Abstract
Cluster of Differentiation-200 (CD200) is an anti-inflammatory glycoprotein expressed in neurons, T cells, and B cells, and its receptor is expressed on glia. Both Alzheimer’s disease (AD) patients and mouse models display age-related or amyloid-β peptide (Aβ)-induced reductions in CD200. The goal of this study was to determine if neuronal CD200 expression restores hippocampal neurogenesis and reduces Aβ in the APP mouse model. APP and wild-type mice were injected at 6 months of age with an adeno-associated virus expressing CD200 into the hippocampus, and sacrificed at 12 months. CD200 expression restored neural progenitor cell proliferation and differentiation in the subgranular and granular cell layers of the dentate gyrus and reduced diffuse but not thioflavin-S+ plaques in the hippocampus. In vitro studies demonstrated that CD200-stimulated microglia increased neural differentiation of neural stem cells and enhanced axon elongation and dendrite number. CD200 also enhanced Aβ uptake by microglia. These data indicate that CD200 is capable of enhancing microglia-mediated Aβ clearance and neural differentiation and has potential as a therapeutic for AD.
Keywords: Alzheimer’s disease, microglia, adeno-associated virus, neuroinflammation, transgenic mouse model, CD200
1. Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder (Minati, et al., 2009). It is characterized by the progressive decline of cognitive function and memory, and it presents with a characteristic neuropathology that includes extracellular depositions of amyloid β-peptide (Aβ) (senile plaques) and intracellular accumulations of hyperphosphorylated microtubule-associated protein tau (neurofibrillary tangles) (Alzheimer, et al., 1995,Dickson, et al., 1988). Aβ aggregates are toxic to neurons and synapses and inhibit neuronal differentiation in both in vivo and in vitro studies (Haughey, et al., 2002). They also activate the innate immune response in microglia and astrocytes (McGeer and McGeer, 2010).
The chronic neuroinflammation seen in AD may contribute to the suppression of neurogenesis in the subgranular zone (SGZ) of the hippocampus (Deng, et al., 2010,Hoehn, et al., 2005,Monje, et al., 2003). Adult neurogenesis in the SGZ is important for maintenance of the central nervous system (CNS) and for the formation of new memories (Bruel-Jungerman, et al., 2007,Deng, et al., 2010,Deng, et al., 2009,Tronel, et al., 2010). Neuronal maturation is reduced in the pre-senile post-mortem AD hippocampus and is accompanied by increased neural stem cell proliferation (Boekhoorn, et al., 2006,Jin, et al., 2004). This suggests that the pro-inflammatory cascade suppresses the neurnal maturation and survival of differentiated neurons rather than their proliferation (Bastos, et al., 2008,Ekdahl, et al., 2003,Monje, et al., 2003), making inflammatory suppression of neurogenesis a worthwhile therapeutic target for AD.
Cluster of differentiation-200 (CD200) is a type I transmembrane glycoprotein with an immunoglobulin superfamily domain (Clark, et al., 1985). It is expressed on T cells, B cells, dendritic cells, and on neurons in the CNS (Barclay, et al., 2002). The CD200R is expressed on cells of the myeloid lineage, namely microglia, macrophages, and dendritic cells, and also on astrocytes and oligodendrocytes (Barclay, 1981,Barclay, et al., 2002,Chitnis, et al., 2007,Koning, et al., 2010). The CD200-CD200R interaction negatively modulates inflammatory activation through binding of Downstream of Tyrosine Kinase 2 (Dok2) to the phosphorylated NpxY motif on CD200R. This results in Ras GTPase inhibition of nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) activation (Mihrshahi, et al., 2009). An alternate pathway by which CD200 reduces pro-inflammatory signaling is through coupling to the CD200 receptor-like protein, CD200R3 (aka CD200RLb), which then utilizes TYRO protein tyrosine kinase binding protein (Tyrobp, also known as DNAX-activating protein of 12 kD; DAP12) as an adaptor protein. This leads to inhibition of Toll-like receptor and NFκB signaling (Kojima, et al., 2007,Wright, et al., 2003). Tyrobp is essential for the signaling of triggering receptor expressed on myeloid cells 2 (Trem2) (see (Ivashkiv, 2008) for review). Recent studies reveal that homozygous or heterozygous missense mutations in Trem2 increase the risk for developing late-onset AD, Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and frontotemporal dementia (FTD) (Borroni, et al., 2014,Cady, et al., 2014,Cuyvers, et al., 2014,R. Guerreiro, et al., 2013,R.J. Guerreiro, et al., 2013,Jonsson, et al., 2013,Rayaprolu, et al., 2013). CD200 mediates immunosuppression as a neuron-glia interaction through CD200 in neurons and CD200R in glia, but glia-glia interactions also mediate immunosuppression (Koning, et al., 2009). CD200 expression decreases with age when it is simultaneously associated with increased microglial activation in rodent models (Lyons, et al., 2007). It is likewise decreased in the brains of AD patients and in the brains of Aβ-treated mice (Lyons, et al., 2009,Walker, et al., 2009).
Interleukin-4 (IL-4), an anti-inflammatory cytokine, increases the expression of CD200. In both in vitro and in vivo studies from human and mouse tissues, an increase in IL-4 is associated with an increase in CD200 and vice-versa (Koning, et al., 2010,Lyons, et al., 2007,Lyons, et al., 2009). IL-4 treatment in animal models restored the Aβ-induced decrease in CD200 expression (Lyons, et al., 2007,Lyons, et al., 2009). Our laboratory and others have shown that pro-inflammatory mediators reduce the phagocytic response of primary microglia, whereas IL-4 supports the phagocytic response and enhances Aβ clearance (Koenigsknecht-Talboo and Landreth, 2005,Yamamoto, et al., 2008). We have also shown that IL-4 gene delivery to a transgenic mouse model of AD (APP+PS1 mice) restored the deficit in neurogenesis in the SGZ and enhanced Aβ clearance, but only in the pre-symptomatic stages of the disease (Kiyota, et al., 2010). These studies suggest that IL-4 signaling may be compromised at later disease stages due to decreased expression of CD200. Thus, CD200 is potentially a more plausible molecule than IL-4 for the treatment of AD. We hypothesize that the age-related or Aβ-induced reduction in CD200 leads to a pro-inflammatory glial environment that results in reduced neurogenesis and reduced Aβ phagocytosis in the hippocampus, and that this can be restored by genetic over-expression of CD200.
In this study, we injected an adeno-associated virus serotype 2/1 (AAV2/1) to increase neuronal expression of CD200 into the hippocampal region in a post-symptomatic mouse model of AD. We then examined its effect on neurogenesis, Aβ levels, and inflammation in the hippocampus. These findings were further explored using in vitro co-culture models of primary murine microglia and neural stem cells. Our findings suggest that CD200 has potential value as an anti-inflammatory therapeutic for AD.
2. Materials and Methods
2.1 Animals
APP mice expressing the Swedish familial AD mutant of human APP695 under the control of a hamster prion promoter (Tg2576) were obtained from Drs. G. Carlson and K. Hsiao-Ashe through the Mayo Medical Venture (Hsiao, et al., 1996). PS1 mutant mice (M146L line 6.1) were provided by Karen Duff (Columbia University, New York, NY) (Duff, et al., 1996) and maintained as transgene homozygotes. More information on APP and APP+PS1 mice are in Supplementary Information.
2.2 Plasmid construction and AAV2/1 generation
Development of AAV-IL-4 and AAV-IL-10 are described in previous publications (Kiyota, et al., 2011b,Kiyota, et al., 2010). For development of AAV2/1-GFP and AAV2/1-full-length CD200 (CD200FL; referred to as CD200 here), pAAV2-cytomegalovirus-tetracycline-controlled transactivator (pAAV2-CMV-tTA), AAV2 inverted terminal repeats flanking tetracycline-response element (TRE)/minimal cytomegalovirus (CMV) promoter, multiple cloning site, and Woodchuck hepatitis post-transcriptional regulatory element, and the bovine growth hormone polyadenylation site (pAAV2-TRE-MCS-WPRE), and pAAV2-TRE-GFP were constructed as previously described (Stone, et al., 2012). We previously showed that this CMV promoter and AAV1 combination tends to express primarily in neurons but is rarely positive in astrocytic cells (Kiyota, et al., 2009a). More details on construction of pAAV2-CMV-tTA and pAAV2-TRE-CD200 can be found in Supplementary Information.
2.3 Stereotactic Injection
2 × 109 viral particles (VP) each of AAV2/1-CMV-tTA and AAV2/1-TRE-GFP or – CD200 viruses were bilaterally injected into the CA1 region of the hippocampus at 6 months of age as described (Kiyota, et al., 2009a). A total of 6–9 mice were injected per group. Details of the stereotactic injection are described in Supplementary Information.
