Abstract
Purpose
With ethical requirements to the enrollment of lower risk subjects, osteoporosis trials are underpowered to detect reduction in hip fractures. Different skeletal sites have different levels of fracture risk and response to treatment. We sought to identify fracture sites which cluster with hip fracture at higher than expected frequency; if these sites respond to treatment similarly, then a composite fracture endpoint could provide a better estimate of hip fracture reduction.
Methods
Cohort study using Veterans Affairs and Medicare administrative data. Male Veterans (n=5,036,536) aged 50-99 years receiving VA primary care between1999-2009 were included. Fractures were ascertained using ICD9 and CPT codes and classified by skeletal site. Pearson correlation coefficients, logistic regression and kappa statistics, were used to describe the correlation between each fracture type and hip fracture within individuals, without regards to the timing of the events.
Results
595,579 (11.8%) men suffered 1 or more fractures and 179,597 (3.6%) suffered 2 or more fractures during the time under study. Of those with one or more fractures, rib was the most common site (29%), followed by spine (22%), hip (21%) and femur (20%). The fracture types most highly correlated with hip fracture were pelvic/acetabular (Pearson correlation coefficient 0.25, p<0.0001), femur (0.15, p<0.0001), and shoulder (0.11, p<0.0001).
Conclusions
Pelvic, acetabular, femur, and shoulder fractures cluster with hip fractures within individuals at greater than expected frequency. If we observe similar treatment risk reductions within that cluster, subsequent trials could consider use of a composite endpoint to better estimate hip fracture risk.
Keywords: Fractures, correlation, osteoporosis, Veterans
Introduction
Current guidance from both the U.S. Food and Drug Association and the European Medicines Agency require that new osteoporosis pharmacotherapies seeking registration have anti-fracture efficacy demonstrated in an 18-24 month randomized, placebo-controlled trial[1, 2]. However, the ethics of using a placebo control in subjects at high risk for fracture have been widely questioned, since currently available pharmacotherapies reduce fracture risk by 30-75%. A consensus conference sponsored by the American Society for Bone and Mineral Research, the International Society for Clinical Densitometry, and the National Osteoporosis Foundation suggested that enrolling high risk patients in placebo controlled osteoporosis trials could be ethical provided there was clear documentation that they understood their risk, that they had failed prior therapy, or did not have access to standard treatment [3]. However, other opinion leaders and ethics boards have concluded that it is difficult to identify and recruit such patients, and point out that investigators have conflicts of interest which render it nearly always unethical to recruit subjects at highest risk [4, 5]. It has therefore been argued that trials should focus on those at lower risk (e.g., those with osteopenia and/or no prior fracture) or compare two active treatment arms to assess non-inferiority.
As a result of this shift, recent osteoporosis trials are frequently underpowered to detect differences in hip fracture rates, because they are enrolling a lower risk population who have fewer events or are utilizing an active comparator arm. This is particularly problematic for trials in special populations such as men, or trials with specific co-morbidities in which patient enrollment tends to be lower than in initial registration trials [6, 7]. Even in large trials which successfully show a reduction in hip fractures, the estimate for reduction in hip fracture rates is imprecise; for example in the FREEDOM trial comparing fracture rates in post-menopausal women with osteoporosis treated with denosumab vs. placebo, the hazard ratio was 0.60, but the 95% confidence interval ranged from a 0.37-0.97 [8]. Since hip fractures are the most clinically devastating and costly type of fracture, the lack of a precise estimate of reduction in hip fracture rates for a given treatment is problematic for clinicians, patients, and policy makers seeking to make informed care decisions.
We may be able to learn about hip fracture risk by examining other types of fractures. Skeletal sites have differing properties, such as the relative proportions of cortical and trabecular bone, which result in varying fracture risks and differential responses to treatment. For example, population based qCT and finite element analysis studies of bone microarchitecture and strength have revealed that age-related changes in trabecular and cortical bone loss vary by skeletal site and gender[9]. Animal studies have shown differential trabecular and cortical response to treatment with various osteoporosis pharmacotherapies by skeletal site[10]. If we could identify skeletal sites with similar fracture risks and responses to therapy as the hip, then a composite fracture endpoint incorporating hip plus related fracture types could be used to improve the power and precision of the estimate of reduction in hip fracture rates.
