Abstract
Background
Diabetes damages peripheral tissues, however, its effects on the lung are less known. Lung diffusing capacity (DLCO) is influenced by alveolar-capillary membrane conductance (DM) and pulmonary capillary blood volume (VC), both of which are reduced in adults with type-1 diabetes (T1D).
Objective
We sought to determine if diabetes duration affects DLCO, DM, VC, and cardiac output (Q).
Methods
24 type-1 diabetics (10.7 – 52.8 years) and 24 non-diabetic controls were recruited and had DLCO, DM, VC, and Q measured at rest and exercise (40, 70 and 90% VO2max).
Results
When stratified into two groups based on age (young < 20.6 years old), there were no significant differences in DLCO, DM, VC, or Q (all of which were normalized to body surface area [BSA]) in the young group, or in the old group. When stratified by diabetes duration (short duration 0.33 – 8.9 years, long duration 9.6 – 28 years), the T1D in the long duration group had lower DLCO/BSA and DM/BSA compared to the controls (P<0.05). There were no differences in any of the variables in the short duration group.
Conclusions
This study has shown that duration of diabetes is associated with decrements in diffusing capacity and its components.
Keywords: Altitude, Cardiac Output, Exercise, Pulmonary Diffusing Capacity, Type 1 Diabetes Mellitus
Introduction
The American Diabetes Association recommends that people with type-1 diabetes (T1D) engage in physical activity [1, 2]. It is unclear whether the effects on the lung limit the increasing number of people with diabetes who are participating in ‘adventure’ activities at moderate to high altitude [3]. Adults with type 1 diabetes have pulmonary diffusion limitations [4, 5] that are particularly applicable when increased altitude reduces the alveolar-arterial gradient for oxygen transfer [6, 7]. Exercise exacerbates this challenge by increasing cardiac output and reducing the capillary transit times available for oxygen transfer in the lung [8]. Consequently, adventure activities at moderate to high altitude may result in more arterial hypoxemia, limiting the ability of individuals with diabetes to sustain vigorous activities at increased altitude.
The diffusing capacity of the lung (DLCO) is determined by two components, the alveolar-capillary membrane diffusing capacity (DM) and the pulmonary capillary blood volume (VC) [9]. Diabetes thickens the pulmonary capillary basal lamina [10, 11] and increases endothelial permeability [12], reducing DM. Niranjan et al. [13] showed that during exercise DLCO was lower in healthy type 1 diabetic adults and that the reduction in DLCO was attributable to reduced DM. They also showed that five years of intensive glycemic control prevented further deterioration of DLCO. It is unclear whether adolescents with diabetes have the same impairment in pulmonary diffusion as adults. Van Gent et al. [14] found that DLCO/VA was not different from age-dependent norms in 27 children with T1D. In contrast, Cazzato et al. [15] found a lower DLCO in children with T1D than in their age-matched controls but found no correlation with diabetes duration or HbA1c. However Villa et al. [16] found DLCO was reduced in adolescents with diabetes and DLCO was inversely associated with HbA1c. These data suggest that age, magnitude, or duration of hyperglycemia impairs pulmonary diffusion by altering the alveolar capillary membrane, but these changes to the diabetic lung are preventable.
Studies in diabetic adults suggest that reduced VC [13, 17] causes reduced DLCO. VC is determined by the number of pulmonary capillaries in contact with ventilated alveoli. During exercise, lung volumes and pulmonary blood flow increase, causing an increase in DLCO. Exercise is needed to evaluate VC because only a small percentage of the pulmonary vasculature is used at rest, therefore, resting measures do not adequately assess microvascular reserve. An attenuated increase in cardiac output during exercise is well described in T1D [13, 18], and could reduce VC by limiting recruitment and distension of pulmonary vessels [19, 20, 21, 22]. Furthermore, microangiopathy in the lung in T1D may impair pulmonary vascular recruitment and distension by not allowing the normal increase in VC [5].
The purpose of this study was to compare resting and exercising DLCO, DM and VC, in children and adults with and without T1D at 7000 ft (2, 130 meters, barometric pressure ~600 mmHg) to examine the effect of age and duration of disease on lung diffusing capacity and its components. We tested two hypotheses. The first was that older people with T1D would have a lower DLCO compared to controls, but younger people with T1D would not. The second hypothesis was that subjects with a longer duration of diabetes (independent of age) would have a lower DLCO compared to controls.
