Abstract
Introduction
We examined prevalence of mild cognitive impairment (MCI) and dementia in the Atherosclerosis Risk in Communities (ARIC) Neurocognitive study.
Methods
Beginning in June, 2011, we invited all surviving ARIC participants to undergo cognitive, neurologic, and brain imaging assessments to diagnose MCI or dementia and assign an etiology for the cognitive disorder.
Results
Of 10,713 surviving ARIC participants (age range, 69–88 years), we ascertained cognitive diagnoses in 6471 in person, 1966 by telephone interviews (participant or informant), and the remainder by medical record review. The prevalence of dementia was 9.0% and MCI 21%. Alzheimer's disease (AD) was the primary or secondary etiology in 76% of dementia and 75% of MCI participants. Cerebrovascular disease was the primary or secondary etiology in 46% of dementia and 32% of MCI participants.
Discussion
MCI and dementia were common among survivors from the original ARIC cohort. Nearly 30% of the ARIC cohort received diagnoses of either dementia or MCI, and for the majority of these individuals, the etiologic basis was attributed to AD.
Keywords: Alzheimer's disease, Cerebrovascular disease, Dementia, Mild cognitive impairment, Epidemiology, Prevalence
1. Introduction
The prevalence of dementia has been extensively studied over the past 25 years [1]. Although there are fewer prevalence studies in African-Americans [2], [3], those studies are generally concordant with those from European or European-American cohorts. Of the many prevalence studies, only a few (Framingham [4], Honolulu [5]) recruited persons at middle age and examined them in later life for dementia. Among the major contributions of the investigations begun in midlife has been the documentation of the importance of midlife vascular risk factors and midlife cognition for later life risks for cognitive impairment.
In 1987–1989, the Atherosclerosis Risk in Communities (ARIC) study recruited a bi-racial group of 15,792 individuals, ages of 45 and 64 years, from four US communities in Maryland, North Carolina, Mississippi, and Minnesota. Cognitive assessments were introduced in the second ARIC examination in 1990–1992. A comprehensive dementia surveillance was performed at the fifth ARIC examination, the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS), in 2011–2013. The goals of ARIC-NCS were to study the relationships between midlife health and later-life cognitive impairment. We report here on the prevalence of clinical diagnoses of mild cognitive impairment (MCI), dementia, and their attributed etiologies. We also describe the methodological bases of ARIC-NCS to facilitate future ARIC-NCS publications.
2. Methods
2.1. Participants
The ARIC study was initiated in 1987. A random sample of individuals between 45 and 64 years were recruited from four communities: Washington County Maryland, Forsyth County North Carolina, Jackson Mississippi, and suburban Minneapolis Minnesota [6]. The overall goals of the ARIC study were to assess the role of midlife cardiovascular risk factors on health outcomes. An extensive cardiovascular examination was carried out at the initial visit (ARIC visit 1, “v1”). From the beginning of the study in 1987, participants were interviewed annually by phone, and discharge codes were recorded for all reported hospitalizations and all hospitalizations occurring within ARIC communities.
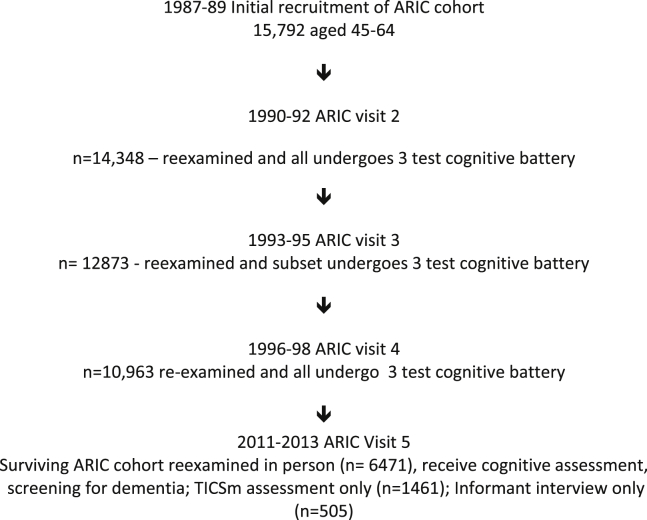
At ARIC visit 2 (v2) in 1990–1992, all participants underwent a 3-instrument cognitive assessment in addition to the entire cardiovascular assessment [7]. The 3-instrument cognitive testing was repeated in a subset of the ARIC cohort who underwent magnetic resonance imaging (MRI) in 1994–1995; in the entire ARIC cohort at visit 4 (1996–1999) [8], [9]; in a subset at the ARIC-MRI examination in 2004–2006 (Jackson and Forsyth County sites only); and again in the full cohort at the latest visit (visit 5). ARIC participants had APOE genotyping performed using the TaqMan assay (Applied Biosystems, Foster City, CA) once that assay became available. All surviving ARIC participants were invited to an in-person assessment at ARIC-NCS visit 5 between June, 2011, and August, 2013. If they were unable or unwilling to undergo an in-person assessment in clinic, they were offered an in-person assessment in their home or long-term care facility. If they were unwilling or unable to participate in any in-person assessment, a telephonic cognitive assessment was offered. If they were unable or unwilling to unable to undergo a telephonic cognitive assessment, and if cognitive impairment was suspected based on hospital discharge codes or annual telephone interviews, a family member was invited to participate in an interview about the participant's cognitive and functional status. Fig. 1 shows the flow of participants in ARIC.
Fig. 1.
Timeline for participants in the ARIC-NCS. Abbreviations: ARIC-NCS, Atherosclerosis Risk in Communities Neurocognitive study.
Institutional review boards of each ARIC center have approved the ARIC study protocol over its 28-year existence. Participants provided written informed consent for their participation at each study visit. Consent was obtained from a designated proxy along with the participant's assent in participants with a known diagnosis of dementia, impaired mental status (determined in the examination), or where our trained staff deemed that the participant had diminished capacity to provide informed consent.
This work was funded by the US National Institutes of Health who played no role in the preparation or final approval of this article.
2.2. Instruments
Participants who were evaluated in person at ARIC-NCS underwent a sequential evaluation. The following cognitive, behavioral, and functional assessments were completed for all participants at ARIC-NCS.
2.2.1. Core in-person assessments
-
1.
The centers for epidemiological studies-depression scale [10] was administered. A score of >8 was considered as suggestive of depression.
-
2.
The three ARIC cognitive instruments that have been administered beginning with ARIC visit 2 were the delayed word recall task, digit symbol substitution from the Wechsler Adult Intelligence Scale -Revised (WAIS-R), and a letter fluency task [7]. Normative data [7], [11] and longitudinal data [12], [13] have been presented.
