yolk sac macrophages throughout the embryo are gradually replaced by circulating fetal monocytes during development, the macrophage population in the brain remains entirely untouched by fetal monocytes. This differential precursor requirement for microglia is likely due to the establishment of the blood brain barrier during embryonic development (E13.5) and inaccessibility of the tissue to circulating precursors28. Complementary results were found in a separate study using the KitMercreMer fate mapping system, although the precise precursor populations (“early” vs. “late” EMPs) were not identified27. Taken together, these studies suggest that microglia are the only tissue-resident macrophage population derived from the yolk sac without a monocyte intermediate. Furthermore, fetal monocytes generated in the fetal liver (but originating from the yolk sac) give rise to the majority of tissue macrophages, independently of HSCs16 (Figure 1).
Macrophage origins during inflammation: in situ expansion versus monocyte recruitment
A diverse array of challenges result in proliferation of tissue-resident macrophages to restore or increase the macrophage pool. For example, macrophage depletion via diphtheria toxin or clodronate-loaded liposomes results in proliferation of bone marrow, splenic red pulp, and alveolar macrophages29. Langerhans cells also demonstrate proliferation after depletion, forming local clonal clusters30,31. Additionally, proliferation is observed to repopulate tissue-resident macrophages during more physiological macrophage depletion such as influenza infection29 or zymosan-induced peritonitis32,33. Conditions of tissue stress, including pregnancy34, central nervous system injury and neurodegeneration35, as well as atopic dermatitis36, also result in local proliferation of macrophage populations. Contrary to most infections which mobilize monocytes (see below), helminth infection promotes dramatic macrophage proliferation37. Furthermore, proliferation is not a feature only associated with tissue-resident macrophages, but can also be characteristic of monocyte-derived cells, such as during zymosan-induced peritonitis32, pancreatic injury38, ultraviolet irradiation-induced skin damage39, and perhaps atherosclerosis as well40.
Although many tissues do not require input from monocytes at steady state, monocytes are clear contributors to the myeloid cell pool during inflammatory conditions such as infection41. Monocytes are comprised of two distinct populations that differ both phenotypically and functionally. These subsets were originally defined in mice by either high or low expression of the chemokine receptor CX3CR142,43. Further characterization defined the CX3CR1hi population as Ly6CloCCR2− “patrolling” monocytes (CD14+CD16++ in human) and the CX3CR1lo population as Ly6ChiCCR2+ “inflammatory” monocytes (CD14++CD16− in human). These definitions were based on initial observed differences in recruitment and effector functions during acute challenge and homeostasis, although it is now clear that “inflammatory” monocytes can be recruited in the absence of overt inflammation and can also serve anti-inflammatory functions. Nevertheless, “inflammatory” monocyte recruitment during challenge is likely due to the wide array of chemokine receptors expressed by these cells, making them highly sensitive to signals in damaged or infected tissues42. Patrolling monocytes were first defined as anti-inflammatory due to their contribution to tissue repair in the myocardium44 and rare extravasation into tissues45. However, with the discovery that Ly6Clo monocyte differentiation requires the transcription factor Nr4a1/Nur7746, genetic tools allowed closer investigation of this cell subset. Evidence now shows that these cells are “housekeepers” of the vasculature, scavenging the luminal side of vessels and promoting the disposal of aged endothelial cells47. They are also involved in immune complex uptake through their abundant expression of Fc receptors48. Furthermore, it has been proposed that this CX3CR1hiLy6Clo population may not make up a second bona fide circulating monocyte subset, but instead could represent a terminally differentiated blood-resident macrophage population49. Indeed, CX3CR1hiLy6Clo monocytes can be derived from CX3CR1loLy6Chi monocytes during steady state, suggesting their terminal differentiation50.
Monocyte recruitment is also a central aspect of non-infectious inflammatory states, even in tissues that normally maintain their macrophage populations independently of monocytes. For example, monocytes contribute minimally to many tissue macrophage populations, including those in the splenic red pulp29. However, during hemolysis and erythrocyte damage, monocytes significantly contribute to the red pulp macrophage pool51. Similarly, during steady state conditions in the heart, monocytes only contribute to a small subset of tissue macrophages, but after macrophage depletion or cardiac inflammation, monocytes contribute to all known cardiac macrophage populations10. In addition, stromal macrophages in the pancreas are largely derived from embryonic precursors23,52, yet pancreatic injury results in profound monocyte recruitment38. Aging may also represent a non-infectious physiological state that requires monocyte recruitment to replenish the macrophage pool53. In the heart, increased monocyte input can be observed with age and it has been proposed that this new dependence on circulating monocytes is due to a loss of self-renewal capacity in tissue-resident macrophages3,53. Whether age-dependent changes are also relevant in other tissues remains to be examined. Inflammatory monocytes are also recruited to resolving tissue to assist in tissue remodeling and repair. While still derived from Ly6ChiCCR2+ monocytes, the gene expression signatures of these cells are anti-inflammatory54,55. There are several scenarios where this recruitment has been observed, including atherosclerosis56, spinal cord and skeletal muscle injury57,58, regression of fibrosis54, and allergic skin reactions59. These studies and others demonstrate a commonality of diverse inflammatory states: transient monocyte recruitment to macrophage compartments, even in tissues that normally maintain their macrophage pool independently of monocytic precursors.