The purpose of this study, therefore, was twofold: to identify correlations between hip and other major fracture types in a large population of older men; and to identify major fracture types which clustered with hip fractures at greater than expected frequency. If these fracture types are subsequently shown to have similar response to therapy, a composite fracture endpoint could be used to provide a valid estimate of hip fracture reduction with smaller sample size requirements.
Methods
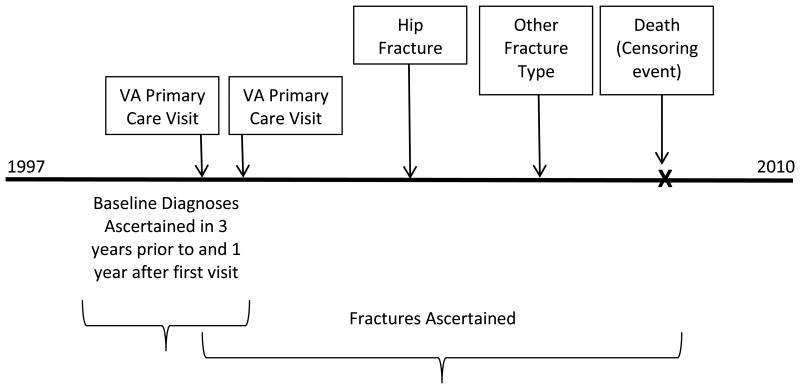
The sample was derived from administrative data bases from a population-based retrospective cohort study of all male Veterans ages 50-99 years receiving primary care in the Veterans Affairs (VA) health care system between 1997 and 2010. Subjects were included if they had at least 2 primary care clinic visits within a 2 year period during the observation period (n=5,036,536) and their VA Medical Center was offering DXA screening. Baseline diagnoses were ascertained in the 3 years prior to and 1 year following the first primary care visit, while fracture assessment began at the first primary care visit and continued until the end of the study period or death (figure 1). The database was created to explore the impact of osteoporosis screening on outcomes in older men; therefore subjects with a diagnosis of osteoporosis or a fracture code in the 3 years prior to the study period (1996 - 1999) were excluded. Subjects who were enrolled in Medicare managed care plans during the study period were also excluded.
Figure 1. Cohort schematics for follow-up time and event ascertainment. Note that the order of the fracture events did not matter for this analysis.
While all 5,036,536 of these patients received VA primary care services, a majority of Veterans also use their Medicare benefit, particularly for acute events such as fractures. Therefore administrative data from the VA and Center for Medicare and Medicaid Services (CMS) Medicare Parts A and B was combined with the VA data. Data sources included inpatient and outpatient treatment files, medications (VA only), and select labs (VA only). Fractures were ascertained using ICD9 and CPT codes from inpatient and outpatient encounters, and classified as hip (femoral neck, intertrochanteric, subtrochanteric fractures; ICD9 codes 820.0-820.9, procedure codes 79.05-79.65, and CPT codes 27235-27269), forearm (radius, ulna, or both; ICD9 codes 813.0-813.93, procedure codes 79.02-79.62, CPT codes 25505-25652 and 24650-24635), spine (thoracic and lumbar only; ICD9 codes 805.2-806.9 and CPT codes 22305-22525 and 22851), shoulder (humerus; ICD9 codes 812.X, procedure codes79.01-79.61 and CPT codes 23605-24582), pelvic/acetabular (ICD9 codes 808.0-808.9 and CPT codes 27193-27228), rib/clavicle (ICD9 codes 807.0-807.19, 810.0-810.9 and CPT codes 21800-21810, 23500-23515), distal femoral (ICD9 codes 821.0-821.39 and CPT codes 27500-27514), tibial/fibular (ICD9 codes 823.0-823.9, procedure code 79.06, and CPT codes 27530-27828), and other. Skull, facial digital, and pathological fracture codes were excluded. To avoid double counting due to repeated coding of the same event over time, each individual was counted as having a fracture type no more than once. Distal femoral fractures that occurred within 6 months of a hip fracture were excluded to decrease the probability of misclassification of hip fractures as femur fractures. Because high trauma might lead to fracture types occurring together without a common underlying risk, we defined potentially traumatic fractures as those in which more than 1 type of fracture was coded within 7 days of another fracture type. However, these were excluded in sensitivity analyses.