-
3.
A neuropsychology test battery [11], logical memory immediate and delayed recall, and incidental learning from the Wechsler Memory Scale-III, trail making test parts A and B, WAIS-R digits span backward, Boston naming test, and animal naming. The mini-mental state examination (MMSE) [14] was also administered. Robust age, race, and education-specific normative data for most of the measures in the battery were developed within ARIC [11]. Comparable normative data for the Boston naming test and digit span backward were derived from data obtained from the National Alzheimer's Coordinating Center [15]. As previously reported [16], we constructed Z-scores for each of four cognitive domains (memory, psychomotor speed/executive functioning, language, and visuospatial) by averaging the scores of tests within each domain, subtracting the domain mean, and dividing by the domain standard deviation. A global composite Z-score was also derived from the three domain scores.
2.2.2. More detailed in-person assessments in a subset
For those with complete or near complete data from the mentioned in-person cognitive assessment, we used the following rules to determine who would be invited for more detailed assessments: (1) all participants who had undergone an MRI in 2004–2006 as part of the ARIC-MRI study; (2) all participants who had either (a) a low score on MMSE (<21 for whites and <19 for blacks) or (b) who scored <−1.5 Z in any of five cognitive domains and showed definite cognitive decline based on prior delayed word recall task, digit symbol substitution from the WAIS-R, and a letter fluency task scores administered at prior ARIC visits (i.e., lowest 10 percentile on any test or lowest 20th percentile on at least 2 tests); and (3) a random 10% sample of those who did not meet these criteria (i.e., those who were presumed cognitively normal).
-
1.
Those invited to more detailed evaluations underwent a neurologic examination performed by a study nurse who had been trained by one of the study neurologists (R.F.G.). The examination was used to complete a National Institutes of Health stroke scale [17] and a modified uniform Parkinson's disease rating scale (UPDRS) [18].
-
2.
The informant and the participant underwent a clinical dementia rating (CDR) interview. The CDR scale is a multidimensional rating tool meant to assess severity of impairment from cognitive normality to dementia [19]. It consists of queries in six domains and uses both participant and informant responses.
-
3.
The informant completed the functional activities questionnaire (FAQ), a 10-item instrument [20] that surveys ability to perform 10 common daily activities.
-
4.
The neuropsychiatric inventory (NPI) [21] was administered. It measures 12 neuropsychiatric symptom complexes.
-
5.
A Hachinski ischemic score [22] was also completed by the trained study personnel based on the history and examination of the participant.
-
6.
A focused neurologic history was also completed. It included questions about events and symptoms that could be linked to causes of dementia.
-
7.
All participants who were evaluated in the second stage who did not have contraindications were invited to have a brain MRI.
2.2.3. Assessment instruments for those not seen in person
For those not evaluated in person, but who agreed to a telephone interview, a telephonic instrument of cognitive status-modified (TICS-m) [23], [24] was administered to assess their cognition.
For those not evaluated in person and unable to undergo a telephone interview, an informant interview was completed primarily where there was suspicion of cognitive impairment or inadequate data to rule it out, more specifically if (1) follow-up interviewer suspected cognitive impairment, (2) follow-up interviewer reported hearing loss, (3) International Classification of Diseases, Ninth Revision (ICD-9) dementia discharge code at any point since the start of cohort surveillance, (4) self-report of dementia diagnosis on the follow-up interview (starting January 1, 2012), (5) proxy contacted for most recent follow-up interview, or if the participant was part of (6) an age comparable random sample of 100 participants not otherwise meeting the mentioned criteria.
2.3. Magnetic resonance imaging
Brain MRI was performed for the dual purposes of obtaining quantitative imaging features for analysis and supplementing the clinical etiologic diagnoses by documenting cerebrovascular lesions such as infarcts and white matter hyperintensities. As previously detailed [16], all ARIC-NCS participants selected for second-stage assessments without contraindications were offered a brain MRI. The MRI scans obtained were performed at each site on 3-T Siemens (various models) scanners using a common set of sequences that included a fluid-attenuated inversion recovery (FLAIR) sequences. For the diagnostic purposes in this report, the FLAIR scans were reviewed by both a neuroradiologist and the clinical diagnostic adjudicators. The two lesions of particular interest for a diagnosis of cerebrovascular etiology of a cognitive disorder were white matter hyperintensities and infarcts. Other structural abnormalities were also considered as they arose.
2.4. Diagnoses of cognitive normality, MCI, or dementia for participants undergoing in-person assessments
To standardize and describe the diagnoses of cognitively normal, MCI, or dementia, we devised an algorithm as a guide for diagnosis. The algorithm was based on the formulations of MCI and dementia laid out in the National Institute on Aging–Alzheimer's Association (NIA-AA) workgroups [25], [26] and Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) [27]. The algorithm used the following scores: MMSE, the sum of the six individual domain ratings in the CDR (“CDR sum of boxes”), z-scores from the current neuropsychological test battery, and change scores from the serial 3-test ARIC cognitive assessments and the FAQ.
The algorithmic approach generated 37 possible combinations of cognitive—functional profiles. The combinations of cognitive and functional profiles were divided into those that were internally consistent (concordant impression of cognitive status) and those that were internally inconsistent (discordant impressions of cognitive status). A listing of profiles is given in Appendix 1.
Concordant profiles for cognitive normality were defined as not meeting criteria for MCI or dementia. Specifically, the diagnosis of cognitive normality required that all ARIC-NCS cognitive domain scores were better than −1.5 Z and that there was an absence of decline below the 10 percentile on one test or below the 20th percentile on two tests in the serial ARIC cognitive battery. The CDR sum of boxes (the sum of the six domain scores in the CDR) was required to be ≤0.5 and the FAQ ≤5.
Concordant profiles for MCI were defined as at least one domain score worse than −1.5 Z, a CDR sum of boxes >0.5 and ≤3, an FAQ ≤5, and decline below the 10 percentile on one test or below the 20th percentile on two tests in the serial ARIC cognitive battery.
Concordant profiles for dementia were defined as >1 cognitive domain worse than −1.5 Z, a CDR sum of boxes >3 and FAQ >5, and decline below the 10 percentile on one test or below the 20th percentile on two tests in the serial ARIC cognitive battery. In addition, a low MMSE score (<21 for whites or <19 for African-Americans), even in the absence of more complete cognitive testing, was regarded as diagnostic of dementia.