Macrophage origins in tumor development: trTAMs versus tiTAMs
Tumor development represents a special challenge to the host, being a sterile insult yet broadly pathogenic. Macrophages constitute the dominant myeloid cell population in most solid tumors and studies of both human and mouse malignancies have associated macrophage density with tumor growth60–62. Experiments using both transplantation of fluorescently-labeled bone marrow63,64 and tracking of microsphere-labeled monocytes65 suggested a monocyte origin for tumor-associated macrophages (TAMs). However, in light of the recent findings that macrophages originate from dual origins, macrophage ontogeny in carcinogenesis has been revisited. To date, developmental origins of TAMs have been best studied in mouse tumor models of the breast and lung. In the polyoma middle T (PyMT) oncogene-driven mouse model of breast cancer, macrophages represent the dominant myeloid cell population9,66,67. The major TAM population (MHCIIhiCD11blo), as well as the mammary tissue-resident macrophage population (mammary tissue macrophages, MTMs, MHCIIhiCD11bhi), originate from Ly6ChiCCR2+ monocytes9. However, TAMs also expand their population through proliferation, and therefore require a lower monocyte input compared to MTMs, which show low levels of proliferation. These findings indicate that both monocyte infiltration and proliferation play a role in macrophage maintenance during tumor growth9. Furthermore, it was shown that this differentiation of TAMs from monocytes requires Notch signaling through the transcriptional regulator, recombination signal binding protein for the immunoglobulin kappa J (Rbpj)9. This study was the first to show a distinct monocyte differentiation pathway triggered by tumor development, however whether Notch signaling represents a monocyte to macrophage differentiation pathway in other tumor types has yet to be determined68. In another spontaneous breast cancer model, MMTV-Neu, two macrophage populations were identified, CD11bhiF4/80loMHCIIhi and CD11bloF4/80hiMHCIIint, and both were also found to be derived from monocytes, with the CD11blo population heavily dependent on proliferation69 akin to the findings in the MMTV-PyMT model. Importantly, TAM proliferation and a correlation between TAM numbers and levels of CCL2 (the ligand for CCR2 expressed on inflammatory monocytes) can be observed in human breast cancer patients, suggesting both proliferative capacity and monocytic origin of TAMs in human breast cancer70,71. While proliferation appears to be an important means of TAM maintenance, factors controlling TAM survival and retention within tumors necessitate further examination.
High macrophage density is also found in lung tumors, and similar to their counterparts in breast carcinomas, TAMs in the lung are monocyte-derived. In the Lewis lung carcinoma (LLC) model of non-small cell lung cancer, monocytes labeled with latex microspheres were found to differentiate into TAMs72. In the genetic KrasLSL-G12D/+p53fl/fl model, fluorescently tagged monocyte precursors were also found to differentiate into macrophages in developing tumors73. Furthermore, this study suggests that TAMs are derived from a splenic reservoir of monocytes through HSC accumulation in the spleen driven by Angiotensin II. However, in the LLC model, the bone marrow was found to be much more efficient at deploying monocytes during tumor development compared to the spleen, suggesting that the capacity of this organ to act as a reservoir of macrophage precursors may vary depending on the particular microenvironment74.