We calculated frequencies, percentages, means and standard deviations to describe characteristics, including fracture incidence, of the study population. We calculated these statistics overall, and for those who had a hip plus at least 1 other type of fracture (the population used in the latent class analysis). Pearson correlation coefficients for each pair of hip × (femur, forearm, pelvic/acetabular, rib, shoulder, spine, tibia) fracture type were calculated for the full cohort. These correlations reflected the co-occurrence of the fracture types within individuals, and without regards to the order or timing of the events. Kappa statistics were also calculated to describe the proportion of potential agreement beyond chance. The Mantel-Haenszel method was used to calculate the odds of each fracture type among patients with hip fractures compared to the odds of the same fracture type among those without hip fractures, along with associated 95% confidence intervals (95% CIs) around these odds ratios (ORs). Because we were interested in the correlation of fractures within individuals, regardless of their individual characteristics or level of risk, no patient level covariates were adjusted for in the Mantel Haenszel analyses. Latent class analysis[11] was used to identify clusters of highly correlated fractures in the group of subjects with hip plus at least one other fracture type; this method is used to identify unmeasured class membership among subjects using categorical or continuous observed variables, and in this case reflected “classes” of fracture types where the conditional probability that groups of fracture types co-occurred in greater than expected rates. All analyses were performed using SAS v9.2 software.[12]
Results
Characteristics of the study population are described in Table 1. The follow-up time for the n=5,036,536 men in this study ranged from 0 to 10 years, with an average of 5.4 years (± 3.1 std. dev.). During follow-up, 595,579 (11.8%) of men in the study population experienced 1 or more fractures and 179,597 (3.6%) experienced 2 or more types of fractures (range 0-9 fracture types) over the study period. Rib fracture was the most common specific fracture type (29% of individuals with fracture), followed by spine (22%), hip (21%) and femur (20%). Of these fractures, 107,619 (2.1%) were potentially traumatic as defined above. Compared to the full cohort, those with hip and at least 1 other fracture type were older, more likely to be white, have a history of alcohol dependence and glucocorticoid use >90 days; given the large sample size, all comparisons were statistically different.
Table 1. Patient characteristics and fracture incidence in the study cohort of Veterans followed from 1999-2009.
| Characteristic | Overall Cohort (n=5,036,536) N |
Male Veterans with Hip and ≥ 1 Other Fracture Type during Study Period (n=60,614) N |
|---|---|---|
|
| ||
| Mean age, years (SD) | 66 (10.0) | 76 (9.5) |
|
| ||
| Race/Ethnicity (%) | ||
| White, non-Hispanic | 3,432,497 (68.2) | 44,810 (73.9) |
| Black, non-Hispanic | 575,131 (11.4) | 3,271 (5.4) |
| Other | 204,827 (4.1) | 1,888 (3.1) |
| Unknown | 824,081 (16.4) | 10,645 (17.6) |
|
| ||
| Mean BMI, kg/m2 (SD) | 29.0 (5.5) | 26.7 (5.0) |
|
| ||
| Alcohol Dependence (%)a | 1,004,278 (19.9) | 13,913 (23.0) |
|
| ||
| Glucocorticoid use ≥ 90 days (%) | 73,834 (1.5) | 1,614 (2.7) |
|
| ||
| One or more fractures during follow-up (%) | ||
| Any fracture | 595,579 (11.8) | 60,614 (100) |
| Hip | 125,479 (2.5) | 60,614 (100) |
| Rib | 174,859 (3.5) | 16,347 (27.0) |
| Distal femur | 120,713 (2.4) | 21,498 (35.5) |
| Forearm | 65,617 (1.3) | 7,256 (12.0) |
| Shoulder | 58,318 (1.2) | 10,357 (17.1) |
| Tibia/fibula | 52,439 (1.0) | 5,410 (8.9) |
| Pelvis/acetabulum | 32,906 (0.7) | 16,894 (27.9) |
| Spine | 130,950 (2.6) | 14,613 (24.1) |
| Otherb | 92,618 (1.8) | 8,258 (13.6) |
|
| ||
| Potentially traumatic fractures during follow-up | 107,619 (2.1) | 37,282 (61.5) |
All comparisons statistically significant at P<0.001.