Eight ARIC clinicians (four physicians: D.S.K., B.G.W., R.F.G., and Guy McKhann and four neuropsychologists: M.S.A., T.H.M., L.C., and Ola Selnes) comprised an expert dementia classification committee who reviewed materials on the examinations that had been collected at each ARIC site. One physician and one neuropsychologist independently reviewed each participant whose algorithmic profiles were concordant or discordant for MCI or dementia. The reviewers then rendered syndromic and etiologic diagnoses. A small number of profiles that were concordant for cognitive normality were also reviewed. Preliminary experience showed that participants whose algorithmic diagnosis was “normal or suspected normal cognition” were invariably viewed by the clinician reviewer panel as normal, and subsequently only one reviewer was assigned to review the data from those who were “normal or suspected normal” by the algorithm. For individuals in whom the two primary reviewers disagreed on cognitive syndrome, primary etiology, or cerebrovascular disease (CVD) etiology, a third reviewer (D.S.K. or M.S.A.) evaluated the participant's case materials and rendered a deciding vote.
2.5. Diagnoses of dementia or not dementia among participants not undergoing in-person assessments
For those ARIC participants who were alive at the time of ARIC-NCS but who declined to be seen in person, we used three strategies to establish diagnoses of dementia: the TICSm score, an informant interview, and review of ICD-9 hospital discharge diagnostic codes.
A diagnosis of dementia based on TICSm scores was made when the TICSm was ≤23. We adjusted for education around this cut point in the following way: 5 points were added to the score for those with <eighth grade education, 2 points were added for those who completed grades 8–10, and 2 points were subtracted for 4+ years of college. We did not use the TICSm to diagnose MCI, based on prior experience [24]; hence, participants who were not demented according to their TICSm scores were not considered in enumerations of MCI.
For those participants who did not complete either an in-person assessment or a TICSm but who were alive at the beginning of ARIC-NCS but had telephonic informant-based assessments, we diagnosed dementia if the sum of the informant ratings of the six domains of the CDR ≥3 and the FAQ >5. We did not attempt to diagnose MCI based on informant interviews alone.
The ARIC study routinely collected ICD-9 discharge diagnosis codes for all hospitalizations of as well as diagnostic codes from death certificates for all ARIC participants. These codes, available at the time of article preparation through the year 2012, were used to diagnose dementia for participants without other components of assessment. The codes are given in Appendix 2. No attempt was made to diagnose MCI using ICD-9 diagnostic codes.
2.6. Summary of syndromic diagnostic procedures
There was one source for a diagnosis of MCI (the examination including full neuropsychological assessment and CDR/FAQ/NPI). There were multiple sources for a dementia diagnosis: (1) full neuropsychological assessment only; (2) full neuropsychological assessment plus an FAQ, a CDR interview, and an NPI interview; (3) TICSm only; (4) informant interviews using the CDR; and (5) hospital discharge codes or diagnostic codes from death certificates.
2.7. Etiologic diagnoses
Etiologic diagnoses were also assigned by the panel of physicians and neuropsychologists, only for individuals who were seen in person and who were given diagnoses of MCI or dementia. The reviewers were allowed to diagnose more than one etiology but they were required to designate one diagnosis as primary.
2.7.1. Alzheimer disease–related MCI/dementia
The diagnosis of Alzheimer's disease (AD) as an etiologic diagnosis of MCI or dementia in ARIC as a primary diagnosis is a clinical one and is based on the presence of the cognitive syndrome that is not of abrupt onset and includes memory impairment and the absence of features of other specific diagnoses sufficient to cause the cognitive impairment, such as those detailed in the following. The criteria from the NIA-AA workgroups [25], [26] were followed.
2.7.2. CVD-related MCI/dementia
The diagnosis of CVD as an etiology was defined by an algorithm (Appendix 3) based in the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria [28] that used the following information: (1) history of stroke regardless of temporal relationship to cognitive decline; (2) history of stroke specifically temporally related to an abrupt onset of the cognitive disorder as operationalized by an affirmative answer to the question: “Was the cognitive impairment of abrupt onset following a stroke?”; (3) bilateral or multiple infarcts or extensive white matter hyperintensities on imaging; and (4) physical examination evidence of a typical stroke pattern of neurologic signs. If imaging was not available, a history of stroke and a history of abrupt onset were considered sufficient to generate an etiologic diagnosis of CVD-related cognitive impairment.
2.7.3. Lewy body disease–related MCI/dementia
A diagnosis of Lewy body disease (LBD) as a primary etiologic diagnosis was made based on published criteria [29], [30] when there were at least two of the following: (1) Parkinson's disease (diagnosed as per the history or suggested by the UPDRS; or on anti-Parkinson medications), (2) history of fluctuations in alertness or cognition, (3) dream enactment behavior reported by an informant, or (4) hallucinations. If only one of the features was present, the diagnosis of LBD could be applied only as a secondary diagnosis.
2.7.4. Other diagnoses
Other diagnoses are listed in Appendix 4 but will not be discussed in this report.
2.8. Analyses
Distribution of demographic characteristics is presented by assessment type and diagnosis. For participants with multiple assessments, the most reliable assessment type was reported, i.e., reviewer diagnosis was preferred over algorithmic diagnosis among those participants assessed in person, an in-person diagnosis was preferred over the TICSm and all assessments (including informant interview) were preferred over surveillance data. Continuous and categorical variables were presented as means (standard deviations) and percentages, respectively.
Diagnostic results were compared in participants with multiple assessment types: reviewer versus algorithmic diagnosis and TICSm versus in-person diagnosis. Combined assessments were compared with surveillance of hospitalizations and deaths. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% confidence intervals (CIs) were calculated treating the more reliable assessment as the gold standard.
Age-, race-, and sex-specific prevalence of MCI were reported for those ARIC participants who were seen in person. Baseline-category logit models were used to test hypotheses that the prevalence of MCI would rise with advancing age, would be similar in men and women, and would be greater in African-Americans than whites, controlling for age group, sex, and race. Age group-, race-, and sex-specific prevalence of dementia are reported for all ARIC participants alive at the start of ARIC-NCS. Logistic regression models were used to test similar hypotheses. Analyses were performed in SAS 9.3 (SAS Institute, Cary, NC, USA).
Distributions of etiologic diagnoses were compared between MCI and dementia diagnoses using a Pearson χ2 test.