Whether TAM ontogeny in the breast and lung represents a general phenomenon or is specific to these tissues is an open question. Untransformed mammary tissue is constantly replenished by monocytes (our unpublished observations), however alveolar macrophages, which make up the major tissue-resident macrophage population in the lung, are seeded early in development and maintained by proliferation15,23,27,50,75,76. Despite the differential contribution of monocytes to these tissues at steady state, major TAM populations in both the breast and lung are monocyte-derived, suggesting this may be a general mechanism of tumor-elicited inflammation68. Indeed, it has been suggested that TAMs in murine hepatocellular carcinomas are also monocyte-derived77, although definitive experiments have yet to be done. Studies to uncover TAM origins in the brain are also in progress. As in other tumor types, gliomas, especially grade IV gliomas (glioblastoma multiforme) contain large numbers of macrophages. As we discussed above, microglia are thought to be the only population of tissue-resident macrophages that are derived exclusively from yolk sac macrophages without a monocyte intermediate. Interestingly, several studies using transplantation of fluorescently-labeled bone marrow suggest a significant proportion of the TAM population in gliomas is derived from monocytes63,78,79, again suggesting common TAM origins, independent of the steady state contribution of monocyte-derived macrophages. In addition, evidence for proliferation of TAMs in glioblastoma was implied by a study using a CSF-1R inhibitor to deplete TAMs79. While CSF-1R inhibition did not result in a decrease in the overall number of TAMs, a decrease in circulating CD11b+Ly6G− cells (likely monocytes) was observed, suggesting that maintenance of the TAM population after treatment was achieved via proliferation. Nonetheless, more rigorous examination of TAM origin and proliferative capacity in the brain awaits fate mapping studies and/or identification of reliable markers to distinguish monocyte-derived and tissue-resident TAMs.
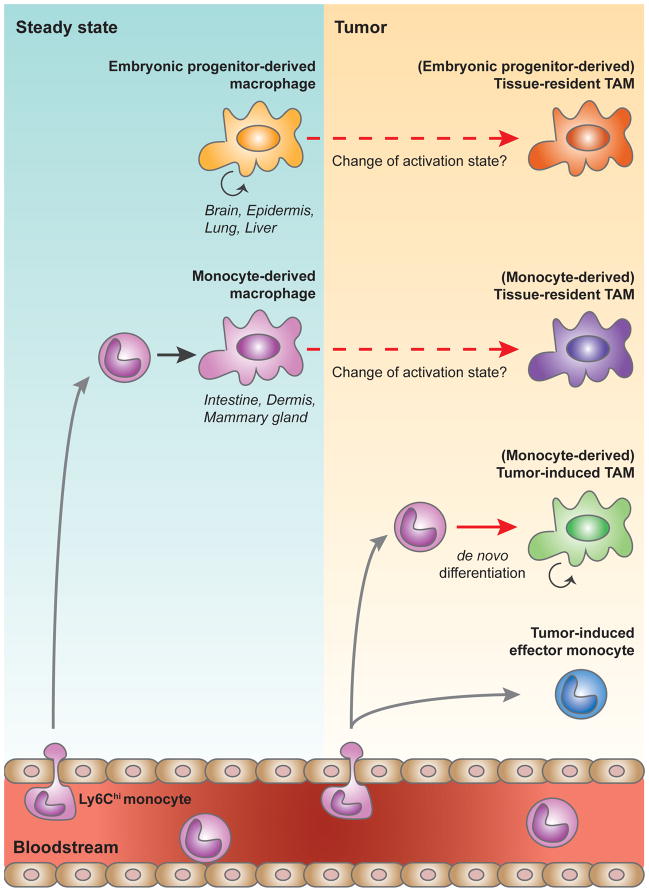
Together, these data suggest two possible developmental routes for TAMs in a given tissue: i) tissue-resident macrophages of either embryonic or monocytic origin that may undergo a change in phenotype/activation state during carcinogenesis (Tissue-resident TAMs, trTAMs), or ii) monocytes that undergo a distinct differentiation step to become macrophages in response to tumor growth (Tumor-induced TAMs, tiTAMs) (Figure 2). These two populations may both be present simultaneously in a particular tumor, or alternatively, trTAMs may dominate at early stages of tumor growth, while tiTAMs become prominent at later stages. In addition, monocytes that enter tumor tissues may undergo phenotypic changes in response to the tumor microenvironment without terminal differentiation into macrophages (Figure 2). This population of tumor-induced effector monocytes is observed in many tumor subtypes and is analogous in origin to effector monocytes found in other inflammatory conditions, such as infection. These monocytic cells in tumors have also been classified as a subpopulation of “myeloid-derived suppressor cells,” which compose a heterogeneous myeloid cell population with unique tumor-associated patterns of activation80. In addition to further elucidating the functional roles of TAMs derived from different origins, it will be important to investigate whether tumor-induced monocytes have redundant or distinct roles during tumor development.
Figure 2. Tumor-associated macrophage origins.
During steady state, tissue-resident macrophages are derived either from embryonic progenitors that seed tissues during development and maintain their population independently from blood-borne precursors in the adult, or from hematopoietic stem cells (HSC)-derived circulating monocytes. In developing tumors, tumor-associated macrophages (TAMs) can originate from tissue-resident macrophages of either embryonic or monocytic origin that may undergo a change in phenotype/activation state during carcinogenesis (Tissue-resident TAMs), or from monocytes that undergo a distinct differentiation step to become macrophages in response to tumor growth (Tumor-induced TAMs). Monocytes can also enter tumor tissue and undergo phenotypic changes without differentiation into macrophages (tumor-induced effector monocyte). Gray arrows signify migration, solid arrows (black and red) denote differentiation, and dashed arrows represent possible polarization/change in activation state.