Alcohol dependence defined as 1 or more of ICD9 codes 290.X, 291.X, 303.X, 305.00, or CPT code 4320F.
Other includes all other fractures except skull, hand, and foot.
The fracture types most highly correlated with hip fracture were pelvic/acetabular (Pearson correlation coefficient 0.25, p<0.0001), femur (0.15, p<0.0001), and shoulder (0.11, p<0.0001). Mantel Haenszel odds ratios for the association between hip fractures and each remaining fracture type, as well as kappa statistics (reflecting the proportion of potential agreement above chance) for each fracture type with hip fracture are reported in Table 2. Latent class analysis revealed good loading onto single factors (rho estimates <0.10 or >0.90) but no convergence over 8 clusters. This suggests a generalized association between all of the fracture types, with no independent fractures which are not related to any other fracture types.
Table 2. Odds ratios (ORs) and 95% Confidence Intervals (CIs) for the association between specified fracture type and hip fracture, and Pearson Correlation Coefficients, and Kappa statistics for the correlation between hip fracture and other fracture types.
| Fracture Types | Odds Ratiosa All Fractures (95% CI) | Odds Ratiosa Excluding Potential Trauma (95% CI) | Kappab All Fractures | Kappab Excluding Potential Trauma | Pearson Correlation Coefficientc All Fractures | Pearson Correlation Coefficientc Excluding Potential Trauma |
|---|---|---|---|---|---|---|
| Pelvis/Acetabulum n=32,906 | 47.6 (46.5, 48.6) | 12.0 (11.5, 12.5) | 0.21 | 0.05 | 0.25 | 0.07 |
| Femur n=120,713 | 10.0 (9.9, 10.2) | 4.5 (4.4, 4.7) | 0.15 | 0.05 | 0.15 | 0.05 |
| Shoulder n=58,318 | 9.1 (8.9, 9.3) | 4.6 (4.5, 4.8) | 0.10 | 0.04 | 0.11 | 0.04 |
| Spine n=130,950 | 5.4 (5.3, 5.5) | 3.7 (3.7, 3.8) | 0.09 | 0.05 | 0.09 | 0.05 |
| Forearm n=65,617 | 5.1 (5.0, 5.2) | 3.0 (2.9, 3.1) | 0.06 | 0.02 | 0.06 | 0.03 |
| Tibia/Fibula n=52,439 | 4.7 (4.5, 4.8) | 2.8 (2.6, 2.9) | 0.05 | 0.01 | 0.05 | 0.02 |
| Rib n=174,859 | 4.5 (4.4, 4.6) | 3.2 (3.1, 3.2) | 0.08 | 0.05 | 0.08 | 0.05 |
| Other n=92,618 | 4.0 (3.9, 4.1) | 2.3 (2.2, 2.4) | 0.06 | 0.02 | 0.06 | 0.02 |
For the odds of specified fracture in patients with hip fractures compared to the same odds in patients without hip fractures
The chance adjusted association between specified fracture type and hip fracture
For the correlation between the specified fracture type and hip fracture
Sensitivity analyses removing potentially traumatic fractures from the analysis decreased the magnitude of the association between hip fracture and each other fracture type by 1.4 to 4.0 times, although the direction of the association remained the same (Table 2). The addition of race to the models resulted in only very minor changes to the odds ratios for the association of each fracture type with hip fracture, indicating that there was no meaningful confounding of the associations between hip and other fracture types by race.