3. Results
Table 1 lists the demographic characteristics of the inception cohort from ARIC v1 in 1987–1989 according to their vital status and assessment type at the time of ARIC-NCS in 2011–2013, and Fig. 1 shows the history before 2011 of the ARIC cohort. Forty-one percent (n = 6471) of the inception cohort were examined in person in ARIC-NCS. MRI scans were usable in 1906. Some information on cognitive status was available on another 3743 individuals through either TICSm (n = 1461), an informant interview (n = 505), or information from hospital discharge or death codes recorded during the time frame of ARIC-NCS (n = 1777). An additional 499 participants were not assessed and had no reported hospitalizations or death. Nearly one-third (n = 5031, 32%) of ARIC participants in the inception cohort had died before ARIC-NCS. The incidence of dementia among the decedents will be reported separately. Higher age at baseline and lower educational attainment characterized those who were deceased, or who, by virtue of suspicion of cognitive impairment, were assessed by informant telephonic interview or had at least one hospitalization during the period covered by ARIC-NCS. Lower ARIC v2 test scores from the ARIC serial test battery were observed in those who had died or who were assessed by informant telephone interview.
Table 1.
Demographic characteristics of ARIC inception cohort participants by vital status and assessment type
| Characteristic | Deceased before NCS | Completed NCS in-person assessment | TICS-m assessment | Informant call assessment | At least one hospitalization or death | No diagnostically useful information |
|---|---|---|---|---|---|---|
| n | 5031 | 6471 | 1461 | 505 | 1777 | 499 |
| % Women | 45 | 59 | 66 | 59 | 58 | 62 |
| % African-American | 33 | 24 | 24 | 29 | 26 | 27 |
| Age at visit 5∗ | — | 75.3 (5.2) | 76.7 (5.6) | 79.6 (5.7) | 77.9 (5.7) | 75.5 (5.5) |
| Age at visit 1∗ | 56.9 (5.5) | 52.1 (5.2) | 53.4 (5.5) | 56.3 (5.6) | 54.7 (5.7) | 52.4 (5.4) |
| < High school education, % | 34 | 15 | 19 | 36 | 27 | 20 |
| High School graduate or equivalent, % | 38 | 42 | 47 | 33 | 41 | 44 |
| ≥1-y college, % | 28 | 43 | 33 | 30 | 32 | 35 |
| APOE ε4: one allele, % | 30 | 27 | 26 | 36 | 29 | 27 |
| Two alleles, % | 3 | 2 | 2 | 5 | 3 | 2 |
| V2 DWRT Z-score∗ | −0.29 (1.04) | 0.17 (0.95) | 0.12 (0.98) | −0.14 (0.93) | −0.04 (0.98) | 0.13 (0.94) |
| V2 DSST Z-score∗ | −0.39 (1.01) | 0.25 (0.95) | 0.12 (0.92) | −0.28 (0.98) | −0.06 (0.92) | 0.16 (0.96) |
| V2 WFT Z-score∗ | −0.19 (1.03) | 0.15 (0.98) | 0.02 (0.96) | −0.11 (1.04) | −0.09 (0.95) | 0.03 (0.99) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities study; TICS-m, telephonic instrument of cognitive status-modified; APOE, apolipoprotein E.
NOTE. Data are missing for the following covariates: education (n = 26); APOE ε4 (n = 680); DWRT (n = 1582); DSST (n = 1632); and WFT (n = 1602). The columns are mutually exclusive and hierarchically defined.
Mean (standard deviation).
Demographic characteristics by MCI and dementia diagnosis are presented in Table 2 according to the assessment method. Among those evaluated in-person, the expected relationships with lower educational attainment, carriage of an apolipoprotein E (APOE) ε4 allele, and lower scores on ARIC v2 cognitive testing were observed in those with dementia. These relationships were not as strong in persons with dementia identified through means other than in-person assessment, probably reflecting the reasons that an in-person assessment was not completed and greater diagnostic uncertainty.
Table 2.
Distribution of participant characteristics by assessment type and diagnosis; ARIC-NCS study
| Characteristic | Completed ARIC-NCS in-person assessment (n = 6471) |
TICS-m assessment (n = 1461) |
Informant call assessment (n = 505) |
Surveillance (n = 2276) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | MCI | Dementia | Normal | Dementia | Normal | Dementia | Normal | Dementia | |
| Overall | 4743 | 1371 | 342 | 1361 | 100 | 169 | 336 | 2089 | 187 |
| Gender | |||||||||
| Male | 1858 (39)∗ | 651 (47) | 147 (43) | 468 (34) | 33 (33) | 79 (47) | 127 (38) | 866 (41) | 69 (37) |
| Female | 2885 (61) | 720 (53) | 195 (57) | 893 (66) | 67 (67) | 90 (53) | 209 (62) | 1223 (59) | 118 (63) |
| Race | |||||||||
| White | 3647 (77) | 1080 (79) | 199 (58) | 1070 (79) | 44 (44) | 113 (67) | 247 (74) | 1551 (74) | 129 (69) |
| Black | 1096 (23) | 291 (21) | 143 (42) | 291 (21) | 56 (56) | 56 (33) | 89 (26) | 538 (26) | 58 (31) |
| Education | |||||||||
| < High School education | 647 (14) | 193 (14) | 130 (38) | 240 (18) | 40 (40) | 69 (41) | 114 (34) | 521 (25) | 62 (33) |
| High School grad or equivalent | 1962 (41) | 610 (44) | 114 (34) | 650 (48) | 41 (41) | 49 (29) | 119 (35) | 877 (42) | 75 (40) |
| ≥1-y college | 2125 (45) | 568 (41) | 96 (28) | 469 (35) | 19 (19) | 51 (30) | 103 (31) | 687 (33) | 49 (26) |
| APOE ε4 | |||||||||
| 0 | 3326 (73) | 894 (68) | 168 (52) | 949 (73) | 68 (69) | 114 (72) | 173 (53) | 1413 (70) | 97 (53) |
| ≥1 | 1228 (27) | 417 (32) | 156 (48) | 354 (27) | 30 (31) | 45 (28) | 152 (47) | 594 (30) | 85 (47) |
| V2 DWRT Z-score | |||||||||
| <−1.0 | 607 (13) | 277 (20) | 118 (35) | 212 (17) | 32 (38) | 36 (24) | 79 (25) | 386 (22) | 47 (27) |
| −1.0 to <0 | 951 (21) | 364 (27) | 71 (21) | 297 (24) | 20 (24) | 48 (32) | 81 (26) | 411 (23) | 39 (23) |
| ≥0 | 3044 (66) | 712 (53) | 145 (43) | 748 (60) | 32 (38) | 66 (44) | 156 (49) | 981 (55) | 85 (50) |
| V2 DSST Z-score | |||||||||
| <−1.0 | 391 (9) | 169 (12) | 109 (33) | 137 (11) | 35 (42) | 38 (25) | 73 (23) | 270 (15) | 40 (24) |
| −1.0 to <0 | 1043 (23) | 485 (36) | 115 (35) | 365 (29) | 25 (30) | 60 (40) | 119 (38) | 591 (33) | 61 (36) |
| ≥0 | 3165 (69) | 699 (52) | 107 (32) | 753 (60) | 23 (28) | 52 (35) | 124 (39) | 915 (52) | 69 (41) |
| V2 WFT Z-score | |||||||||
| <−1.0 | 423 (9) | 224 (17) | 82 (25) | 152 (12) | 32 (39) | 34 (23) | 46 (15) | 281 (16) | 31 (18) |
| −1.0 to <0 | 1527 (33) | 543 (40) | 133 (40) | 479 (38) | 35 (42) | 67 (45) | 128 (41) | 712 (40) | 64 (37) |
| ≥0 | 2652 (58) | 586 (43) | 116 (35) | 625 (50) | 16 (19) | 47 (32) | 142 (45) | 782 (44) | 76 (44) |
Abbreviations: ARIC-NCS, Atherosclerosis Risk in Communities Neurocognitive study; MCI, mild cognitive impairment; TICS-m, telephonic instrument of cognitive status-modified; APOE, apolipoprotein E.