Macrophage Function
Macrophage function in healthy tissues
In order to maintain tissue homeostasis, macrophages perform a number of trophic functions including removal of apoptotic cells and debris, regulation of angiogenesis, and production of components necessary for extracellular matrix (ECM) turnover and tissue remodeling81–83 (Figure 3). Most tissue-resident macrophage populations performing these functions in the absence of inflammation are derived from embryonic precursors rather than adult monocytes, yet the rationale for dual macrophage origins in certain settings is unclear. Tissue macrophage populations either require influx of precursors or proliferation to expand and maintain their populations29,35,36,50. These two distinct methods of macrophage maintenance beg the question: are different sources of macrophages functionally distinct due to developmental origin (“nature”) or do they serve largely overlapping functions that are instead dictated by environmental cues (“nurture”)? The former was recently proposed based on observations that monocyte-derived cells tend to produce higher levels of inflammatory mediators, through activation of the NF-κB pathway13,84,85. In support of the latter, recent studies investigating enhancer landscapes in different macrophage populations suggest that tissue environment may have a greater influence on macrophage function than developmental ontogeny86,87. In addition, macrophages exhibit distinct phenotypic and transcriptional profiles depending on the tissue type in which they reside, suggesting the importance of environmental signals in macrophage programming86–88.
Figure 3. Macrophage function during steady state and inflammation.
Macrophages serve diverse functions to maintain tissue homeostasis. During steady state, both monocyte-derived and embryonic progenitor-derived macrophages perform generalized homeostatic roles as well as functions specialized to specific demands of the tissue. During infection, macrophages participate in pathogen recognition and host defense responses. In most infectious settings, monocyte-derived macrophages are recruited to tissues to engage Type I responses. In contrast, a subset of pathogens elicits a Type II immune response, which is largely orchestrated by expansion of tissue-resident macrophages. Tumor-associated macrophages (TAMs) contribute to tumor growth through dysregulated homeostatic functions and modulation of the adaptive immune system. Monocyte-derived tumor-induced TAMs (tiTAMs) are largely responsible for these protumor functions. The role of tissue-resident TAMs (trTAMs) in tumors remains to be determined.
Alongside their general role in the maintenance of tissue homeostasis, macrophages perform specialized functions depending on the demands of the tissue (Figure 3). For example, in the brain, microglia contribute to learning and memory89 through postnatal synapse remodeling90,91. Splenic red pulp macrophages phagocytose erythrocytes and recycle heme in order to maintain iron homeostasis92,93. Peritoneal macrophages induce production of immunoglobulin A (IgA) in the intestine through communication with peritoneal B1 cells94. In addition to the identification of compartment-specific functions of macrophages, several of the environmental factors that regulate these functional programs have also been identified, such as heme induction of Spi-c in red pulp macrophages51,93 and retinoic acid induction of Gata6 in peritoneal macrophages94. Moreover, the importance of local signals in determining macrophage identity and function is demonstrated by experiments in which peritoneal macrophages transplanted into lungs of recipient mice acquire a gene expression profile similar to lung-resident alveolar macrophages87. Interestingly, these transferred cells do not convert completely to an alveolar macrophage phenotype87. The mechanisms that control the plasticity of some features and rigidity of others remain to be explored.
Macrophage control of immune defense
Macrophages participate in defense responses by their ubiquitous presence in tissues where they serve as sentinels, surveying their surroundings for signs of infection. Microbial signals are largely comprised of pathogen-associated molecular patterns (PAMPs) detected by pattern-recognition receptors (PRRs) including Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (Nod)-, leucine-rich repeat-containing receptors (NLRs), RIG-I-like receptors (RLRs), C-type lectin receptors (CLRs) and intracellular sensors of nucleic acids95,96. These innate signals can activate antimicrobial effector functions and in concert with other tissue-resident cells, including dendritic cells (DCs), macrophages help initiate inflammatory responses. This includes the active recruitment of other innate immune defenders and in many instances, activation of adaptive immunity.