Methods
Subjects
Twenty-four individuals with type-1 diabetes and 24 non-diabetic controls matched for age, self-reported fitness history, and gender participated in this study. Subjects were aged 11 – 53 years, were non-smoking and showed no evidence of cardiovascular disease (Bruce protocol), microvascular impairment (proteinurea or retinopathy) or autonomic dysfunction. Three T1D participants were taking ACE inhibitors, however no participants were hypertensive (>140/90 mm Hg). The protocol was reviewed and approved by the Northern Arizona University Institutional Review Board and all subjects signed a written informed consent prior to the study. In addition, adolescents filled out a self-assessment for Tanner stage of maturity [23].
Procedure
The study required two visits to the lab. During the first visit, height, weight and body fat percentage were determined using a seven-site skinfold method [24] on adults, and a two-site skinfold method on adolescents [25]. Standard spirometry testing was performed using a Medical Graphics CPX/D (St. Paul, MN) metabolic cart. Peak oxygen uptake (VO2peak) (ParvoMedics TrueOne 2400 Sandy, Utah) was then determined on a stationary cycle ergometer (Corival Lode B.V., The Netherlands).
Peak oxygen uptake was assessed using an incremental protocol. Adolescents pedaled at 60 – 70 revolutions per minute (rpm) at an initial workload of 25 watts for subjects less than 14 years old and 40 watts for those greater than 14 years old. Workload then increased 15 watts each minute until exhaustion. Adults cycled 70 – 80 rpm. Females started at a workload of 50 watts and males at 100 watts and workload increased by 50 watts and then 25 watts every 2 minutes until exhaustion to make the total exercise duration between 8 and 14 minutes. Since the test was performed on a cycle ergometer, classification of peak VO2 was based on either; 1) an increase in VO2 of less than 250 mL with an increase in workload, or 2) two of the following three situations; respiratory exchange ratio (RER) greater than 1.10, the subject’s inability to maintain the pedal rate, and a heart rate within 10 beats per minute of age-predicted maximum.
During the second visit, cardiac output (Q), DLCO, and lung diffusing capacity for nitric oxide (DLNO) were measured using a rebreathing method. Two maneuvers were performed at rest and during each of the three exercise workloads (40%, 70%, and 90% of VO2peak) for the measurement of DLCO and DLNO, and the determination of DM [26], while simultaneously measuring Q. Subjects rebreathed a gas mixture containing 0.6% C2H2, 0.28% C18O, 9% He, 36.1% O2, and 40 ppm NO from a 5-liter anesthesia bag for eight breaths. Breathing was set at a rate of 32 breaths/min using a metronome until respiratory rate exceeded 32 breaths/min, at which point the metronome was set at the subject’s preferred breathing rate. Gases were sampled with a Perkin Elmer MGA-1100 mass spectrometer (Wesley, MA) and Sievers 280i Nitric Oxide Analyzer (GE, Boulder, CO), and analyzed on a PC using custom software (KC Beck, Liberty, UT) for the measurement of DLCO, DLNO, and Q. The bag volume corresponded to the subject’s normal tidal volume plus 200 mL of the gas mixture. During rebreathing, DLCO, DLNO, and Q were measured from the slope of the end-tidal exponential disappearance of C18O, NO, and acetylene respectively, with respect to helium over time. These data were used to calculate the two components of DLCO, DM and VC, using the Roughton and Forster [9] equation.
Statistical Analysis
Data are reported as mean ± SD. Statistical analyses were performed using SigmaStat 3.5 (Systat Software, Inc., Point Richmond, CA). A two-way analysis of variance with repeated measures was used to determine differences in Q, DLCO, DM, and VC (dependent variables) between groups, exercise intensity, and the interaction of group and condition (independent variables). The post-hoc test, Holm-Sidak, was used when appropriate. Results were considered statistically significant if p< 0.05.