Number (column-wise percent). The columns are mutually exclusive and hierarchically defined.
3.1. Relationship between syndromic algorithmic diagnosis and final adjudicated diagnoses
Among participants evaluated in person, algorithmic diagnoses were compared with the opinion of the clinician reviewers. The clinician reviewers agreed with the algorithmic diagnosis of dementia in 275 of 293 (94%) persons. The clinician reviewers disagreed in 14 persons with an algorithmic diagnosis of dementia, assigning 13 a diagnosis of MCI and one a diagnosis of cognitively normal. In four instances, the clinician reviewers did not have enough information to make a syndromic diagnosis. For algorithmic diagnoses of MCI, the clinician reviewers disagreed with the algorithm in 9% (111 of 1220). When they disagreed, they were more likely to diagnose cognitive normality (62%, 69 of 111) than dementia (34%, 38 of 111). The reviewers were unable to arrive at a diagnosis in four participants.
3.2. Validity of TICSm diagnoses of dementia versus in-person assessments
There were 248 participants who underwent both an in-person assessment and a TICSm. Dementia was diagnosed in person in 22 of them (8.8%). At a cut score of 23, the TICSm had a sensitivity of 50% (95% CI, 28%–72%), a specificity of 94% (95% CI, 90%–97%), a PPV of 44% (95% CI, 24%–65%), and an NPV of 95% (95% CI, 91%–98%). Because the cut score of 23 was selected by the investigators to achieve greater diagnostic specificity in the group assessed only by interview, the lower sensitivity was anticipated.
3.3. Validity of hospital/death code diagnoses of dementia versus in-person assessments
Across all methods of ascertainment (in person, TICSm, and telephonic informant interviews), there were 8437 assessments with 778 dementia diagnoses among them. The sensitivity of hospital and death diagnostic codes for dementia was 25% (95% CI, 22%–29%) and the specificity 99% (95% CI, 99%–99%). The PPV of the hospital/death codes was 70% (95% CI, 64%–75%) and NPV was 93% (95% CI, 92%–93%). However, if analyses were restricted to those participants with a hospitalization or death within 1 year of an in-person diagnosis, TICSm assessment, or informant telephone interview, sensitivity rose to 67% (95% CI, 62%–73%) and PPV to 82% (95% CI, 76%–86%), whereas specificity (97%; 95% CI, 96%–98%) and NPV (94%; 95% CI, 92%–95%) changed very little.
3.4. Age-, sex-, and race-specific prevalence of MCI and dementia
Table 3 presents age-, race-, and sex-specific prevalences of MCI based on in-person assessments. Table 4 presents age-, race-, and sex-specific prevalences of dementia based on participants alive at the start of ARIC-NCS. As expected, prevalence of both MCI and dementia increased with advancing age (P < .001 for both). Prevalence of MCI was higher in men than women (24% vs. 19%, P < .001). Prevalence of dementia was higher in women overall versus men (9.2% vs. 8.7%) but was not statistically significant (P = .09). In the 65–69 year age bracket, less than 16% of ARIC survivors were considered to have cognitive impairment (i.e., either MCI or dementia), whereas in the oldest age bracket, over half were cognitively impaired, although most had MCI, not dementia. Dementia was more prevalent in blacks than whites (P < .001), whereas there were no racial differences for MCI prevalence.
Table 3.
Mild cognitive impairment prevalence by race, sex, and age group
| Group | 65–69 | 70–74 | 75–79 | 80–84 | 85–89 | Total |
|---|---|---|---|---|---|---|
| Total n | 1150 | 2261 | 1681 | 1065 | 314 | 6471 |
| All, % | ||||||
| Male | 15 | 24 | 27 | 28 | 34 | 24 |
| Female | 11 | 16 | 22 | 27 | 29 | 19 |
| Total | 13 | 19 | 24 | 27 | 31 | 21 |
| White, % | ||||||
| Male | 16 | 25 | 28 | 29 | 36 | 26 |
| Female | 11 | 16 | 21 | 27 | 33 | 19 |
| Total | 13 | 20 | 24 | 28 | 34 | 22 |
| Black, % | ||||||
| Male | 13 | 21 | 21 | 23 | 22 | 19 |
| Female | 12 | 16 | 25 | 27 | 19 | 19 |
| Total | 12 | 18 | 24 | 25 | 20 | 19 |
Abbreviation: ARIC-NCS, Atherosclerosis Risk in Communities Neurocognitive study.
NOTE. P values for Wald tests of main effects adjusted for other covariates: gender P < .0001; race P = .9139; age P < .0001; Wald test of the race × gender interaction was marginally significant (P = .0497). Estimates based on participants evaluated in person at ARIC-NCS.
Table 4.
Dementia prevalence by race, sex, and age group
| Group | 65–69 | 70–74 | 75–79 | 80–84 | 85–89 | Total |
|---|---|---|---|---|---|---|
| Total n | 1642 | 3383 | 2777 | 2096 | 815 | 10,713 |
| All, % | ||||||
| Male | 3.2 | 4.8 | 7.5 | 15 | 24 | 8.7 |
| Female | 2.4 | 4.3 | 9.7 | 15 | 26 | 9.2 |
| Total | 2.7 | 4.5 | 8.8 | 15 | 25 | 9.0 |
| White, % | ||||||
| Male | 2.6 | 3.4 | 5.1 | 14 | 22 | 7.6 |
| Female | 1.9 | 3.0 | 7.5 | 13 | 22 | 7.7 |
| Total | 2.2 | 3.2 | 6.5 | 14 | 22 | 7.7 |
| Black, % | ||||||
| Male | 4.7 | 9.8 | 18 | 19 | 33 | 13 |
| Female | 3.3 | 7.4 | 16 | 22 | 40 | 13 |
| Total | 3.7 | 8.2 | 16 | 21 | 38 | 13 |
Abbreviation: ARIC-NCS, Atherosclerosis Risk in Communities Neurocognitive study.