Although not a unique feature among immune cells, macrophages demonstrate functional polarization in response to the local cytokine milieu97,98. A broad spectrum of activation states allows macrophages to respond and adapt to a wide array of stimuli and environmental conditions. When this inherent plasticity was first observed, macrophages were defined as two extremes, “classically activated” or “M1” macrophages, which are polarized in settings of local interferon gamma production during Th1 responses (Type I), or “M2” or “alternatively activated” macrophages responding to cytokines characteristic of Th2 responses, such as IL-4 and IL-13 (Type II)99. While meaningful to model macrophage polarization in vitro and in the context of certain acute infection settings in vivo, this strict bimodal polarization scheme is not observed in complex multi-stage immune responses and should be used to define macrophage activation with caution97,98. Importantly, however, this paradigm first allowed the plasticity of macrophage functional states to be appreciated.
In contrast to helminth infection, which results in tissue-resident macrophage proliferation in response to the Type II cytokine IL-437 (Figure 3), microbial infections typically engage Type I responses by promoting inflammatory monocyte recruitment41,100 (Figure 3). Upon entering tissues, monocytes can remain “immature” or can differentiate into mature cells, including macrophages, to perform various effector functions. Perhaps the best-characterized recruitment of inflammatory monocytes is their mobilization to the spleen during L. monocytogenes infection and differentiation into TNF- and iNOS-producing cells known as “Tip-DCs”101. These cells and their inflammatory monocytic progenitors are also known to be important for defense against Mycobacterium tuberculosis and Brucella melitensis infection102,103, as well as providing protection against a number of other pathogens both through direct effector mechanisms and activation of adaptive immune responses104–108. While important for host resistance, Type I responses resulting from monocyte infiltration can also contribute to tissue pathology, as is observed during Trypanosoma brucei and influenza virus infections109,110. The recruitment of monocytes in the settings described above suggests that tissue-resident macrophages are not sufficient for antimicrobial activity and monocyte recruitment and differentiation into effector cells is required for robust immune responses.
Macrophage function in tumors
Early on, it was believed that macrophages were present in high numbers at the tumor site to reject the growing tumor. However, it soon became clear that despite high levels of infiltration, macrophages are unable to stimulate an effective anti-tumor response and are instead generally associated with poor patient prognosis, with colorectal cancer as the exception to this trend111,112. High macrophage density is associated with poor survival in carcinoma of the thyroid, lung, breast, prostate, endometrium, uterus, brain and liver, identifying macrophages as promising therapeutic targets61,113. In fact, studies in mice show that macrophages help support tumor development by promoting angiogenesis, tissue invasion, and metastasis114 (Figure 3). In the PyMT mammary tumor model, genetic ablation of the macrophage survival factor CSF-1 results in reduced TAM density, delayed malignant progression, and decreased metastasis, demonstrating the contribution of macrophages to these processes67. TAMs also enhance tumor growth by regulating angiogenesis through direct and indirect production of VEGF115–117 and association with blood vessels118. In addition, TAMs promote tumor progression through production of growth factors and stimulation of tissue remodeling66,119. TAMs have also been found to modulate the adaptive immune response (T cell responses in particular), preventing effective antitumor immunity9,120,121 (Figure 3). These and other protumoral mechanisms of macrophages are discussed in greater detail elsewhere114,122,123.
As we uncover TAM origins in different tumor types, studies to clarify the contributions of distinct TAM populations to tumor progression are increasingly important. To begin addressing the different roles of tissue-resident and tumor-induced TAM populations, we used genetic models on the PyMT tumor background to delete either the MTM (trTAM) or TAM (tiTAM) populations. While inhibition of tiTAM differentiation resulted in decreased tumor growth, trTAM depletion was inconsequential9. These data suggest that in some tissues, the trTAM population may have a minor effect, if any, on tumor development. Instead, the tumor-induced inflammatory response characterized by monocyte infiltration may be the dominant mechanism of tumor promotion by TAMs. In addition, these data provide evidence that “nature,” or developmental origin, plays a significant role in determining TAM phenotype. If conditioning by the tumor microenvironment, or “nurture,” was solely responsible for TAM function, trTAMs would likely be the more dominant constituent of growing tumors, analogous to expansion of the tissue-resident macrophage population during helminth infection. It is also important to note that in the PyMT model, tiTAMs, unlike trTAMs, do not exhibit characteristics of “M2” or “alternatively activated” macrophages9, thus expanding on the recent notion that not all TAMs are “M2”-polarized and highlighting the heterogeneity of TAM populations61,68,123,124. Importantly, these findings also suggest that in some contexts M2 macrophages are not tumor-promoting9.