Discussion
Accurately estimating the effect of osteoporosis therapies on hip fracture risk is important for clinical decision making and healthcare policy. This estimation is particularly challenging in today's context of current trials enrolling lower risk populations, and may become more challenging with a shift toward comparative effectiveness studies in the future. In other fields, such as cardiology, composite clinical endpoints have been used successfully to address this issue.[13] This analysis is the first step in defining a rational composite fracture endpoint that could be used to help approximate the true hip fracture risk reduction rate. We identified several fracture types that are strongly associated with hip fracture within individuals, with odds ratios ranging from 9.1 to 47.6; specifically distal femur, pelvis/acetabular, and humerus fractures are highly correlated with hip fracture. If treatment-related risk reduction is found to be similar among hip and these additional 3 fracture types, the 3 additional fracture types might serve as proxies for hip fractures. Combining these proxies with hip fractures could increase the effective number of ‘events' in a trial, reduce sample size requirements, and improve the precision of the estimates of effectiveness of a therapy for reduction in “hip fracture-like” fractures.
We were not able to identify clusters or “latent classes” of fracture types that group with hip fracture and no other fracture types. This is not surprising since osteoporosis affects the entire skeleton, albeit to varying degrees and the underlying risk for falls generally remains elevated within individuals over time and predisposes to all types of fractures. Nevertheless, the odds ratios for pelvic, humerus, and distal femur fracture in association with hip fracture were approximately 10 or more, and were nearly double the odds ratios for other fracture types. The magnitude of these associations suggests that these fracture types are more strongly associated with hip fracture than other sites in the skeleton. While correlation coefficient of 0.25 is usually considered modest, in the context of rare events within individuals, and with consideration of the associated odds ratios, we believe that the observed values are clinically meaningful.
This study has several important strengths. Identifying correlations within fracture types requires a very large sample with adequate follow-up time and nearly complete fracture acquisition. We included more than 5 million older men and merged both VA and Medicare data over 10 years of follow-up. Prior studies have suggested that accurate fracture ascertainment for non-vertebral fractures in administrative data exceeds 95%, with high sensitivity and specificity; for example a recent study found 97% sensitivity for hip fracture in Medicare data alone [14]. While 2/3 of vertebral fractures are clinically silent, others have demonstrated that the positive predictive value of a vertebral fracture claim is 87% [15]. Since clinical vertebral fractures are also an important study endpoint, this cohort provides valuable information despite the lack of availability of silent vertebral fracture information.
There are also limitations which should be considered. The cohort included only men, and these correlations need to be examined in women as there may be important gender-related differences in bone characteristics. Veterans have a higher risk of co-morbidities and other fracture risk factors than non-Veterans [16-19], therefore the generalizability of our findings to populations outside of the VA can be questioned. However, while Veterans' absolute fracture risk may be higher than that of other men due to higher risks of co-morbidities and other factors, this study looked at correlations of fractures within individuals, and there is no clear physiologic rationale as to why the correlations among fracture types should be different in Veterans than non-Veterans. In administrative data there is a possibility of miscoding or double-counting the same fracture; we took steps to minimize potential study bias by limiting coding to a single fracture of each type per individual, and excluding fractures close physical proximity (e.g., hip and distal femur) when they occurred within 6 months. Because this analysis only examined correlations between fracture types and not fracture rates, there is no bias introduced from counting each individual as having a fracture type no more than once, but the total number of fractures reported here for the cohort may be lower than actually occurred. While we excluded pathologic fracture codes, the cohort may have included fractures related to malignancy or infection. There is no accurate way to distinguish high trauma fractures from low trauma fractures in administrative data, and our definition of “potentially traumatic fractures” (2 or more fracture types within 7 days) likely misclassifies some osteoporotic fractures as traumatic. However when we excluded potentially traumatic fractures in a sensitivity analysis, the magnitude of our odds ratios and Kappa values were reduced, but the order was not changed, suggesting that the association between these fracture types remains relevant.