Results
Subject testing starting in February 2011 and was completed in September 2012. Whole group subject characteristics are shown in table 1. The T1D and control groups were of similar age, height, weight, percent body fat, and activity level. There were no group differences in VO2peak, RER and heart rate at maximal exercise, or pulmonary function. DLCO/BSA, DM/BSA, VC/BSA, and Q/BSA were measured at rest, and low, moderate, and high exercise intensities. DLCO/BSA, DM/BSA, and Q/BSA increased with increased exercise intensity. DLCO/BSA (p = 0.167), DM/BSA (p = 0.494), VC/BSA (p = 0.569), or Q/BSA (p = 0.112) were not different across the testing conditions (Table 2). Relative exercise intensities were not different between groups (p = 0.390).
Table 1.
Subject Characteristics, Data are means ± SD
| Diabetics | Controls | p-value | |
|---|---|---|---|
| Age | 23.8±12.2 | 24.2±12.9 | 0.238 |
| Gender (% male) | 45.8 | 45.8 | NA |
| Height (cm) | 166.6±14.7 | 166.8±12.2 | 0.936 |
| Weight (kg) | 64.1±18.5 | 65.4±20.2 | 0.593 |
| BMI | 22.5±3.3 | 23.0±4.3 | 0.482 |
| Body Fat Percentage | 23.8± | 22.9±7.0 | 0.463 |
| Activity Level (hrs/week) | 5.0±2.8 | 6.0±3.7 | 0.139 |
| Diabetes Duration (yrs) | 9.8±7.8 | ---- | ---- |
| HbA1c | 7.9±1.3 (n=18) | ---- | ---- |
| VO2max (mL/kg/min) | 34.9±5.8 | 36.3±7.7 | 0.307 |
| Max Heart Rate (bpm) | 181±13.8 | 187.0±14.1 | 0.136 |
| RER at max | 1.1±0.1 | 1.1±0.1 | 0.951 |
| FVC (% predicted) | 100.4±12.1 | 104.9±11.3 | 0.146 |
| FEV1.0 (% predicted) | 97.6±15.9 | 100.9±11.6 | 0.379 |
Table 2.
Resting and Exercise Variables Measured in Diabetics and their Matched Controls, Data are means ± SD
| Diabetics | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Exercise Intensity | Rest | Low | Moderate | High | Rest | Low | Moderate | High |
| % of VO2max | 15.6±3.1 | 43.4±3.9 | 72.2±4.6 | 92.5±6.0 | 15.7±4.1 | 43.3±5.9 | 70.3±8.7 | 89.3±10.4 |
| DLCO/BSA | 12.9±2.5 | 15.7±2.7 | 17.9±3.4 | 19.5±4.3 | 13.4±2.2 | 16.3±3.5 | 19.5±3.8 | 21.5±4.6 |
| DM/BSA | 18.1±5.4 | 20.8±3.6 | 23.9±4.9 | 28.2±7.0 | 18.4±4.8 | 21.4±5.3 | 25.9±6.3 | 28.8±6.3 |
| VC/BSA | 56.7±70.9 | 64.2±40.2 | 73.3±59.9 | 60.8±24.9 | 56.9±30.2 | 68.8±30.8 | 73.2±30.6 | 75.4±21.5 |
| Q/BSA | 3.1±0.6 | 5.9±1.3 | 7.4±1.4 | 8.6±1.7 | 3.2±0.8 | 6.2±1.4 | 8.3±1.5 | 9.5±1.5 |
The effect of age on lung diffusing capacity was determined by stratifying subjects around the median age (20.7 years) of the sample. The average age of the young and older groups were 15.3 ± 4.0 years and 32.3 ± 11.8 years respectively (P< 0.05). In the young group, DLCO/BSA, DM/BSA, and Q/BSA, but not Vc/BSA increased with increasing exercise intensity. In the older group, DLCO/BSA, DM/BSA, VC/BSA, and Q/BSA increased with increasing exercise intensity. There were no differences in DLCO/BSA, DM/BSA, VC/BSA, and Q/BSA between the young and older T1Ds and their age-matched controls (table 3).
Table 3.