Although the functional capacity and inherent plasticity of monocytes makes them the dominant source of macrophages in many tumors, TAMs are not phenotypically homogeneous61,124, providing evidence that local environmental factors also have a role in TAM identity and function123. For example, in both transplantable and autochthonous mouse breast tumors, two phenotypically different TAM populations can be found, localized in either well-oxygenated or hypoxic regions65,72,125, despite both populations originating from inflammatory monocytes. Furthermore, this localization has functional consequences, as preventing migration of a tumor-promoting TAM population into a region of hypoxia diminishes its pro-tumor effects126. Similarly, blocking the association of a subpopulation of angiogenic TAMs with blood vessels inhibits their pro-angiogenic functions and limits tumor growth118. Phenotypically distinct TAM populations are also found localized in discrete regions of the tumor, such as the core or the periphery69,120,127–129. Additionally, heterogeneous TAM subsets have been observed in tumors of the lung65,72,130, pancreas63,131, and brain63,132, and gene expression analyses of TAM populations isolated from multiple tumor types demonstrate key transcriptional differences between the subsets9,65,127,130,133. Many of these differences are consistent between tumor types, suggesting redundant mechanisms of TAM programming. The impact of this heterogeneity across tumor types requires further investigation.
Therapeutic targeting of tumor-associated macrophages
Conventional cancer therapies, including surgery, radiation, and cytotoxic chemotherapy, aim to eradicate malignant cells. However, tumor cells do not grow in isolation and accessory cells within the tumor microenvironment must often be targeted for effective therapeutic outcomes134. This kind of therapy largely depends on the potential activity of the immune system and early preclinical and clinical data show encouraging results135. Immunotherapy acts in a fundamentally different way than classical therapies. Rather than destroying tumor cells directly, immunotherapy promotes tumor cell killing via the host’s own immune system. This result can be achieved directly via the main effectors of the immune system, cytotoxic CD8+ T cells (as is employed in checkpoint blockade strategies), or indirectly via targeting other immune cell types, such as macrophages. A current approach in TAM targeting that has shown efficacy is inhibition of CSF-1/CSF-1R signaling, as this axis is required for macrophage survival136,137. Blockade of this pathway depletes TAMs and stimulates CD8+ T cell responses, resulting in decreased tumor progression in mouse models of breast and cervical cancers138,139. In a model of glioblastoma, CSF-1R inhibition decreases tumor growth and even promotes regression of established tumors79. Surprisingly, this is not due to depletion of TAMs, but rather, phenotypic reprogramming, suggesting “reeducation” as a plausible means of achieving clinical success123,140. Additional methods of immune modulation have shown promise, including activation of CD40 to promote antitumor immunity through reprogramming of TAM function141. These treatments and others are discussed more thoroughly in recent reviews60,123,140,142.
Although not their intention, both radiation and chemotherapeutic agents are also known to influence the activity of other cell types found within the tumor mass. TAMs, in particular, are dramatically altered by these treatments, and can either enhance or limit the efficacy of therapeutic strategies depending on the treatment and tumor type60,143–145. In fact, clinical trials using CSF-1R blockade (mentioned above) were first initiated due to the increased efficacy of the chemotherapeutic paclitaxel when combined with CSF-1R inhibition138. A key challenge of many of these immunotherapeutics, both alone or in combination with chemotherapy, is to recognize “responders” prior to treatment. For example, in some patients, checkpoint blockade is curable and in others, there is no effect. What makes responders and non-responders different? Would non-responders to one therapy be better suited for another immunotherapeutic? Improved understanding of the immune mechanisms at work in different tumor subtypes may be the key to identifying the most effective therapy for each patient. Additionally, in light of the recent findings that TAMs are both monocyte-derived and have the capacity to proliferate, combination therapy to block both differentiation and proliferation may be required to effectively target these cells.
Concluding Remarks
Studies in mouse and man identify macrophages as potential therapeutic targets in the treatment of cancer, but several open questions remain (see Outstanding Questions). For instance, what are the individual contributions of tiTAMs and trTAMs to tumor growth in different tumor subtypes? In tumors where tiTAMs are present, what signaling pathways are responsible for their differentiation from monocytes? Moreover, are these pathways common between tumors in different tissues? The extent of TAM proliferation in different tumor types is also unclear; is expansion by proliferation a shared feature of TAMs? Another key question relates to the plasticity of TAMs; to what degree can TAMs be re-programmed or “re-educated”? A major challenge as we look forward is to investigate macrophage origins, differentiation, and maintenance in humans. There is a paucity of data in human cancer patients and the extent to which the mechanisms observed in mice are present in humans remains largely unknown. In addition, the heterogeneity and functional characteristics of human TAMs must be explored in order to better inform prognosis and treatment options. However, with the recent advances in the fields of macrophage biology and tumor immunology, we have every reason to be hopeful. Current research has contributed significantly to our understanding of cancer development and additional insight into the mechanisms by which TAMs accumulate in tumors and influence carcinogenesis will provide novel ways to harness the immune system in the fight against cancer.