2.4 BrdU Administration and Tissue Preparation
Six months after AAV2/1 injection, bromodeoxyuridine (BrdU) was injected intraperitoneally (i.p.) (50 mg/kg body weight) twice daily eight hours apart for 2.5 days to mark proliferating cells as described with some modification (Butovsky, et al., 2006). Mice were euthanized three weeks after the last injection and tissue was harvested for immunohistochemistry or ELISA as described in Supplementary Information.
2.5 Immunohistochemistry
Conventional immunohistochemistry was used to examine neurogenesis, Aβ, and neuroinflammation using primary antibodies outlined in Table S1. Details of immunohistochemical staining are described in Supplementary Information. Briefly, five 30-µm sections at 150 µm intervals per mouse were quantified from four to five brains per group using image analysis software (ImageJ, NIH, Bethesda, MD). The numbers of BrdU+ or Dcx+ cells in the granular cell layer (GCL) and SGZ were counted over the length of the SGZ of the dentate gyrus (DG). F4/80 was used to label microglia-like cells in the brain. NOS2+/F4/80+ and YM1+/F4/80+ cells were counted over the area of the granular cell layer (GCL) and SGZ regions combined. Percent area occupied by Aβ plaques in the hippocampus was quantified by determining the area occupied by plaques over the total area of the hippocampus for each section.
2.6 ELISA
Biochemical analysis of hippocampal tissue for detection of human Aβ40 and Aβ42 was performed with two to five samples per group and performed in duplicate using commercially available enzyme immunoassays according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA).
2.7 Cell Culture
Primary neurons were cultured as previously described (Yamamoto, et al., 2007). Cytokine stimulation of neurons was performed with conditioning medium containing IL-10 or IL-4 for 24 hours followed by cell lysis. For Aβ stimulation, neurons were treated with oligomeric Aβ42 with or without 2 ng/ml IL-4 followed by cell lysis. AAV-293 cells and primary neurons were transduced with AAV2/1 by adding AAV directly into media. Cells were then harvested after 14 days with lysis buffer, briefly sonicated, and subjected to immunoblot analysis.
Primary neural stem cells were prepared from embryonic E14 CD-1 mice (Charles River Laboratory, Wilmington, MA) as described (Kiyota, et al., 2011a). Primary microglia were isolated from P0 mice as described (Kiyota, et al., 2009b). For the co-culture neural differentiation study, microglia were pre-treated for one hour with 100 ng/ml soluble CD200Fc ligand prior to indirect co-culture with neural stem cells for seven days to allow for neuronal differentiation. For analysis of phagocytosis, microglia were pre-treated with 5 µM Aβ42 prior to one-hour treatment with CD200Fc ligand (100 ng/ml). After this, cells were stimulated with fluorescein isothiocyanate (FITC)-tagged Aβ42 (Aβ42Fitc) for phagocytosis, followed by preparation for flow cytometry analysis. For examination of microglial activation and growth factor analysis by RT-PCR, microglia were serum-starved overnight prior to five-hour incubation with 100 ng/ml CD200Fc ligand. RNA was harvested using the RNeasy Mini Kit (Qiagen, Valencia, CA).
More details on cell culture, maintenance, stimulation conditions, and lysis, can be found in Supplementary Information.
2.8 Immunocytochemistry
Immunocytochemistry of neural stem cells and microglia was performed to examine neural differentiation and Aβ phagocytosis. Primary antibodies to β-III tubulin (β3T) and glial fibrillary acidic protein (GFAP) were used to label mature neurons and astrocytes, respectively. Details of the immunocytochemical procedure are described in Supplementary Information. Differentiation was quantified by counting the number of β3T+ versus GFAP+ cells over total number of cells. The NeuronJ plug-in was used on ImageJ (NIH Bethesda, MD) to measure the length of axons and number of dendrites emanating from the cell soma (Meijering, et al., 2004).
2.9 Flow Cytometry
Cells were labeled for microglia-specific marker CD11b conjugated to allophycocyanin (APC) (CD11bAPC) and examined by flow cytometry using a BD LSR II instrument with BD FACS Diva 6.2.1 software (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (FlowJo LLC, Ashland, OR).
2.10 RT-PCR
After RNA isolation from primary microglia, gene expression for microglial activation was analyzed using specific primers against CD200R1, CD200R3, Trem2, Tyrobp, TGF-β1, and TGF-βR1 and compared against the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Specific growth factors were also analyzed to determine if CD200 induced growth factor expression in microglia. These included insulin-like growth factor 1 (IGF-1), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), and glial cell-derived neurotrophic factor (GDNF). Primer sequences and references can be found in Supplementary Information.
2.11 Immunoblotting
We performed immunoblotting to measure the amount of CD200 or GFP protein production after AAV2/1-CD200 or –GFP transduction. Details of the immunoblotting procedure can be found in Supplementary Information. Immunoreactive bands were quantified by normalizing band intensities relative to controls on scanned films using ImageJ software (NIH, Bethesda, MD).
2.12 Statistics
All data are presented as mean values ± standard error of the mean (SEM). For comparison between two groups, data were analyzed by Student’s t-test. For multiple mean comparisons, data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey or Bonferroni post-hoc analysis using statistics software (Prism 4.0, Graphpad Software, Inc., San Diego, CA). P-values ≤ 0.05 were considered statistically significant.
3. Results
3.1 CD200 expression is down-stream of IL-4
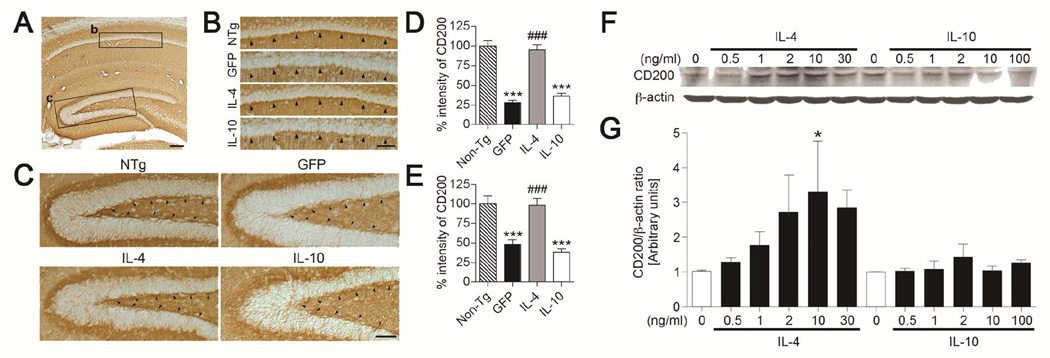
Immunohistochemistry of CD200 shows its expression in hippocampal neurons throughout the neuropil in WT mice (Fig. 1A) and particularly in pyramidal neurons in the stratum radiatum of the CA1 region (Fig. 1B,D) and the SGZ of the DG (Fig. 1C,E). CD200 expression was significantly reduced in AAV-GFP- and AAV-IL-10-injected APP+PS1 mice (296% and 203% of AAV-GFP-injected group, respectively). AAV-IL-4 injection into the hippocampus restored CD200 immunoreactivity in APP+PS1 mice both in the CA1 and DG.
Figure 1. IL-4 but not IL-10 increases CD200 expression in the hippocampus in vivo and in primary cultured neurons in vitro.
(A) CD200 expression throughout the neuropil in the hippocampus of non-transgenic (Tg) mice (NTg). (B, C) High magnification of boxes B and C in Fig. 1A; strong immunoreactivity of CD200 in pyramidal neurons at the stratum radiatum of the CA1 region (B, arrowheads) and the SGZ of the dentate gyrus (C, arrowheads) in non-Tg (NTg) and AAV-IL-4-injected APP+PS1 (IL-4), but not in AAV-GFP (GFP) or AAV-IL-10-injected APP+PS1 groups (IL-10). Scale bars represent 200µm in A and 100µm in B. (D, E) Intensity of CD200 expression in the stratum radiatum of the CA1 region (D) and SGZ (E) *** or ### denotes p < 0.001 vs. non-Tg or AAV-GFP group (n = 5 per group, 5 sections per brain). (F) Immunoblotting of CD200 and β-actin in primary cultured murine cortical neurons after IL-4 or IL-10 stimulation. (G) Quantification of immunoreactive bands in F. * denotes p<0.05 vs. non-treated control as determined by one-way ANOVA and Newman–Keuls post-test, respectively (n=3).
We also studied the effects of IL-4 and IL-10 on CD200 expression in primary cultured murine neurons. Direct stimulation of neurons by IL-4, but not IL-10, significantly increased CD200 levels in a dose-dependent manner (up to 321% increase over basal level) (Fig. 1F,G). These data support previous findings that CD200 expression is down-stream of IL-4, and that IL-4 can restore CD200 expression in the presence of Aβ (Lyons, et al., 2007,Lyons, et al., 2009). We demonstrate this by showing that a reduction in CD200 expression can be restored by AAV-IL-4 gene delivery into the APP+PS1 mouse brain, which has high levels of Aβ (Holcomb, et al., 1998). Restoring CD200 expression in APP+PS1 mice is an effect specific to IL-4 and not anti-inflammatory cytokines in general, such as IL-10.