Resting and Exercise Variables in Diabetics and Controls Stratified by Age, Data are means ± SD
| Young | Older | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetics | Controls | Diabetics | Controls | |||||||||||||
| Exercise Intensity | Rest | Low | Moderate | High | Rest | Low | Moderate | High | Rest | Low | Moderate | High | Rest | Low | Moderate | High |
| DLCO/BSA | 13.1±3.2 | 15.7±3.5 | 17.4±3.9 | 19.4±5.0 | 13.7±1.9 | 15.6±3.8 | 19.6±3.9 | 20.9±4.9 | 12.9±2.0 | 15.7±1.8 | 18.3±3.0 | 19.7±3.7 | 13.0±2.5 | 17.0±3.3 | 19.5±3.9 | 22.0±4.4 |
| DM/BSA | 17.2±6.1 | 20.9±4.6 | 23.2±6.0 | 28.5±8.7 | 19.5±5.0 | 20.2±4.6 | 25.8±5.3 | 27.9±6.2 | 18.7±4.9 | 20.7±2.6 | 24.5±3.7 | 28.0±5.2 | 17.5±4.8 | 22.5±6.0 | 26.1±7.4 | 29.6±6.5 |
| VC/BSA | 81.8±105.9 | 61.4±29.0 | 87.6±83.8 | 55.2±18.5 | 64.1±40.2 | 71.8±38.5 | 72.4±38.6 | 71.1±25.4 | 37.9±10.3 | 67.1±50.2 | 60.2±19.8 | 66.4±29.8 | 50.9±18.3 | 65.9±22.1 | 73.9±23.0 | 78.9±17.9 |
| Q/BSA | 3.3±0.6 | 6.1±1.2 | 7.1±1.0 | 8.4±2.0 | 3.3±0.6 | 5.9±1.3 | 8.5±1.6 | 9.4±1.5 | 2.9±0.6 | 5.8±1.3 | 7.7±1.7 | 8.8±1.3 | 3.1±0.9 | 6.4±1.4 | 8.2±1.3 | 9.5±1.6 |
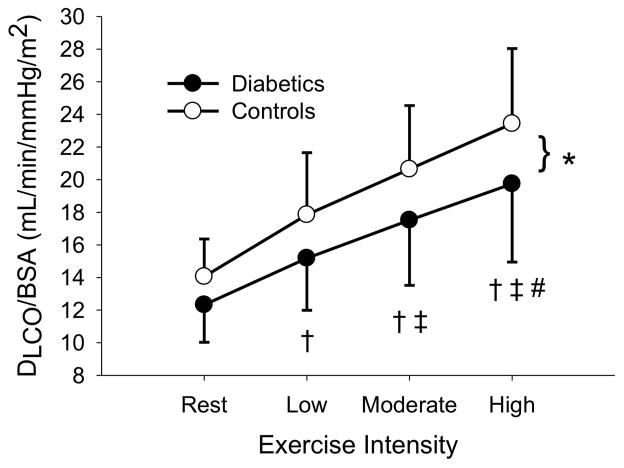
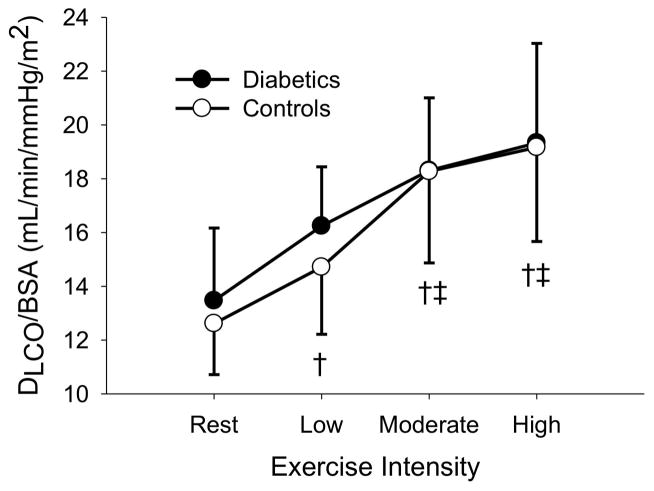
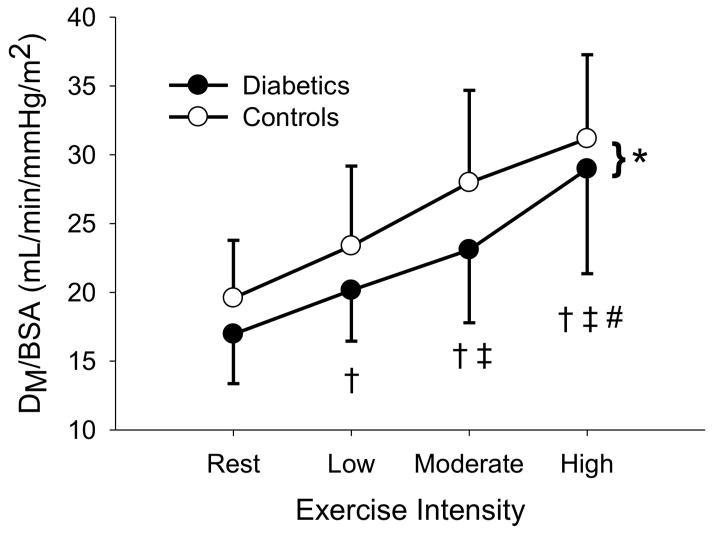
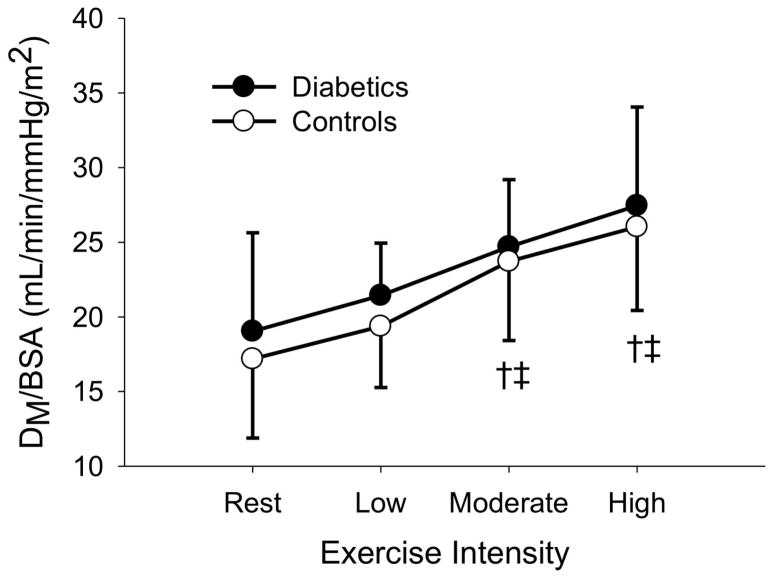
The sample was then stratified around the median duration of diabetes (9.3 years) and those above and below the median duration were compared against their assigned age-and sex-matched controls. In both groups, DLCO/BSA, DM/BSA, and Q/BSA increased