Trends Box.
Macrophages originate from both embryonic precursors and adult monocytes.
Both proliferation and differentiation contribute to macrophage homeostasis.
Monocyte-derived tiTAMs are prominent among many tumors.
tiTAM differentiation can be controlled by discrete differentiation pathways.
TAM origin helps determine its function in the control of tumor development.
Outstanding Questions Box.
What are the individual contributions of tiTAMs and trTAMs and how do they impact each other in different tumor models?
What signaling pathways, such as Notch, promote the aberrant differentiation of monocytes into tiTAMs in different tumor types?
Is in situ proliferation a common feature of TAMs or is this capacity restricted to tumors of a particular tissue, such as the breast?
To what extent is the phenotype or transcriptional program of TAMs “reprogrammable” or “reversible”?
How relevant are the above mechanisms in human cancer patients?
Acknowledgments
We apologize to the authors whose work we could not cite due to space constraints. We thank R. Medzhitov for insightful discussions. This work was supported by the Cancer Research Institute Tumor Immunology Predoctoral Fellowship Training Grant (R.A.F), the CRI Irvington Postdoctoral Fellowship (R.A.F), the Geoffrey Beene Cancer Research Center of Memorial Sloan Kettering Cancer Center (M.O.L.), the National Institute of Health (RO1 CA198280-01 to M.O.L.), and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nature immunology. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self-renewal. Immunological reviews. 2014;262:56–73. doi: 10.1111/imr.12224. [DOI] [PubMed] [Google Scholar]
- 4.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 5.Jakubzick C, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamoutounour S, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Bain CC, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nature immunology. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zigmond E, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Franklin RA, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epelman S, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nature reviews. Immunology. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 13.Gomez Perdiguero E, Geissmann F. Myb-independent macrophages: a family of cells that develops with their tissue of residence and is involved in its homeostasis. Cold Spring Harbor symposia on quantitative biology. 2013;78:91–100. doi: 10.1101/sqb.2013.78.020032. [DOI] [PubMed] [Google Scholar]
- 14.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeffel G, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeffel G, Ginhoux F. Ontogeny of Tissue-Resident Macrophages. Frontiers in immunology. 2015;6:486. doi: 10.3389/fimmu.2015.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frame JM, McGrath KE, Palis J. Erythro-myeloid progenitors: “definitive” hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells. Blood cells, molecules & diseases. 2013;51:220–225. doi: 10.1016/j.bcmd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y, Yoder MC, Yoshimoto M. Lymphoid progenitor emergence in the murine embryo and yolk sac precedes stem cell detection. Stem cells and development. 2014;23:1168–1177. doi: 10.1089/scd.2013.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 20.Kieusseian A, Brunet de la Grange P, Burlen-Defranoux O, Godin I, Cumano A. Immature hematopoietic stem cells undergo maturation in the fetal liver. Development. 2012;139:3521–3530. doi: 10.1242/dev.079210. [DOI] [PubMed] [Google Scholar]
- 21.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 22.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 24.Mucenski ML, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 25.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kierdorf K, et al. Microglia emerge from erythromyeloid precursors via Pu.1-and Irf8-dependent pathways. Nature neuroscience. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 27.Sheng J, Ruedl C, Karjalainen K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 2015;43:382–393. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghigo C, et al. Multicolor fate mapping of Langerhans cell homeostasis. The Journal of experimental medicine. 2013;210:1657–1664. doi: 10.1084/jem.20130403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Davies LC, et al. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nature communications. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies LC, et al. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. European journal of immunology. 2011;41:2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- 34.Tagliani E, et al. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. The Journal of experimental medicine. 2011;208:1901–1916. doi: 10.1084/jem.20110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature neuroscience. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 36.Chorro L, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. The Journal of experimental medicine. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Gassen N, et al. Macrophage dynamics are regulated by local macrophage proliferation and monocyte recruitment in injured pancreas. European journal of immunology. 2015;45:1482–1493. doi: 10.1002/eji.201445013. [DOI] [PubMed] [Google Scholar]
- 39.Ginhoux F, et al. Langerhans cells arise from monocytes in vivo. Nature immunology. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins CS, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nature medicine. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews. Immunology. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 43.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Molecular and cellular biology. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of experimental medicine. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 46.Hanna RN, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nature immunology. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlin LM, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biburger M, et al. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011;35:932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Mildner A, Yona S, Jung S. A close encounter of the third kind: monocyte-derived cells. Advances in immunology. 2013;120:69–103. doi: 10.1016/B978-0-12-417028-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 50.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haldar M, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calderon B, et al. The pancreas anatomy conditions the origin and properties of resident macrophages. The Journal of experimental medicine. 2015;212:1497–1512. doi: 10.1084/jem.20150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molawi K, et al. Progressive replacement of embryo-derived cardiac macrophages with age. The Journal of experimental medicine. 2014;211:2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramachandran P, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3186–3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stables MJ, et al. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. The Journal of clinical investigation. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnold L, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of experimental medicine. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shechter R, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS medicine. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Egawa M, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38:570–580. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 60.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 64.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nature medicine. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 65.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer research. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 66.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. The Journal of experimental medicine. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franklin RA, Li MO. The ontogeny of tumor-associated macrophages: a new understanding of cancer-elicited inflammation. Oncoimmunology. 2014;3:e955346. doi: 10.4161/21624011.2014.955346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tymoszuk P, et al. In situ proliferation contributes to accumulation of tumor-associated macrophages in spontaneous mammary tumors. European journal of immunology. 2014;44:2247–2262. doi: 10.1002/eji.201344304. [DOI] [PubMed] [Google Scholar]