NOTE. P values for Wald tests of main effects adjusted for other covariates: gender P = .903; race P < .0001; age P < .0001; Wald test of the race × gender interaction was not significant (P = .461). Estimates based on all participants alive at the start of ARIC-NCS.
3.5. Etiology-specific diagnoses in MCI and dementia
Among the participants examined in person, the assigned etiologies for those diagnosed with MCI or dementia are listed in Table 5. AD had the highest proportion of primary etiologies (58%) of dementia and the highest proportion as either a primary or secondary etiology (76%). CVD as a primary etiology of dementia made up 24% and as a primary or secondary diagnosis in 46%. LBD was rare as a primary etiology (5%) but was more common as a secondary etiology for dementia (12%, 43 of 342). The majority (58%) of dementia cases received more than one etiologic diagnosis, with the combination of AD and CVD most common (n = 141, 41%).
Table 5.
Etiologic diagnoses by syndrome in participants examined in person; ARIC-NCS
| Etiology | Syndromic diagnoses |
Total | |
|---|---|---|---|
| MCI, n (%) | Dementia, n (%) | ||
| Pure AD | 427 (31) | 73 (21) | 500 |
| AD with CVD | 302 (22) | 78 (23) | 380 |
| AD with LBD | 100 (7) | 33 (10) | 133 |
| AD with other | 82 (6) | 14 (4) | 96 |
| AD primary | 911 (66) | 198 (58) | 1109 |
| AD primary or secondary | 1021 (75) | 261 (76) | 1282 |
| Pure CVD | 14 (1) | 8 (2) | 22 |
| CVD with AD | 110 (8) | 63 (18) | 174∗ |
| CVD with LBD | 9 (1) | 10 (3) | 19 |
| CVD with other | 2 (0) | 0 (0) | 2 |
| CVD primary | 135 (10) | 81 (24) | 216 |
| CVD primary or secondary | 437 (32) | 159 (46) | 596 |
| Pure LBD | 5 (0) | 4 (1) | 9 |
| LBD with other | 42 (3) | 13 (4) | 55 |
| LBD primary | 47 (3) | 17 (5) | 64 |
| LBD primary or secondary | 156 (11) | 60 (18) | 216 |
| Other | 22 (2) | 2 (1) | 24 |
| Insufficient information | 256 (19) | 44 (13) | 306† |
| Total | 1371 | 342 | 1720 |
Abbreviations: ARIC-NCS, Atherosclerosis Risk in Communities Neurocognitive study; AD, Alzheimer's disease; MCI, mild cognitive impairment; CVD, cerebrovascular disease; LBD, Lewy body disease.
One case of uncertain cognitive syndrome excluded.
Six cases of uncertain cognitive syndrome excluded.
Etiologic diagnoses in MCI were more weighted toward AD as a primary one (66%), with proportionately fewer assigned a primary etiology of CVD (10%) and fewer given more than one etiologic diagnosis (47%, 647 of 1371). AD as a primary or secondary etiologic diagnosis was about the same proportion in MCI as in dementia (75% vs. 76%, χ2 = 0.49, P = .48), but CVD as a primary or secondary etiologic diagnosis was less frequent in MCI (32%) compared with dementia (32% vs. 46%, χ2 = 25.8, P < .001). Overall, more MCI participants were assigned a single etiology of AD, CVD, or LBD compared with the dementia participants (33% vs. 25%, χ2 = 7.5, P = .006).
4. Discussion
The purpose of this report was to show prevalence data in the well-described ARIC cohort and describe the method used to diagnose MCI and dementia in participants alive at the start of ARIC-NCS. Although nearly one-third of the inception cohort had died, we examined in person nearly two-thirds of survivors. The prevalence of dementia in ARIC-NCS was in line with prior reports [1], [31]. The prevalence of MCI in ARIC-NCS was also comparable with other groups [32], [33], [34]. Although there were race and sex differences in MCI and dementia prevalence, these differences were dwarfed by the powerful effect of age on cognitive impairment. Using recent formulations for etiologic diagnoses using clinical or clinical and MRI, AD was the dominant etiology for both MCI and dementia while CVD was also common. Even with the imprecision inherent in etiologic diagnoses made with limited information, cognitive impairment attributed to more than one etiology occurred in more than half the cases. As expected, participants with dementia were more likely to have more than one etiology compared with MCI.
The prevalence of cognitive impairment at one point in time underestimates the lifetime burden of disease because of differential survival from middle age. With extensive cardiovascular and cognitive evaluations over the long history of ARIC as well as knowledge of what happened to members of the ARIC cohort who were no longer alive at ARIC-NCS in 2011–2013, we are in a position of depicting the complex interplay of cognition, cardiovascular health, cerebrovascular health, and mortality from middle age into the eighth decade of life in a longitudinal cohort. The current analysis is the foundation for subsequent reports that will go into detail about these relationships.
The prevalence of dementia in ARIC-NCS cohort among those evaluated in person was lower than prior reports [1], [31], but when all sources of information were used, the overall prevalence of dementia in living ARIC participants corresponded closely to estimates from the literature [1], [31]. By simultaneously accounting for MCI prevalence as well, we present a comprehensive view of cognitive functioning in a large representative cohort. The fact that nearly as many patients with dementia were identified by informant interview as by in-person examinations demonstrates the tendency of persons with dementia to decline participation in observational studies [35]. Had those whose informants were contacted by phone not had a prior relationship with ARIC, it is doubtful that we would have been able to obtain any information about the 505 persons who were alive at the time of ARIC-NCS but unable or unwilling to come for an in-person assessment. Use of the TICSm assessments and the hospital/death codes identified additional dementia cases, albeit with imperfect sensitivity, but those other surveillance methods allowed us to include more cases defined with high specificity in future analyses of midlife risk factors.
MCI was prevalent in ARIC-NCS, and the prevalence of MCI was similar to other recent studies [32], [33]. Had MCI been detectable by telephonic means, it might have included an even larger number of individuals, but currently only in-person techniques can be used to diagnose MCI. Because different MCI definitions result in marked variations in MCI prevalence, it is difficult to compare precisely our findings to other studies. We used consensus normative cut points to base diagnoses of MCI or dementia [25], [26], [27], but operational variations across studies is inevitable. Longitudinal studies of incident dementia in this cohort are necessary to provide validation for our MCI definitions.