3.2 AAV2/1-CD200 transduction expresses CD200 in cultured cells
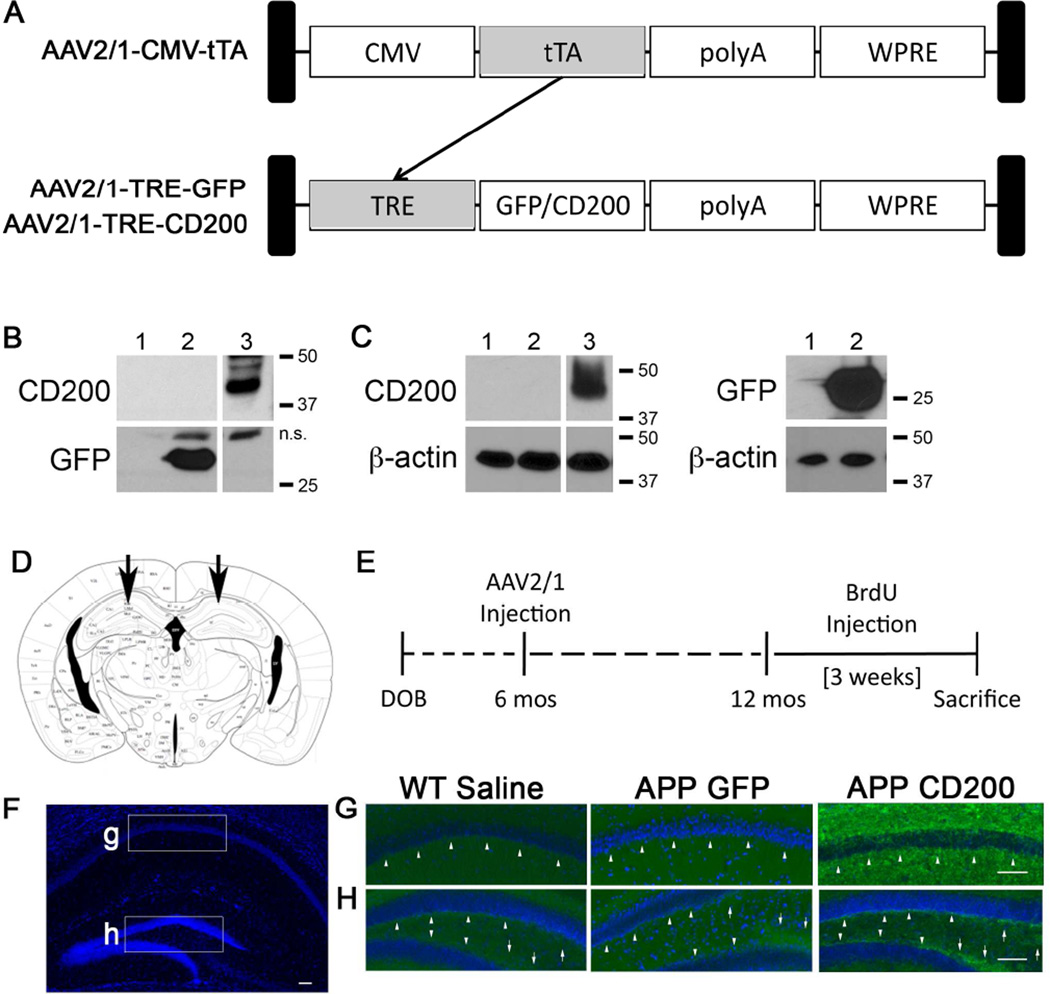
We established an AAV-mediated gene delivery system for tetracycline-inducible CD200 (Fig 2A). This promoter is shorter than the CMV/chicken β-actin hybrid promoter, allowing our CD200 cDNA to be inserted into our AAV2 vector. This makes the total length of the AAV gene cassette less than 4.7 kb, which is essential for AAV packaging (Grieger and Samulski, 2005). The AAV2/1-CMV-tTA contains a cytomegalovirus (CMV) promoter that controls the expression of the tetracycline transactivator protein (tTA). tTA activates any genes under a tetracycline response element (TRE). In this injection paradigm, we activate expression of TRE-controlled GFP or CD200 (AAV2/1-TRE-GFP or AAV2/1-TRE-CD200) by co-injection with AAV2/1-CMV-tTA. AAV-293 cells and primary cultured mouse neurons were infected with AAV-CMV-tTA and AAV-TRE-GFP or AAV-TRE-CD200FL, and harvested 14 days post-infection before immunoblot analysis. GFP and CD200 were expressed in both AAV-293 cells and neurons (Fig. 2B,C), demonstrating that our gene delivery system works in vitro.
Figure 2. AAV2/1-TRE-CD200/AAV2/1-CMV-tTA-co-administration induces CD200 expression in vitro and in vivo.
(A) Schematic for our AAV2/1-CMV-tTA (tetracycline transactivor) and AAV2/1-TRE (tetracycline response element)-GFP/CD200 gene induction system. tTA gene expression under the CMV promoter induces expression of genes of interest (GFP or CD200) under the control of TRE and can be controlled by doxycycline (Tet-off system). (B, C) Each column has been given the following code: 1) Uninfected, 2) AAV2/1-TRE-GFP (1×109 VP/ml), and 3) AAV2/1-TRE-CD200FL (4×108 VP/ml)-infected samples. AAV293 cells (1×105 cells, B) and Primary murine cortical neurons (1×105 cells, C) were lysed 14 days after virus infection by RIPA buffer for immunoblot for CD200, GFP and β-actin. n.s: nonspecific bands. (D) The scheme depicts the location of the bilateral stereotactic injection into the CA1 region of the hippocampus in vivo (E) Experimental paradigm for in vivo study of AAV-injection into APP mice. (F–H) Hippocampal images taken from 12 month-old WT or APP mice injected with saline, AAV2/1-GFP or AAV2/1-CD200 at six months of age. Fixed brain tissue sections were immunostained for CD200 (green) and counter-stained with Hoechst 33342 (blue). Mice injected with AAV2/1-CD200 show increased intensity of CD200 staining in pyramidal neurons in the stratum radiatum of the CA1 (arrowheads) (G) and in the SGZ (arrowheads) and infrapyramidal and suprapyramidal mossy fiber neurons (arrows) of the DG (H). Scale bars represent 100 µm.
3.3 AAV2/1-CD200 gene transfer increases CD200 expression in the hippocampus
To determine if our gene delivery system also worked in vivo, 6 month-old Tg2576 APP mice or non-Tg littermates (WT) were given bilateral stereotactic co-injections of AAV2/1-CMV-tTA and AAV2/1-TRE-GFP or AAV2/1-TRE-CD200FL viruses (hereafter abbreviated as AAV2/1-GFP or AAV2/1-CD200) or saline directly into the CA1 region of the hippocampus (Fig. 2D). This was followed by BrdU injection at twelve months and euthanasia three weeks later (see Fig. 2E for experimental paradigm). CD200 immunoreactivity in the hippocampus shows low baseline expression of CD200 with relatively higher expression in the SGZ and the suprapyramidal and infrapyramidal mossy fiber neurons in saline-injected WT mice and AAV2/1-GFP-injected APP mice (Fig. 2 F–H). AAV2/1-CD200 injection into APP mice increased CD200 levels in the hippocampus, particularly within the SGZ, pyramidal neurons in the stratum radiatum of the CA1 region, and suprapyramidal and infrapyramidal mossy fiber neurons. Sustained enhanced immunoreactivity six months after the injection demonstrates long-term expression of the recombinant CD200 protein in the DG.