- 70.Bonapace L, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 71.Campbell MJ, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast cancer research and treatment. 2011;128:703–711. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laoui D, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer research. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 73.Cortez-Retamozo V, et al. Origins of tumor-associated macrophages and neutrophils. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shand FH, et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7771–7776. doi: 10.1073/pnas.1402914111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guilliams M, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. The Journal of experimental medicine. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schneider C, et al. Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nature immunology. 2014;15:1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 77.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 78.Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature medicine. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews. Immunology. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends in immunology. 2006;27:244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 82.Pollard JW. Trophic macrophages in development and disease. Nature reviews. Immunology. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wynn TA. Cellular and molecular mechanisms of fibrosis. The Journal of pathology. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature neuroscience. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 85.Saijo K, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gosselin D, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lavin Y, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature immunology. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 91.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chow A, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature medicine. 2013;19:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohyama M, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nature immunology. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annual review of immunology. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 97.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews. Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gordon S. Alternative activation of macrophages. Nature reviews. Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 100.Lauvau G, Chorro L, Spaulding E, Soudja SM. Inflammatory monocyte effector mechanisms. Cellular immunology. 2014;291:32–40. doi: 10.1016/j.cellimm.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 102.Copin R, De Baetselier P, Carlier Y, Letesson JJ, Muraille E. MyD88-dependent activation of B220-CD11b+LY-6C+ dendritic cells during Brucella melitensis infection. Journal of immunology. 2007;178:5182–5191. doi: 10.4049/jimmunol.178.8.5182. [DOI] [PubMed] [Google Scholar]
- 103.Peters W, et al. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nature immunology. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hohl TM, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell host & microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 107.Narni-Mancinelli E, et al. Inflammatory monocytes and neutrophils are licensed to kill during memory responses in vivo. PLoS pathogens. 2011;7:e1002457. doi: 10.1371/journal.ppat.1002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. The Journal of experimental medicine. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aldridge JR, Jr, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bosschaerts T, et al. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS pathogens. 2010;6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 112.Zhang QW, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PloS one. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends in immunology. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer research. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 117.Yeo EJ, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer research. 2014;74:2962–2973. doi: 10.1158/0008-5472.CAN-13-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mazzieri R, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 119.Gocheva V, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes & development. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Engelhardt JJ, et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer cell. 2012;21:402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Norian LA, et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer research. 2009;69:3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Seminars in immunopathology. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 123.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends in immunology. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 124.Lahmar Q, et al. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochimica et biophysica acta. 2015 doi: 10.1016/j.bbcan.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 125.Ruffell B, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Casazza A, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 127.Broz ML, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Egeblad M, et al. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Disease models & mechanisms. 2008;1:155–167. doi: 10.1242/dmm.000596. discussion 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer research. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 130.Pucci F, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 131.Mitchem JB, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer research. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szulzewsky F, et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PloS one. 2015;10:e0116644. doi: 10.1371/journal.pone.0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Squadrito ML, et al. miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell reports. 2012;1:141–154. doi: 10.1016/j.celrep.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 134.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 135.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 136.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Current opinion in immunology. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 137.Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G. Functional Relationship between Tumor-Associated Macrophages and Macrophage Colony-Stimulating Factor as Contributors to Cancer Progression. Frontiers in immunology. 2014;5:489. doi: 10.3389/fimmu.2014.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeNardo DG, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Strachan DC, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature medicine. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends in cell biology. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. The Journal of experimental medicine. 2015;212:435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nature reviews. Clinical oncology. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]