Before a composite fracture outcome could be considered, it will be important to confirm that pharmacologic treatments result in similar fracture risk reductions for all fracture types within the cluster. Because use of pharmacologic therapy for osteoporosis was not randomized, such analysis would be biased within our cohort; meta-analysis of randomized controlled trials will be the most appropriate data to accomplish this task.
In conclusion, we found that pelvic/acetabular, distal femur, and humerus fractures correlate with hip fractures nearly twice as much as with other fracture types. If these 3 skeletal sites are shown to have risk reductions to that of hip upon osteoporosis therapy, a composite fracture endpoint could potentially be used to increase the number of outcomes of interest to provide a more precise estimate of hip fracture risk in clinical trials.
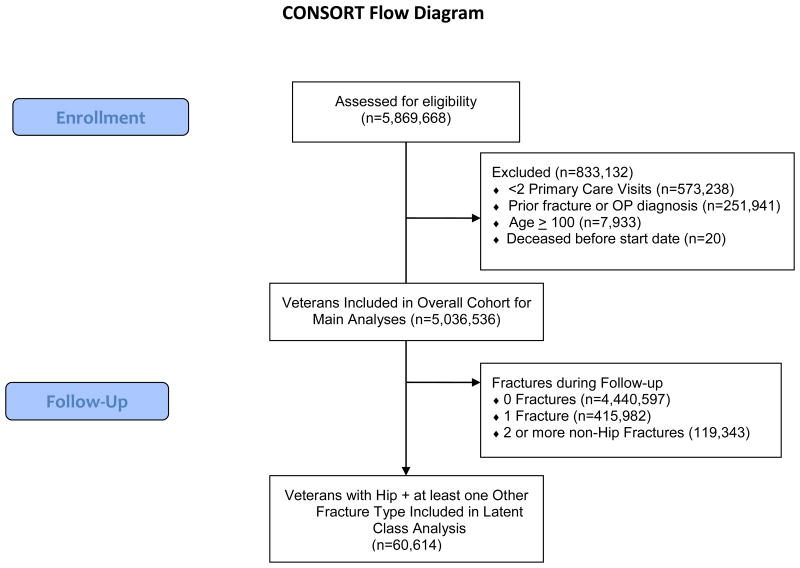
Figure 2. CONSORT diagram illustrating selection of subjects for analysis.
Acknowledgments
This work was funded by Department of Defense, W81XWH-12-2-0093, and NIA 2P30AG028716-06, the Claude D. Pepper Older Americans Independence Center
Footnotes
Conflicts of Interest: Dr. Colón-Emeric is a consultant to Novartis and Amgen, and receives research support from Amgen. Dr. Lyles is a consultant to Novartis, Amgen, and UCB and receives research support from Amgen, Novartis, and Kirin Pharmaceuticals. Dr. Lyles is co-inventors of US patent applications 20050272707 “methods for preventing or reducing secondary fractures after hip fracture, 12532285 “Medication kits and formulations for preventing, treating or reducing secondary fractures after previous fracture. Drs. Colón-Emeric and Lyles are co-inventors of US patent applications 20104717 “Bisphosphonate compositions and methods for treating heart failure, and 61560328 “Bisphosphonate compositions and methods for treating and/or reducing cardiac dysfunction”. They are equity owners of BisCardia, Inc. Carl F. Pieper, Janet Grubber, Lynn Van Scoyoc, Merritt Schnell, Courtney Harold Van Houtven, Megan Pearson, Joanne LaFleur, and Robert Adler declare that they have no conflict of interest.