with increasing exercise intensity (P < 0.05). In the long duration group, VC/BSA, increased with increasing exercise intensity (P < 0.05). There were no differences in DLCO/BSA, DM/BSA, VC/BSA, and Q/BSA (p = 0.328, 0.155, 0.601, and 0.263, respectively) for the short duration T1D (4.0 ± 2.8 years) vs. non-diabetic controls. Similarly, there were no differences in VC/BSA and Q/BSA (p = 0.115 and 0.234, respectively) between the long duration T1D (15.7 ± 6.6 years) vs. matched controls. However, long duration T1D had lower DLCO/BSA (p = 0.014) and DM (p = 0.033) than matched controls (figure 1, figure 2).
Figure 1.
Figure 1A: DLCO/BSA at rest, low, moderate, and high exercise intensities for the long duration diabetics and their matched controls. * (p < 0.05) signifies a difference between diabetics and controls. † (p < 0.05) different from rest. ‡ (p < 0.05) different from low intensity exercise # (p < 0.05) different from moderate intensity exercise.
Figure 1B: DLCO/BSA at rest, low, moderate, and high exercise intensities for the short duration diabetics and their matched controls. † (p < 0.05) different from rest. ‡ (p < 0.05) different from low intensity exercise.
Figure 2.
Figure 2A: DM/BSA at rest, low, moderate, and high exercise intensities for long duration diabetics and their matched controls. * (p < 0.05) Signifies a difference between diabetics and controls. † (p < 0.05) different from rest. ‡ (p < 0.05) different from low intensity exercise # (p < 0.05) different from moderate intensity exercise.