There were differences between blacks and whites in the prevalence of dementia, with blacks generally having higher rates. There were no racial differences in prevalence of MCI. Some prior studies report higher rates of MCI and dementia in blacks [36], [37], [38], [39], but other studies found no differences between blacks and whites [40]. However, differences observed need to be placed in the context of the higher mortality in blacks before evaluation. The advantage of ARIC-NCS over others that have examined dementia prevalence in African-Americans is that members of both races were evaluated simultaneously using the same methods. Note that the methods used different adjustments for blacks and whites, based on normative data from well-defined normative subsamples of black and white participants in prior ARIC studies.
In contrast to some studies [1], [31], [41] but not others [38], [40], [42], there were no consistent differences in dementia prevalence between men and women, but men were consistently more likely to receive a diagnosis of MCI [32]. We accept the view that men are probably at greater risk for cognitive impairment, but because of their premature mortality, the number of prevalent cases in women eventually exceeds that in men [4].
We made etiologic diagnoses for those with cognitive disorders, relying on the informant interviews, neurologic examinations, neuropsychological test result patterns, and imaging in a majority, including nearly all with impairments. We recognize that etiologic diagnoses in epidemiologic contexts are subject to uncertainty. In the absence of amyloid imaging, 18Fluorodeoxyglucose Positron Emission Tomography, or cerebrospinal fluid biomarkers of AD, the etiologic diagnosis of AD in ARIC-NCS was based on a combination of a multidomain amnestic disorder in the absence of convincing evidence of an alternative primary etiology. Thus, a diagnosis of AD as an etiology in a setting such as ARIC-NCS will be imprecise. We used an algorithmic approach to the diagnosis of LBD and CVD, but it is well known that the individual features on which those two diagnoses of LBD [30] and CVD [43] are based are neither sensitive nor specific. With the caveat that we cannot attribute causality by mere presence alone, it is remarkable that CVD features were present in nearly one in four persons with dementia similar to prior epidemiologic [5], [31] and neuropathologic reports [44], [45], [46]. In MCI, only one in 10 had CVD features; we are not aware of other studies that quantified etiologic features in MCI.
Strengths of our study include the 25+ year history of the participants in ARIC, our comprehensive in-person assessments, and the availability of imaging to supplement diagnoses in those with cognitive impairments, representing nearly one-third of the total examined. We used state-of-the-art diagnostic approaches that allow us to deconstruct diagnoses, both syndromic and etiologic, which improved transparency of the relationship between raw data from participants and ultimate diagnoses. We used state-of-the-art diagnostic criteria that were operationally defined in advance. Double review by neurologists or a geriatrician and neuropsychologists with adjudication was also strength; as was the development and use of a clinically derived algorithmic approach to diagnosis. Although the algorithm was meant to reduce between-clinician variation, a clinician could override the algorithmic diagnosis if the data justified it. The algorithm was helpful in the frequent instances where clinical information was internally discordant (i.e., very low cognitive assessment scores in the face of an informant-derived FAQ or CDR indicating no dysfunction). We are not aware of any published strategies to deal with such occurrences. Furthermore, ARIC-NCS had access to the 25-year record of hospitalizations among participants, as well as the rapport built up over that time that enabled us to perform telephonic assessment of cognition or informant interviews among those who were no longer able to come for an in-person assessment.
Weaknesses are ones that are inherent in epidemiologic surveys. There were many nonparticipants, although we were able to account for them, and in most cases document their status at the time of ARIC-NCS. Our diagnostic methods were not as thorough as might be done in clinical practice or in focused research settings such as dementia clinical trials. Diagnoses were made by review of case report forms, not by the direct interview and observation by our diagnostic reviewers. Nuances from the neurologic examination or from face-to-face interviews were, thus, not accessible to our diagnosticians. However, independent reviews by two skilled clinicians for each participant and additional reviews or committee discussions were required for adjudication among disagreements, all of which enhanced the diagnoses in this cohort setting.
Research in context.
-
1.
Systematic Review: We searched PubMed for English-language publications on the prevalence and incidence of mild cognitive impairment or dementia. We cited several specific publications [[1], [2], [3], [4], [5], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]].
-
2.
Interpretation: The Atherosclerosis Risk in Communities (ARIC) Study was initiated in 1987 to study outcomes of cardiovascular and cerebrovascular diseases. Beginning with the second ARIC study visit, cognition was also assessed. In 2011-2013, the ARIC Neurocognitive Study performed both in-person, telephonic and medical record reviews to diagnose prevalent mild cognitive impairment and dementia. Here we describe the ascertainment methodology of the ARIC Neurocognitive Study. We provide prevalence estimates and presumed etiologies for both mild cognitive impairment and dementia. The current report replicates prevalence values for MCI and dementia from other populations.
-
3.
Future Directions: The description of the methodology and of the prevalence estimates provided here will serve as the basis for future reports from the ARIC Neurocognitive Study. The ARIC study is unique because of its ability to link mid-life cardiovascular and cognitive status to late-life cognitive disorders. The ARIC is also able to account for those lost to follow-up.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. This is ARIC article #2120a. The authors would also acknowledge the contributions of Guy McKhann and Ola Selnes who participated in the dementia adjudication panel.
Authors' contributions: D.S.K. generated the first draft and completed the final draft. D.S.K., A.R.S., M.S.A., T.H.M., L.W., and J.C. contributed to the study concept and design. D.S.K., R.F.G., B.G.W., L.H.C., M.S.A., T.H.M., A.A. did the acquisition of data. D.S.K., S.H., and L.W. did the analysis and interpretation. D.S.K., R.F.G., A.R.S., A.L.C.S., B.G.W., L.H.C., M.S.A., T.H.M., A.A., S.H., L.W., and J.C. did the critical revision of the article for important intellectual content. T.H.M. and J.C. obtained funding and contributed to study supervision.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data are collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 with previous brain MRI examinations funded by R01-HL70825.
Footnotes
D.S.K. served as deputy editor for Neurology; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals, and the Alzheimer's Disease Cooperative Study; and receives research support from the National Institutes of Health. R.F.G., A.R.S., A.L.C.S., B.G.W., L.H.C., M.S.A., T.H.M., A.A., S.H., L.M.W., and J.C. have no conflicts of interest to disclose.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2015.12.002.