3.4 CD200 restores hippocampal neurogenesis in APP mice
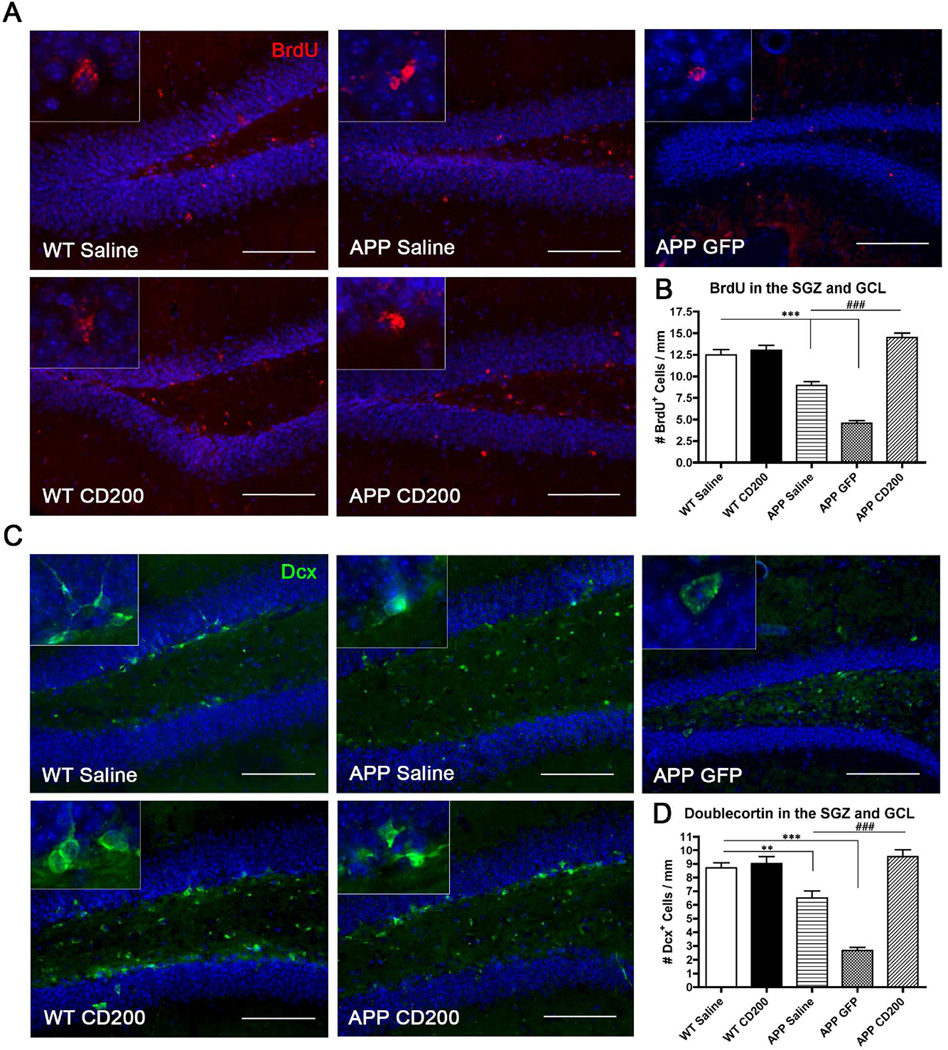
To determine if AAV2/1-CD200 injection increased proliferation in the hippocampi of APP mice, we i.p. injected BrdU to label newly divided cells. Saline- and AAV2/1-GFP-injected APP mice had significantly fewer BrdU+ cells in the SGZ and GCL compared to WT mice (Fig. 3 A,B), contrary to findings in human AD patients (Boekhoorn, et al., 2006,Jin, et al., 2004). AAV2/1-CD200 injection into APP mice restored BrdU+ cell number in the DG. Dcx was used as a marker of newly differentiated neurons. AAV2/1-CD200 injection in APP mice also restored the number of Dcx+-differentiated cells in the DG (Fig. 3 C,D). Restored neurogenesis in the SGZ coincided with the regional increase in SGZ CD200 expression after AAV injection shown in Fig. 2H. These results demonstrate that increasing CD200 expression in APP mice enhances or promotes both proliferation and differentiation of neural stem cells in the DG, thus restoring neurogenesis to WT mouse levels.
Figure 3. AAV2/1-CD200-injection increases hippocampal neurogenesis in vivo.
Mice were injected with bromodeoxyuridine (BrdU) five times three weeks before sacrifice and then immunostained for BrdU (newly proliferated cell marker, red) or doublecortin (Dcx, a newly synthesized neuronal marker, green). (A, B) BrdU immunoreactivity in the hippocampus to mark newly divided cells (A) and quantification of number of BrdU+ cells in the GCL and SGZ of the dentate gyrus (B). *** or ### denote p < 0.001 vs. WT Saline or APP Saline and APP GFP groups as determined by one-way ANOVA and Tukey’s post-test (n=5 per group). (C, D) Dcx immunoreactivity in the hippocampus to mark newly differentiated neurons (C) and quantification of number of Dcx-positive cells in the dentate gyrus (D). ** and *** denote p<0.01 and 0.001, respectively, as determined by one-way ANOVA and Tukey’s post-test vs WT Saline mice. ### denotes p<0.001 as determined by one-way ANOVA and Tukey’s post-test vs APP Saline and APP GFP mice. Scale bars represent 100 µm in (A) and (C).
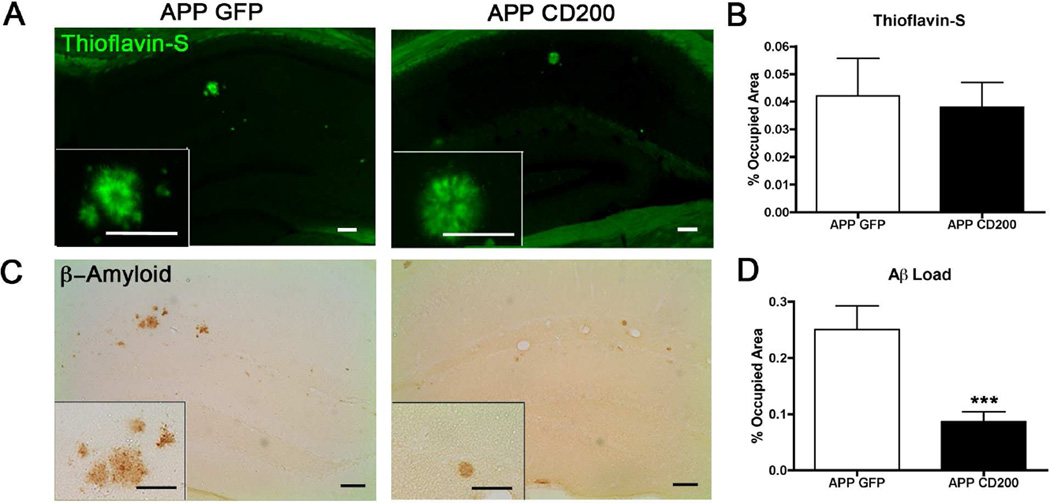
3.5 CD200 expression suppresses β-amyloidosis in APP mice
We analyzed APP mice for amyloid pathology using markers for compact (Thioflavin-S+, TS+) and diffuse Aβ plaques. AAV2/1-CD200-injected APP mice exhibited no change in percent occupied area of TS+ compact plaques compared to AAV2/1-GFP-injected APP mice (Fig. 4A,B). This demonstrates that over-expression of CD200 has no effect on TS+ insoluble amyloid plaque levels. However, diffuse Aβ was significantly reduced in AAV2/1-CD200-injected APP mice as compared to AAV2/1-GFP-injected APP mice (Fig. 4C,D). Hippocampal tissue lysate levels of Aβ40 and Aβ42 were also measured by ELISA (data not shown). There was a trend for reduced Aβ42 levels in the hippocampi of AAV2/1-CD200-injected APP mice, though this was not statistically significant (APP GFP: 0.29 ± 0.08 versus APP CD200: 0.16 ± 0.05 ng/mg, p= 0.35). There was no change in Aβ40 protein levels (APP GFP: 2.29 ± 0.56 versus APP CD200: 1.89 ± 0.38 ng/mg, p= 0.65). These data demonstrate that AAV2/1-CD200 injection into APP mice reduces soluble Aβ42.
Figure 4. AAV2/1-CD200-injection reduces hippocampal Aβ load in APP mice.
Brain sections (n=5 per group) were stained for compact, insoluble Aβ plaques (Thioflavin-S, green) or total amyloid (Aβ, DAB). (A, B) Thioflavin-S staining in the hippocampus demonstrates insoluble plaque area in APP mice (A) and quantification was measured by percent-occupied area (NS as determined by Student’s t-test) (B). (C, D) DAB staining in the hippocampus show total amyloid in APP mice (C) and quantification was performed by measuring percent-occupied area of the whole hippocampus (D). *** denotes p<0.001 as determined by Student’s t-test. Scale bars represent 100 µm for both (A) and (C) and for insets.