Figure 2B: DM/BSA at rest, low, moderate, and high exercise intensities for short duration diabetics and their matched controls. † (p < 0.05) different from rest. ‡ (p < 0.05) different from low intensity exercise.
Discussion
Our study of non-smoking adolescents and adults found that resting and peak exercise pulmonary carbon monoxide diffusing capacity was not significantly different between normals vs. type 1 diabetics. Contrary to our hypothesis, neither younger nor older patients were different from their age- and sex-matched controls. However, when participants were stratified by diabetes duration, we found that people with longer type 1 diabetes diagnoses (>10 years) had reduced DLCO relative to controls, and this difference was exacerbated during high intensity exercise. Simultaneous measurements of DLCO and DLNO in our study showed that the reduction in DLCO was due to lower alveolar-capillary membrane diffusing capacity (DM), but that pulmonary capillary blood volume was not significantly affected. None of the participants had evidence of microangiopathy, and there was no significant relationship between DLCO and HbA1c levels. These findings suggest that diabetes-specific impairment of pulmonary diffusion is a progressive process.
Our finding that age-matched DLCO did not differ between normal and T1D subjects does not agree with some previous reports of impaired resting pulmonary diffusing capacity in adults [4, 17, 27, 28, 29] and adolescents [14, 16, 30] with type 1 diabetes. However, some [17], but not all [16] of these earlier studies also reported abnormal pulmonary function in their subjects, whereas our type 1 diabetic cohort had normal pulmonary function (spirometry). Normal lung volumes and ventilatory flows in our diabetic cohort may have allowed them to transfer CO more effectively whereas the pathology in the previous studies’ cohorts [14, 15, 17, 30] did not. Nonetheless, a study of children with normal spirometry tests has also reported impaired DLCO [16].
Another possibility is that differences in global diabetes control or behavior between our cohort and previous cohorts may have contributed to differences in DLCO observations. Several studies reporting lower DLCO in patients with type 1 diabetes have included nephropathic [29, 31], neuropathic [29] and retinopathic [28, 32] patients. Other cohorts have been composed of patients with higher mean HbA1c values than the present study [30]. Two studies stratified participants with type 1 diabetes into good and poor glycemic control [16, 33] and found that pulmonary diffusion was impaired in those with poor control, but not in those with HbA1c less than 7% (53 mmol). None of the participants in this study had clinically evident microvascular abnormalities, and HbA1c levels were, in relation to previous studies, lower. Considering the association between glycemic control and microvascular complications [34], group differences in DLCO may have been even more evident if our cohort had worse metabolic control. The average HbA1c in our subjects was 7.9, while in studies showing differences in DLCO between diabetics and controls [13, 15, 16, 29, 30, 32, 33, 35], only one study had HbA1c values lower than ours [15], while the rest had an average HbA1c in their subjects of 8.98%. Yet another possibility for the lack of difference within the age groups may be the wide range of durations. The diabetic adolescents ranged from 0.33 to 14 years disease duration, while the adults ranged from 6.5 to 28 years.
Our finding that diabetes duration was associated with reduced pulmonary diffusion expands on the findings of Asanuma et al. [4] who found that the decrease in DLCO was correlated with diabetes duration in adults with T1D. However, retinopathy was also correlated with diabetes duration in their cohort, raising the possibility that microangiopathy, which worsened with diabetes duration, explained the reduction in DLCO rather than uncomplicated diabetes duration. Villa et al. [16] found that glycemic control, but not diabetes duration reduced DLCO in a small cohort of children, however their cohort had a significantly smaller range of diabetes duration than the present study. In the present study, DLCO was lower in a cohort free of microvascular complications with relatively long diabetes duration (14.8 ± 7.3 vs. 5.0 ± 4.4 years), which is considerably longer than previous studies.