Supplementary data
References
- 1.Hy L.X., Keller D.M. Prevalence of AD among whites: A summary by levels of severity. Neurology. 2000;55:198–204. doi: 10.1212/wnl.55.2.198. [DOI] [PubMed] [Google Scholar]
- 2.Fillenbaum G.G., Peterson B., Welsh-Bohmer K.A., Kukull W.A., Heyman A. Progression of Alzheimer's disease in black and white patients: The CERAD experience, part XVI. Consortium to Establish a Registry for Alzheimer's Disease. Neurology. 1998;51:154–158. doi: 10.1212/wnl.51.1.154. [DOI] [PubMed] [Google Scholar]
- 3.Hendrie H.C., Osuntokun B.O., Hall K.S., Ogunniyi A.O., Hui S.L., Unverzagt F.W. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 4.Chene G., Beiser A., Au R., Preis S.R., Wolf P.A., Dufouil C. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015;11:310–320. doi: 10.1016/j.jalz.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White L., Petrovitch H., Ross G.W., Masaki K.H., Abbott R.D., Teng E.L. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- 6.ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 7.Cerhan J.R., Folsom A.R., Mortimer J.A., Shahar E., Knopman D.S., McGovern P.G. Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) study investigators. Gerontology. 1998;44:95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 8.Knopman D., Boland L.L., Mosley T., Howard G., Liao D., Szklo M. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 9.Mosley T.H., Jr., Knopman D.S., Catellier D.J., Bryan N., Hutchinson R.G., Grothues C.A. Cerebral MRI findings and cognitive functioning: The Atherosclerosis Risk in Communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 10.Kohout F.J., Berkman L.F., Evans D.A., Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 11.Schneider A.L., Sharrett A.R., Gottesman R.F., Coresh J., Coker L., Wruck L. Normative data for 8 neuropsychological tests in older blacks and whites from the Atherosclerosis Risk in Communities (ARIC) study. Alzheimer Dis Assoc Disord. 2015;29:32–44. doi: 10.1097/WAD.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knopman D.S., Mosley T.H., Catellier D.J., Coker L.H. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: The ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman R.F., Rawlings A.M., Sharrett A.R., Albert M., Alonso A., Bandeen-Roche K. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: The ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–966. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub S., Salmon D., Mercaldo N., Ferris S., Graff-Radford N.R., Chui H. The Alzheimer's Disease Centers' Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopman D.S., Griswold M.E., Lirette S.T., Gottesman R.F., Kantarci K., Sharrett A.R. Vascular imaging abnormalities and cognition: Mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities-Neurocognitive study. Stroke. 2015;46:433–440. doi: 10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein L.B., Bertels C., Davis J.N. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46:660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 18.Fahn S., Elton R.L., Committee. UD . Unified Parkinson's disease rating scale. In: Fahn S., editor. Recent Developments in Parkinson's Disease. MacMillan Healthcare Information; Florham Park: 1987. pp. 153–163. [Google Scholar]
- 19.Morris J.C. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer R.I., Kurosaki T.T., Harrah C.H., Jr., Chance J.M., Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 21.Kaufer D.I., Cummings J.L., Ketchel P., Smith V., MacMillan A., Shelley T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 22.Rosen W.G., Terry R.D., Fuld P.A., Katzman R., Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 23.Welsh K.A., Breitner J.C., Magruder-Habib K.M. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry, Neuropsychology, Behavioral Neurology. 1993;6:103–110. [Google Scholar]
- 24.Knopman D.S., Roberts R.O., Geda Y.E., Pankratz V.S., Christianson T.J., Petersen R.C. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34:34–42. doi: 10.1159/000255464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R.J., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert M., DeKosky S.T., Dickson D., Dubois B., Feldman H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging– Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, DC: 2013. DSM-5: Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 28.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 29.McKeith I.G., Dickson D.W., Lowe J., Emre M., O'Brien J.T., Feldman H. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 30.Ferman T.J., Boeve B.F., Smith G.E., Lin S.C., Silber M.H., Pedraza O. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77:875–882. doi: 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobo A., Launer L.J., Fratiglioni L., Andersen K., Di Carlo A., Breteler M.M. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S4–S9. [PubMed] [Google Scholar]
- 32.Petersen R.C., Roberts R.O., Knopman D.S., Geda Y.E., Cha R.C., Pankratz V.S. Prevalence of mild cognitive impairment is higher in men than in women. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez O.L., Jagust W.J., DeKosky S.T., Becker J.T., Fitzpatrick A., Dulberg C. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 35.Knopman D.S., Roberts R.O., Pankratz V.S., Cha R.H., Rocca W.A., Mielke M.M. Incidence of dementia among participants and nonparticipants in a Longitudinal Study of Cognitive Aging. Am J Epidemiol. 2014;180:414–423. doi: 10.1093/aje/kwu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fillenbaum G.G., Heyman A., Huber M.S., Woodbury M.A., Leiss J., Schmader K.E. The prevalence and 3-year incidence of dementia in older black and white community residents. J Clin Epidemiol. 1998;51:587–595. doi: 10.1016/s0895-4356(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 37.Katz M.J., Lipton R.B., Hall C.B., Zimmerman M.E., Sanders A.E., Verghese J. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: A report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall K.S., Gao S., Baiyewu O., Lane K.A., Gureje O., Shen J. Prevalence rates for dementia and Alzheimer's disease in African Americans: 1992 versus 2001. Alzheimers Dement. 2009;5:227–233. doi: 10.1016/j.jalz.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzpatrick A.L., Kuller L.H., Ives D.G., Lopez O.L., Jagust W., Breitner J.C. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 41.Canadian Study of Health and Aging Working Group Canadian study of health and aging: Study methods and prevalence of dementia. CMAJ. 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- 42.Knopman D.S., Petersen R.C., Rocca W.A., Larson E.B., Ganguli M. Passive case-finding for Alzheimer's disease and dementia in two U.S. communities. Alzheimers Dement. 2011;7:53–60. doi: 10.1016/j.jalz.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold G., Bouras C., Canuto A., Bergallo M.F., Herrmann F.R., Hof P.R. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry. 2002;159:82–87. doi: 10.1176/appi.ajp.159.1.82. [DOI] [PubMed] [Google Scholar]
- 44.Yu L., Boyle P.A., Leurgans S., Schneider J.A., Kryscio R.J., Wilson R.S. Effect of common neuropathologies on progression of late life cognitive impairment. Neurobiol Aging. 2015;36:2225–2231. doi: 10.1016/j.neurobiolaging.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: A summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18:713–725. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 46.Sonnen J.A., Larson E.B., Crane P.K., Haneuse S., Li G., Schellenberg G.D. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.