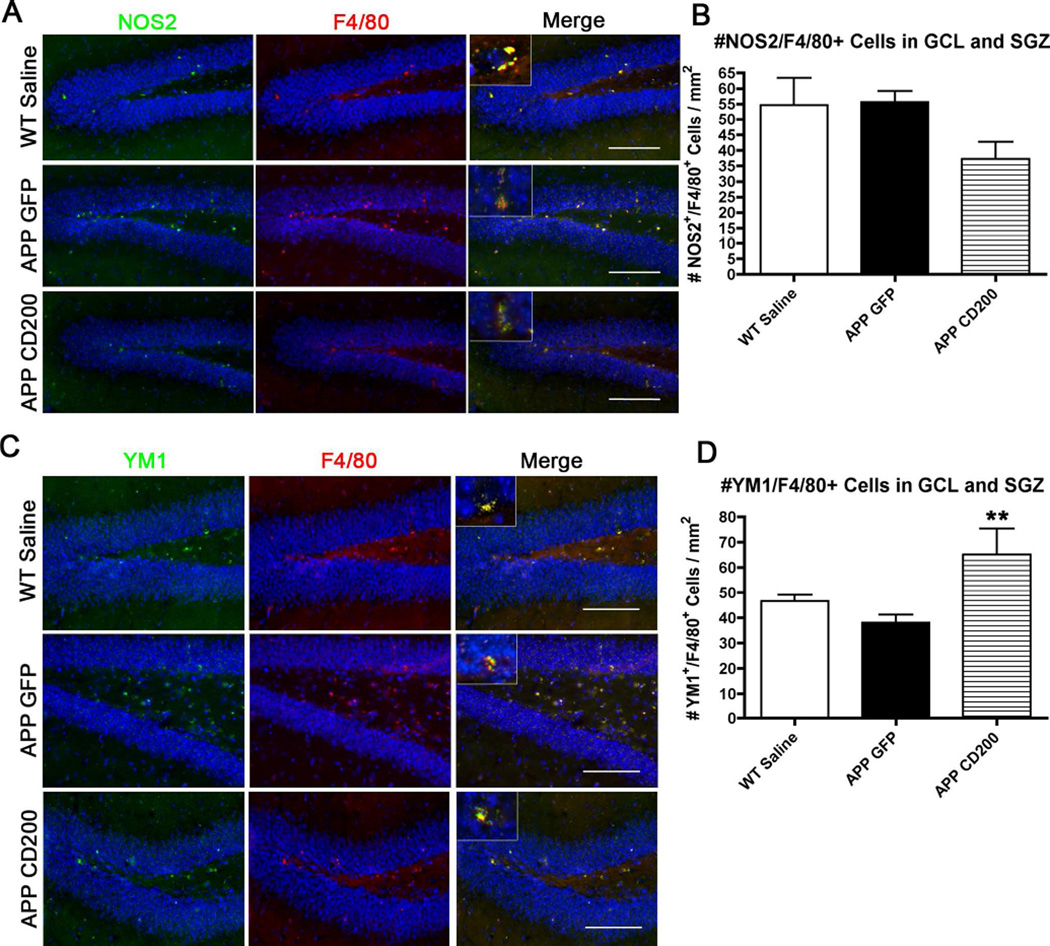
3.6 CD200 expression reduces inflammation in the APP mouse
APP mice injected with AAV2/1-CD200 had a reduced number of NOS2+ microglia-like cells in the GCL and SGZ compared to saline-injected WT mice and AAV2/1-GFP-injected APP mice (Fig 5 A,B), although the difference was not significant. There was a significant increase in the number of YM1+ microglia-like cells in the same regions in AAV2/1-CD200-injected APP mice (Fig 5 C,D). It should be noted that F4/80 stains resident microglia and peripheral macrophages, and we did not differentiate between the two in our quantification. Both cell types are present in our WT and APP mouse brains (see Supplementary Fig S1). We observed a minor population of CD169+ peripheral monocytes in addition to several microglia-specific P2ry12+ microglia in the mouse brain (Fig. S1). We can only conclude that the NOS2+ and YM1+ staining are mononuclear phagocytes (brain microglia and perivascular macrophages). F4/80 immunostaining showed that NOS2 and YM1 expression was predominantly from these mononuclear phagocytes (insets Fig. 5 A,C). This indicates that over-expression of CD200 establishes an anti-inflammatory milieu.
Figure 5. AAV2/1-CD200-injection reduces NOS2+ and increases YM1+ microglia in APP mice.
(A) Brain tissue sections were immunostained for pro-inflammatory marker NOS2 (green) and microglia marker F4/80 (red). Insets depict predominant NOS2 expression in microglia-like cells in the dentate gyrus. Scale bars represent 100 µm. (B) The number of NOS2+ microglia was quantified over the area of the GCL and SGZ. Results showed a trend for a reduction after CD200 treatment. (C) Hippocampal tissue sections were immunostained for anti-inflammatory marker YM1 (green) and F4/80 (red). Insets depict predominant YM1 expression in microglia-like cells. Scale bars represent 100 µm. (D) Quantification of the number of YM1+ microglia in the GCL and SGZ. ** denotes p<0.01 vs WT Saline mice as determined by one-way ANOVA and Tukey post-test (n=4–5 per group).
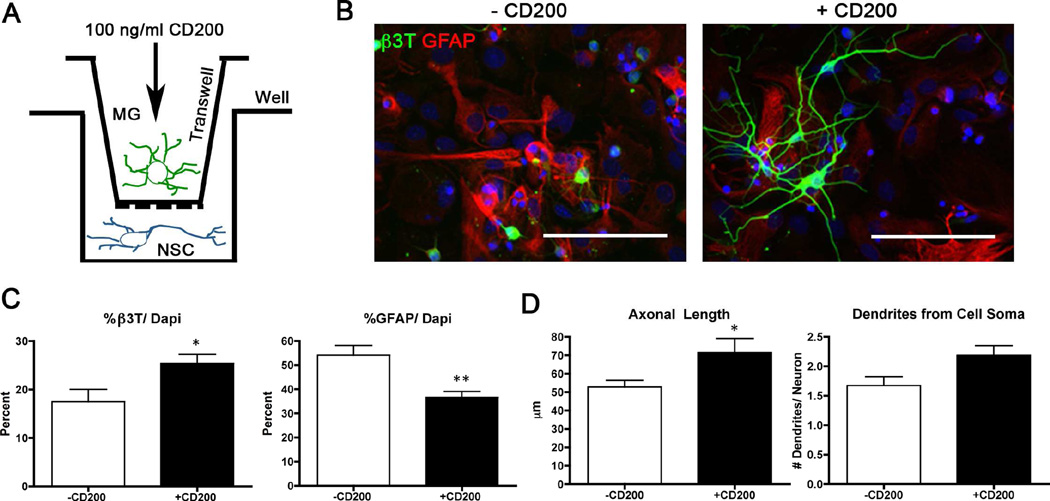
3.7 CD200 stimulation of microglia increases neuronal maturation in vitro
We conducted a co-culture study to determine if CD200-stimulated microglia enhanced neuronal maturation in vitro. Primary cultured murine microglia were pretreated with or without soluble CD200Fc ligand prior to indirect co-culture with primary cultured murine neural stem cells in differentiation conditions for seven days (see Fig. 6A). CD200-treated microglia enhanced neuronal maturation of neural stem cells as compared to untreated microglia (Fig. 6 B,C). These differentiated neurons exhibited more complex structures with longer axons and more dendrites emanating from the cell soma (Fig. 6D). This suggests that CD200 may be beneficial for restoring neurogenesis in vivo by enhancing neuronal maturation and complexity.
Figure 6. CD200-stimulated microglia increase neural differentiation of neural stem cells and Aβ phagocytosis in vitro.
(A) Scheme shows the experimental design for the in vitro co-culture study. Neural stem cells were co-cultured with CD200-pre-treated microglia in transwells for seven days. (B) Images of neural stem cells after co-culture with CD200-treated microglia stained with astrocytic marker GFAP (red) and mature neuronal marker β3T (green). Scale bars represent 100 µm. (C) Quantification of cellular differentiation was performed by counting the percentage of β3T+ or GFAP+ cells over total number of cells. (D) Neuronal complexity was determined by measuring axon length using NeuronJ and total number of dendrites emerging from the cell soma (n= ~50 per group). * and ** denote p<0.05 and 0.01, respectively, as determined by Student’s t-test. (E) Microglia were pre-treated with CD200 followed by incubation with oligomeric Aβ42 for 72 hours. Microglia were stained for Aβ (NU-1, green) and Hoechst 33342 (blue). Scale bar represents 100 µm. * and ** denotes p<0.05 and 0.01, respectively, as determined by Student’s t-test.