References
- 1.European Medicines Agency. Committee for Medicinal Products for Human Use. London: 2006. Guideline on the Evaluation of Medicinal Products in the Treatment of Primary Osteoporosis. [Google Scholar]
- 2.Food and Drug Administration. Food and Drug Administration 2004 Draft guidance for industry on the preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis. Federal Register. 2004;69:6673–6675. [Google Scholar]
- 3.Silverman SL, Cummings SR, Watts NB, for the Consensus Panel of the Asbmr I, Nof Recommendations for the Clinical Evaluation of Agents for Treatment of Osteoporosis: Consensus of an Expert Panel Representing the American Society for Bone and Mineral Research (ASBMR), the International Society for Clinical Densitometry (ISCD), and the National Osteoporosis Foundation (NOF) Journal of Bone and Mineral Research. 2008;23:159–165. doi: 10.1359/jbmr.070905. [DOI] [PubMed] [Google Scholar]
- 4.Stein CM, Ray WA. The Ethics of Placebo in Studies with Fracture End Points in Osteoporosis. New England Journal of Medicine. 2010;363:1367–1370. doi: 10.1056/NEJMsb1006120. [DOI] [PubMed] [Google Scholar]
- 5.Rosen CJ, Khosla S. Placebo-Controlled Trials in Osteoporosis — Proceeding with Caution. New England Journal of Medicine. 2010;363:1365–1367. doi: 10.1056/NEJMsb1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boonen S, Reginster J-Y, Kaufman J-M, et al. Fracture Risk and Zoledronic Acid Therapy in Men with Osteoporosis. New England Journal of Medicine. 2012;367:1714–1723. doi: 10.1056/NEJMoa1204061. [DOI] [PubMed] [Google Scholar]
- 7.Smith MR, Egerdie B, Toriz NH, et al. Denosumab in Men Receiving Androgen-Deprivation Therapy for Prostate Cancer. New England Journal of Medicine. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings SR, Martin JS, McClung MR, et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. New England Journal of Medicine. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: A population-based HR-pQCT study. Journal of Bone and Mineral Research. 2011;26:50–62. doi: 10.1002/jbmr.171. [DOI] [PubMed] [Google Scholar]
- 10.Portero-Muzy NR, Chavassieux PM, Bouxsein ML, Gineyts E, Garnero P, Chapurlat RD. Early effects of zoledronic acid and teriparatide on bone microarchitecture, remodeling and collagen crosslinks: Comparison between iliac crest and lumbar vertebra in ewes. Bone. 2012;51:714–719. doi: 10.1016/j.bone.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Bollen K. Structural Equations with Latent Variables. Wiley; New York City: 1990. [Google Scholar]
- 12.Statistical Applications Software. SAS Institute; Cary, NC: 2004. [Google Scholar]
- 13.Vigen R, O'Donnell CI, Barón AE, et al. ASsociation of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 14.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. Journal of Clinical Epidemiology. 2000;53:183–194. doi: 10.1016/s0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 15.Curtis J, Mudano A, Solomon D, Xi J, Melton M, Saag K. Identification and Validation of Vertebral Compression Fractures using Administrative Claims Data. Medical Care. 2009;47:69–72. doi: 10.1097/MLR.0b013e3181808c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bass E, Pracht E, Foulis P, Bass E, Pracht E, Foulis P. Bone mineral density scans in veterans. Clinical Interventions In Aging. 2007;2:255–261. [PMC free article] [PubMed] [Google Scholar]
- 17.Ohldin A, Floyd J, Ohldin A, Floyd J. Unrecognized risks among Veterans with hip fractures: opportunities for improvements. Journal of the Southern Orthopaedic Association. 2003;12:18–22. [PubMed] [Google Scholar]
- 18.Swislocki A, Green JA, Heinrich G, et al. Prevalence of osteoporosis in men in a VA rehabilitation center. American Journal of Managed Care. 16:427–433. [PubMed] [Google Scholar]
- 19.Yeh SS, Phanumas D, Hafner A, Schuster MW, Yeh S-S, Phanumas D, Hafner A, Schuster MW. Risk factors for osteoporosis in a subgroup of elderly men in a Veterans Administration nursing home. Journal of Investigative Medicine. 2002;50:452–457. doi: 10.1136/jim-50-06-05. [DOI] [PubMed] [Google Scholar]