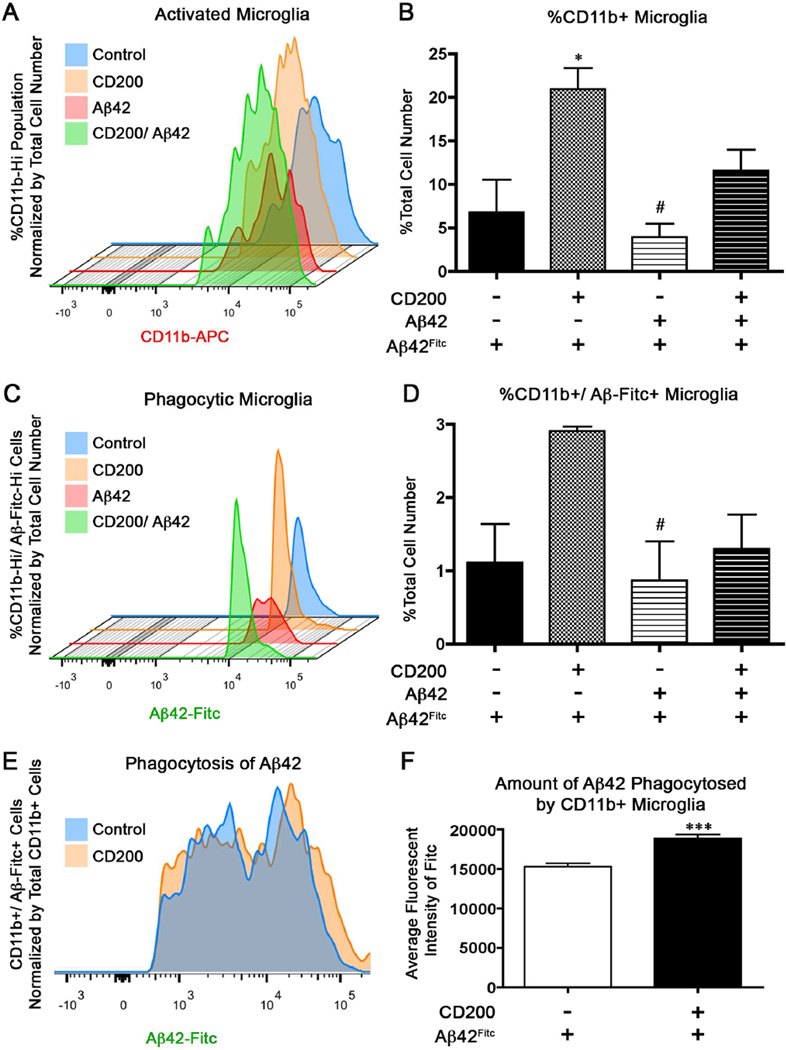
3.8 CD200 enhances the survival of CD11b+ primary microglia in vitro
Flow cytometry analysis of microglia labeled with the activation marker CD11b revealed that microglia treated with CD200 had an increased percentage of CD11b+ microglia compared to untreated microglia and microglia pre-treated with Aβ42 (Fig. 7a and b). Interestingly, pre-treatment of microglia with Aβ42 reduced the percentage of CD11b+ microglia, and this was partially restored by CD200 prior to stimulation with fibrillar Aβ42Fitc. This is consistent with our previous report of Aβ42-induced cytotoxicity in primary cultured murine microglia (Kiyota, et al., 2009b).
Figure 7. CD200-treated microglia show increased phagocytosis of Aβ42 in vitro.
Microglia were pre-treated with untagged Aβ42 prior to treatment with soluble CD200Fc ligand and then stimulated with Aβ42Fitc for phagocytosis. Smoothed histogram represents the percentage of CD11b+ microglia (A), percentage of CD11b+ microglia that phagocytosed Aβ42Fitc (C), and mean fluorescent intensity of Fitc per cell (E). These were quantified in (B), (D), and (F) (n= 3 samples per group). * denotes p<0.05 compared to Control and # denotes p<0.05 compared to CD200-treated group as determined by one-way ANOVA and Tubey’s post-test in B and D. *** denotes p<0.001 as determined by Student’s t-test in (F).
3.9 CD200-treated microglia have enhanced phagocytosis of Aβ42 in vitro
We performed in vitro experiments to determine if CD200 enhanced the ability of primary microglia to phagocytose Aβ42Fitc by flow cytometry. CD200 significantly enhanced the percentage of Aβ42Fitc+CD11b+ microglia compared to microglia pre-treated with Aβ42 alone (Fig. 7C and D). Treatment with CD200 after Aβ42 pre-treatment partially restored the percentage of Aβ42Fitc+CD11b+ microglia. Aβ42Fitc uptake by microglia, as determined by the mean fluorescent intensity of Aβ42Fitc+CD11b+ microglia, was increased by CD200 treatment (Fig. 7E and F), demonstrating that CD200 not only increased the number of phagocytic CD11b+ microglia, but also increased Aβ42Fitc uptake per cell.
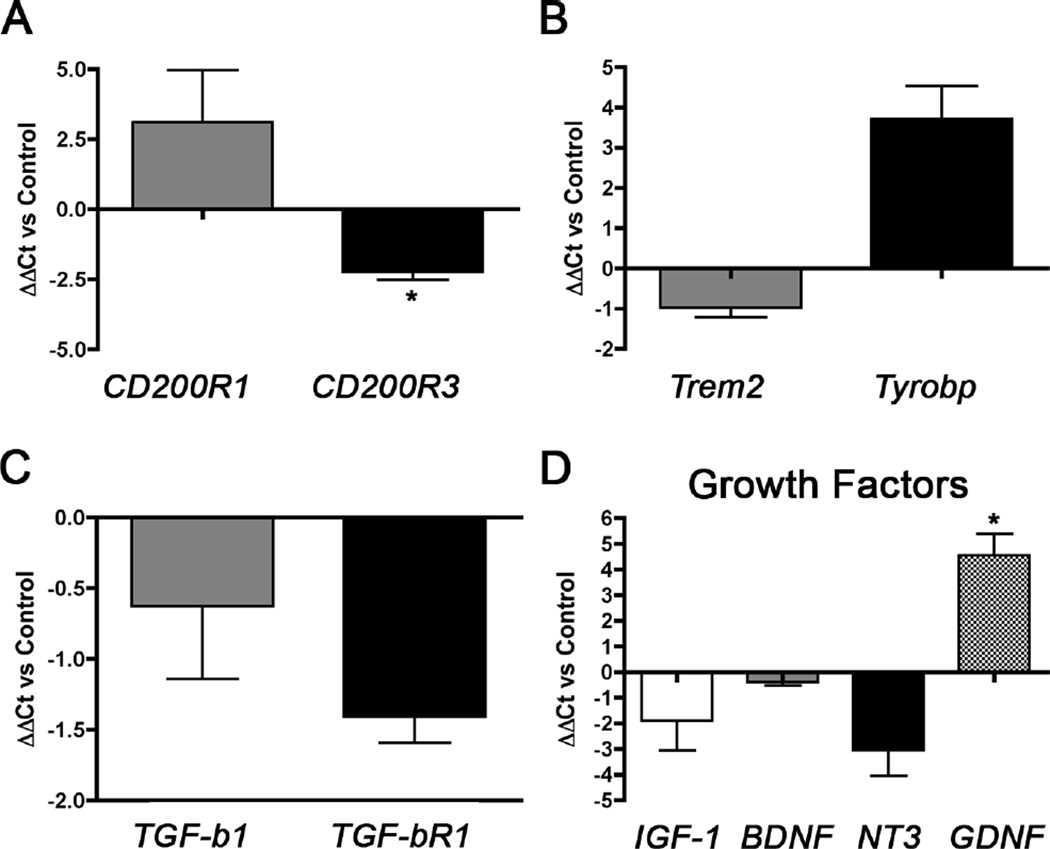
3.10 CD200 alters microglial activation in vitro
To examine the effect of CD200 on the microglial phagocytic mechanism, we analyzed the expression of CD200R1 and CD200R3 (Fig. 8A), Trem2 and Tyrobp (Fig. 8B), and TGF-β1 and TGF-βR1 (Fig. 8C) in CD200-treated microglia in vitro. We found that CD200 treatment increased the expression of CD200R1, but reduced the expression of CD200R3 compared to untreated cells. Despite the reduction in CD200R3 expression, we observed an increase in the expression of the CD200R3 adaptor molecule, Tyrobp. Trem2, a phagocytic molecule that also couples to Tyrobp, did not alter its gene expression. We also observed a reduction in both TGF-β1 and TGF-βR1 expression, though the changes were not significant. These trends indicate a feedback alteration in receptor expression and a skewing into an M2a microglial activation phenotype upon down-regulation of TGF-β1 signaling.
Figure 8. CD200Fc induces an alteration in primary microglial activation and growth factor expression in vitro.
Primary microglia were incubated with 100 ng/ml soluble CD200Fc ligand prior to gene expression analysis of CD200R1 and CD200R3 (A), Trem2 and Tyrobp (B), and TGF-β1 and TGF-βR1 (C) and of specific growth factors IGF-1, BDNF, NT3, and GDNF (D). Data are represented as ΔΔCt versus untreated Control samples (n= 2–3 samples per group and tested in duplicate). * denotes p<0.05 compared to untreated Control samples as determined by Student’s t-test.
3.11 CD200 alters microglial expression of growth factors in vitro
We showed that CD200-treated primary microglia enhanced neuronal differentiation and maturation of indirectly co-cultured neural stem cells in vitro. This suggests that stimulation of microglia with CD200 may induce the expression of growth factors that stimulate or support neurogenesis. We explored potential growth factors induced by CD200 in primary cultured microglia. There were no significant changes in expression of IGF-1, BDNF, and NT3 after treatment of primary murine microglia with CD200. However, GDNF expression was significantly increased by CD200 (Fig. 8D, p < 0.05).
4. Discussion
We show for the first time that Aβ-associated reduction of CD200 is seen in transgenic mouse models of AD. We also demonstrate that the APP+PS1 mouse model undergoes the same IL-4-induced restoration of CD200 expression in vivo as proposed by previous studies (Lyons, et al., 2007,Lyons, et al., 2009). This makes APP+PS1 and Tg2576 mice good mouse models for studying the therapeutic effects of CD200. We report that over-expression of CD200 in the early stages of disease development in APP mice restored neurogenesis in the DG and reduced Aβ in the hippocampal region in vivo. Both of these effects coincided with a reduction in pro-inflammatory activation in microglia-like cells. Our in vitro experiments using primary cultured murine microglia and neural stem cells support these findings and suggest that CD200-stimulated microglia play a role in mediating these effects.
Treatment of primary microglia with CD200 increases GDNF expression in vitro. GDNF is more commonly recognized as a survival factor for dopaminergic neurons, as it promotes their survival, size, and neurite length in vitro (Lin, et al., 1993). However, GDNF also alters microglial activation and protects neurons in the hippocampus from excitotoxic insult (Boscia, et al., 2009). It may therefore play a role in proliferation and survival of microglia (Chang, et al., 2006,Honda, et al., 1999). Interestingly, several studies report the association of CD200 and dopaminergic neurons in the context of PD. Deficits in CD200-CD200R signaling are associated with enhanced microglial activation and loss of dopaminergic neurons in the substantia nigra in aged rats and animal models of PD (Sung, et al., 2012,Wang, et al., 2011,Zhang, et al., 2011). Our data suggest that CD200 may be implicated in the protection of dopaminergic neurons in PD due to its suppression of pro-inflammatory microglial activation and the production of GDNF. CD200 therefore has potential benefits as a therapeutic for both AD and PD.
Since a pro-inflammatory environment is suppressive to neurogenesis (Bastos, et al., 2008,Deng, et al., 2010,Ekdahl, et al., 2003,Hoehn, et al., 2005,Monje, et al., 2003), our data suggest that expression of CD200 alters microglial activation status from pro- to anti-inflammatory and restores homeostatic activities of microglia for neural differentiation and maturation. This is similar to our previous findings, where direct co-culture of neural stem cells with IL-10-treated microglia increased neuronal differentiation and cell survival and reduced glial differentiation (Kiyota, et al., 2011b). Previous studies support this by showing that CD200Fc ligand reduces the production of pro-inflammatory cytokines in the presence of Aβ (Lyons, et al., 2012). We showed that CD200 treatment of microglia increased expression of CD200R1 and significantly reduced CD200R3. It moderately reduced Trem2, TGF-β1, and TGF-βR1 expression. Despite this, we saw an increase in expression of Tyrobp, suggesting that there is a switch in microglial activation that reduces CD200R3, perhaps as part of a feedback mechanism.
Of note, AAV2/1-GFP injection into APP mice reduced proliferation and differentiation in the DG compared to APP saline-injected mice. This indicates a toxic effect of the GFP or tetracycline transactivator (tTA) protein expressed from the AAV cassette. Since we did not observe a toxic effect by GFP expression itself, it is likely to be the effect of tTA or the combination of GFP and tTA expression in the same cells. Transgenic expression of tTA is cytotoxic in certain mouse strains, and this cytotoxicity can be exacerbated by concomitant transgenic expression of APP (Han, et al., 2012). Nonetheless, our AAV2/1-CD200-injected WT and APP mice did not exhibit a reduction in BrdU+ or Dcx+ cells in the DG, suggesting that CD200 expression can overcome any tTA-induced toxicity. Interestingly, increasing CD200 expression did not enhance cellular proliferation or differentiation in WT mice, indicating CD200 may only be effective in the presence of neuroinflammation.
The other major finding is that AAV2/1-CD200 reduced amyloid levels in the hippocampal region of APP mice. This is correlated with an anti-inflammatory change in microglia, as indicated by reduced NOS2+ and increased YM1+ Iba1+ cells. This coincides with previous research demonstrating that microglia treated with anti-inflammatory cytokines are more capable of Aβ phagocytosis than microglia treated with pro-inflammatory mediators (Koenigsknecht-Talboo and Landreth, 2005). Another study demonstrated that pro-inflammatory cytokines interferon-γ and tumor necrosis factor-α suppress Aβ degradation in microglia, whereas anti-inflammatory cytokines IL-4 or TGF-β enhance it (Yamamoto, et al., 2008). Our in vitro experiment suggests that the reduction in Aβ is partially due to increased microglial phagocytosis mediated by CD200. Microglial phagocytosis may also be required for maintenance of the homeostatic environment of the SGZ for neuronal differentiation and survival (Lu, et al., 2011,Paolicelli, et al., 2011). This would suggest that the concomitant increase in neurogenesis and reduction in amyloid is mediated by enhanced microglial phagocytosis of both amyloid and apoptotic neural stem cells in the SGZ.
We found that microglia pre-treated with Aβ42 had a reduced percentage of CD11b+ cells. CD11b is a component of the MAC-1 complex (CD11b/CD18) and is constitutively expressed on the surface of microglia (Akiyama and McGeer, 1990). Its expression can be altered after activation by specific stimulations (Kalaria, 1999,McGeer, et al., 1993,McGeer and McGeer, 1995). CD11b may be required for phagocytosis of amyloid as blocking of CD11b reduces microglial phagocytosis of artificial amyloid deposits consisting of heat-killed yeasts coated with Aβ42 (Choucair-Jaafar, et al., 2011). In addition, we previously showed that incubation of primary cultured murine microglia with high concentrations of Aβ42 induces cytotoxicity (Kiyota, et al., 2009b). This is replicated in this study. CD200 protected microglia against Aβ42-induced cytotoxicity, suggesting that CD200 protects microglia from inflammatory toxicity and induces their CD1 1b expression.
These data should be interpreted with some caution. Other cell types, including astrocytes, oligodendrocytes, and dendritic cells, are known to express the CD200Rs (Barclay, 1981,Barclay, et al., 2002,Chitnis, et al., 2007,Koning, et al., 2010). Thus, it is likely that other CD200R+ cells may contribute to the beneficial effects of CD200 expression in the APP mouse hippocampus. A recent paper by Butovsky et al (Butovsky, et al., 2014) characterized the gene expression profile of adult microglia and found that P2ry12 is a novel microglia-specific maker. Using their custom P2ry12 antibody (for resident microglia) and CD169 (for peripheral monocytes), we observed positive staining for both P2ry12 and CD169 in the WT and APP DG, and therefore cannot discount the contribution of infiltrating peripheral monocytes. We recently demonstrated that there was a depletion of resident microglia and an infiltration of peripheral monocytes into the CNS during the disease stage of aged tau transgenic mice (Asai, et al., 2014). These infiltrating monocytes predominately displayed a pro-inflammatory phenotype, whereas resident microglia within the CNS maintained an anti-inflammatory phenotype (Asai, et al., 2014). This indicates that infiltrating peripheral monocytes may be culprits for inflammatory changes in the AD brain. This could be further augmented by reductions in CD200.
5. Conclusions
In summary, we show that AAV2/1-CD200 injection into the hippocampal region of the APP mouse brain is effective at restoring neurogenesis and reducing AD-like pathology. We posit that this is partly through suppression of pro-inflammatory microglial activation and enhanced phagocytosis. This makes CD200 worthy of further study to understand the mechanisms of its effects and as a therapeutic to treat AD.
Supplementary Material
Highlights.
Doxycycline-inducible adeno-associated viral vector is developed
Adeno-associated virus-mediated CD200 expression restores hippocampal adult neurogenesis and reduce amyloid load in an AD mouse model after post-symptomatic gene delivery
CD200 enhances microglia-mediated neural differentiation of neural stem cells
CD200 enhances microglial phagocytosis of amyloid-beta peptide
Acknowledgments
The authors particularly thank Dr. Susan Leeman for her generous support of this study and guidance in writing of the manuscript. We also thank Dr. K. Ashe and Dr. G. Carlson for providing Tg2576 mice and Dr. K. Duff for providing M146L PS1 mice, and Dr. Butovsky for the anti-P2ry12 antibody. We would also like to thank Henry Chao, Ahmed El-Araby, Jonathan Boucher, and Grant Yonemoto for assistance with tissue processing and data collection. This work was supported in part by National Institute of Health grant 5T32GM008541 (MMV), 7R21AG032600 (TI), Alzheimer’s Art Quilt Institute Award (TI), Coins for Alzheimer’s Research Trust Award (TI), and Alzheimer’s Association (TI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. Journal of neuroimmunology. 1990;30(1):81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer's 1907 paper, "Uber eine eigenartige Erkankung der Hirnrinde". Clin Anat. 1995;8(6):429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Woodbury ME, Yonemoto GM, Cui L, Ikezu T. Accelerated neurodegeneration and neuroinflammation in transgenic mice expressing P301L tau mutant and tau-tubulin kinase 1. The American journal of pathology. 2014;184(3):808–818. doi: 10.1016/j.ajpath.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AN. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981;44(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends in immunology. 2002;23(6):285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- Bastos GN, Moriya T, Inui F, Katura T, Nakahata N. Involvement of cyclooxygenase-2 in lipopolysaccharide-induced impairment of the newborn cell survival in the adult mouse dentate gyrus. Neuroscience. 2008;155(2):454–462. doi: 10.1016/j.neuroscience.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiology of disease. 2006;24(1):1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Borroni B, Ferrari F, Galimberti D, Nacmias B, Barone C, Bagnoli S, Fenoglio C, Piaceri I, Archetti S, Bonvicini C, Gennarelli M, Turla M, Scarpini E, Sorbi S, Padovani A. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiology of aging. 2014;35(4):e7–e10. doi: 10.1016/j.neurobiolaging.2013.09.017. 934. [DOI] [PubMed] [Google Scholar]
- Boscia F, Esposito CL, Di Crisci A, de Franciscis V, Annunziato L, Cerchia L. GDNF selectively induces microglial activation and neuronal survival in CA1/CA3 hippocampal regions exposed to NMDA insult through Ret/ERK signalling. PloS one. 2009;4(8):e6486. doi: 10.1371/journal.pone.0006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18(2):93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M. Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(31):11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady J, Koval ED, Benitez BA, Zaidman C, Jockel-Balsarotti J, Allred P, Baloh RH, Ravits J, Simpson E, Appel SH, Pestronk A, Goate AM, Miller TM, Cruchaga C, Harms MB. TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA neurology. 2014;71(4):449–453. doi: 10.1001/jamaneurol.2013.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YP, Fang KM, Lee TI, Tzeng SF. Regulation of microglial activities by glial cell line derived neurotrophic factor. Journal of cellular biochemistry. 2006;97(3):501–511. doi: 10.1002/jcb.20646. [DOI] [PubMed] [Google Scholar]
- Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT, Khoury SJ. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. The American journal of pathology. 2007;170(5):1695–1712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choucair-Jaafar N, Laporte V, Levy R, Poindron P, Lombard Y, Gies JP. Complement receptor 3 (CD11b/CD18) is implicated in the elimination of beta-amyloid peptides. Fundamental & clinical pharmacology. 2011;25(1):115–122. doi: 10.1111/j.1472-8206.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Gagnon J, Williams AF, Barclay AN. MRC OX-2 antigen: a lymphoid/neuronal membrane glycoprotein with a structure like a single immunoglobulin light chain. EMBO J. 1985;4(1):113–118. doi: 10.1002/j.1460-2075.1985.tb02324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuyvers E, Bettens K, Philtjens S, Van Langenhove T, Gijselinck I, van der Zee J, Engelborghs S, Vandenbulcke M, Van Dongen J, Geerts N, Maes G, Mattheijssens M, Peeters K, Cras P, Vandenberghe R, De Deyn PP, Van Broeckhoven C, Cruts M, Sleegers K. Investigating the role of rare heterozygous TREM2 variants in Alzheimer's disease and frontotemporal dementia. Neurobiology of aging. 2014;35(3):e11–e19. doi: 10.1016/j.neurobiolaging.2013.09.009. 726. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(43):13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH. Alzheimer's disease. A double-labeling immunohistochemical study of senile plaques. The American journal of pathology. 1988;132(1):86–101. [PMC free article] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. Journal of virology. 2005;79(15):9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. TREM2 variants in Alzheimer's disease. The New England journal of medicine. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Lohmann E, Bras JM, Gibbs JR, Rohrer JD, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, Emre M, Singleton A, Hardy J. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA neurology. 2013;70(1):78–84. doi: 10.1001/jamaneurol.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HJ, Allen CC, Buchovecky CM, Yetman MJ, Born HA, Marin MA, Rodgers SP, Song BJ, Lu HC, Justice MJ, Probst FJ, Jankowsky JL. Strain background influences neurotoxicity and behavioral abnormalities in mice expressing the tetracycline transactivator. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(31):10574–10586. doi: 10.1523/JNEUROSCI.0893-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. Journal of neurochemistry. 2002;83(6):1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke; a journal of cerebral circulation. 2005;36(12):2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nature medicine. 1998;4(1):97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Honda S, Nakajima K, Nakamura Y, Imai Y, Kohsaka S. Rat primary cultured microglia express glial cell line-derived neurotrophic factor receptors. Neuroscience letters. 1999;275(3):203–206. doi: 10.1016/s0304-3940(99)00769-7. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nature reviews Immunology. 2008;8(10):816–822. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer's disease. The New England journal of medicine. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN. Microglia and Alzheimer's disease. Current opinion in hematology. 1999;6(1):15–24. doi: 10.1097/00062752-199901000-00004. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Ingraham KL, Jacobsen MT, Xiong H, Ikezu T. FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer's disease and has therapeutic implications for neurocognitive disorders. Proceedings of the National Academy of Sciences of the United States of America. 2011a;108(49):E1339–E1348. doi: 10.1073/pnas.1102349108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2011b doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer's disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24(8):3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Yamamoto M, Schroder B, Jacobsen MT, Swan RJ, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T. AAV1/2-mediated CNS gene delivery of dominant-negative CCL2 mutant suppresses gliosis, beta-amyloidosis, and learning impairment of APP/PS1 mice. Mol Ther. 2009a;17(5):803–809. doi: 10.1038/mt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Yamamoto M, Xiong H, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PloS one. 2009b;4(7):e6197. doi: 10.1371/journal.pone.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(36):8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Obata K, Mukai K, Sato S, Takai T, Minegishi Y, Karasuyama H. Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J Immunol. 2007;179(10):7093–7100. doi: 10.4049/jimmunol.179.10.7093. [DOI] [PubMed] [Google Scholar]
- Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. Journal of neuropathology and experimental neurology. 2009;68(2):159–167. doi: 10.1097/NEN.0b013e3181964113. [DOI] [PubMed] [Google Scholar]
- Koning N, van Eijk M, Pouwels W, Brouwer MS, Voehringer D, Huitinga I, Hoek RM, Raes G, Hamann J. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J Innate Immun. 2010;2(2):195–200. doi: 10.1159/000252803. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, Kipnis J. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nature cell biology. 2011;13(9):1076–1083. doi: 10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Costello DA, Murphy N, Lynch MA. Dok2 mediates the CD200Fc attenuation of Abeta-induced changes in glia. Journal of neuroinflammation. 2012;9:107. doi: 10.1186/1742-2094-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(31):8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, McQuillan K, Deighan BF, O'Reilly JA, Downer EJ, Murphy AC, Watson M, Piazza A, O'Connell F, Griffin R, Mills KH, Lynch MA. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain, behavior, and immunity. 2009;23(7):1020–1027. doi: 10.1016/j.bbi.2009.05.060. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Neuroinflammation in Alzheimer's disease and mild cognitive impairment: a field in its infancy. Journal of Alzheimer's disease : JAD. 2010;19(1):355–361. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG. Microglia in degenerative neurological disease. Glia. 1993;7(1):84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain research Brain research reviews. 1995;21(2):195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2004;58(2):167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- Mihrshahi R, Barclay AN, Brown MH. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J Immunol. 2009;183(8):4879–4886. doi: 10.4049/jimmunol.0901531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minati L, Edginton T, Bruzzone MG, Giaccone G. Current concepts in Alzheimer's disease: a multidisciplinary review. Am J Alzheimers Dis Other Demen. 2009;24(2):95–121. doi: 10.1177/1533317508328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, Hatanpaa KJ, Lomen-Hoerth C, Kertesz A, Bigio EH, Lippa C, Josephs KA, Knopman DS, White CL, 3rd, Caselli R, Mackenzie IR, Miller BL, Boczarska-Jedynak M, Opala G, Krygowska-Wajs A, Barcikowska M, Younkin SG, Petersen RC, Ertekin-Taner N, Uitti RJ, Meschia JF, Boylan KB, Boeve BF, Graff-Radford NR, Wszolek ZK, Dickson DW, Rademakers R, Ross OA. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson's disease. Molecular neurodegeneration. 2013;8:19. doi: 10.1186/1750-1326-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DK, Kiyota T, Mosley RL, Gendelman HE. A model of nitric oxide induced alpha-synuclein misfolding in Parkinson's disease. Neuroscience letters. 2012;523(2):167–173. doi: 10.1016/j.neulet.2012.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Kim SC, Hong HP, Park CY, Shin MS, Kim CJ, Seo JH, Kim DY, Kim DJ, Cho HJ. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson's disease mice. Life sciences. 2012;91(25–26):1309–1316. doi: 10.1016/j.lfs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2010 doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer's disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215(1):5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Zhang S, Yan ZQ, Zhao YX, Zhou HY, Wang Y, Lu GQ, Zhang JD. Impaired CD200-CD200R–mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free radical biology & medicine. 2011;50(9):1094–1106. doi: 10.1016/j.freeradbiomed.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH, Barclay AN. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171(6):3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. The American journal of pathology. 2007;170(2):680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kiyota T, Walsh SM, Liu J, Kipnis J, Ikezu T. Cytokine-mediated inhibition of fibrillar amyloid-beta peptide degradation by human mononuclear phagocytes. J Immunol. 2008;181(6):3877–3886. doi: 10.4049/jimmunol.181.6.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, Zhang YJ, Ding JQ, Chen SD. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson's disease. Journal of neuroinflammation. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.