The most novel aspect of our study design was our measurement of DLCO during peak exercise. Alveolar ventilation and pulmonary capillary perfusion are maximized during peak exercise and the demand for oxygen transfer is greatest in this state. Therefore small, often insignificant differences in resting DLCO can increase when these systems are stressed. Studies in adults have confirmed greater impairment in pulmonary diffusion in the exercised vs. resting state that have been attributed to reduced DM [33], VC [17], or both [13]. However these findings are limited to adults with poor glycemic control [13, 33]. The present study showed a significant reduction in DLCO and DM, but not Vc during exercise in patients (young and older) with a long duration of diabetes, suggesting that diabetes affects the pulmonary capillary membrane in a manner independent of age. The lack of difference in Vc may reflect the fact that cardiac output was not lower in our diabetic cohort, as seen in other studies [13, 33]. Vracko et al. [10] and Weynand et al. [11] described thickened alveolar and capillary basal laminae in post-mortem diabetic lungs, which also occurred in the renal and muscle capillaries. It is generally believed that these permanent changes in basal membrane protein structure accumulate over time [36]. Our data in the diabetic lung support this hypothesis.
One unexpected finding of this study was a reduction in pulmonary capillary blood volume during high intensity exercise in the young subjects with diabetes. We believe the drop to partly be due to the innate variability of the measurement, but believe it could also be due to the subjects exhibiting a mild hypoxic response to the testing being performed at moderate altitude (~2100 meters). Our changes from rest to moderate exercise are of nearly identical magnitude to those described previously [20, 37]. We are not sure why we didn’t see increases from moderate to high intensity exercise, but the possibility of hypoxic vasoconstriction from exercising at moderate altitude, may be one possible explanation to why further increases in VC at the highest exercise intensity were not seen [38].
An unusual aspect of this study is that it was conducted at moderate altitude (2100 m). The lower inspired PO2 found at the moderate altitude impairs oxygen diffusion by reducing the alveolar-arterial O2 gradient [39]. Chance et al. [40] have suggested that the non-symptomatic reduction in DLCO during sea-level rest might become functionally limiting when diabetic individuals exercised at increased altitude. However, we found that our diabetic cohort had similar decreases in DLCO at altitude to those who have performed similar studies at or near sea level [33]. Moreover, any reductions in DLCO were not sufficient to result in a difference in SaO2 (data not shown) or exercise capacity between the groups. This may reflect the relatively minor hypoxia (PA02 ~ 65 – 75 mm Hg) at this altitude and/or the high level of metabolic control and healthy lifestyle of our participants.
This study found that pulmonary diffusing capacity was significantly affected in people with longer diabetes duration, who had reduced DLCO, which was exacerbated by high-intensity exercise. The lower DLCO was associated with a decreased DM, but not Vc, suggesting that the ‘type 1 diabetes effect’ related to changes in the alveolar capillary membrane. Despite the fact that experiments were conducted at moderate altitude, differences in pulmonary diffusion did not result in lower arterial saturation during exercise in people with long-duration diabetes. Our interpretation of these findings is that pulmonary diffusion, though impaired, does not limit aerobic capacity in people with well-controlled type 1 diabetes.
Acknowledgments
This research was supported by National Institutes of Health Grant R15 HL097335-01A1.
References
- 1.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27:2518–2539. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 2.Steppel JH, Horton ES. Exercise in the management of type 1 diabetes mellitus. Rev Endocr Metab Disord. 2003;4:355–360. doi: 10.1023/a:1027302112655. [DOI] [PubMed] [Google Scholar]
- 3.Brubaker PL. Adventure travel and type 1 diabetes: the complicating effects of high altitude. Diabetes Care. 2005;28:2563–2572. doi: 10.2337/diacare.28.10.2563. [DOI] [PubMed] [Google Scholar]
- 4.Asanuma Y, Fujiya S, Ide H, Agishi Y. Characteristics of pulmonary function in patients with diabetes mellitus. Diabetes Res Clin Pract. 1985;1:95–101. doi: 10.1016/s0168-8227(85)80034-6. [DOI] [PubMed] [Google Scholar]
- 5.Hsia CCW, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin. Am J Med. 2005;118:205–211. doi: 10.1016/j.amjmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 6.West JB. Diffusing capacity of the lung for carbon monoxide at high altitude. J Appl Physiol. 1962;17:421–426. doi: 10.1152/jappl.1962.17.3.421. [DOI] [PubMed] [Google Scholar]
- 7.West JB, Lahiri S, Gill MB, Milledge JS, Pugh LGCE, Ward MP. Arterial oxygen saturation during exercise at high altitude. J Appl Physiol. 1962;17:617–621. doi: 10.1152/jappl.1962.17.4.617. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RL., Jr Pulmonary diffusion as a limiting factor in exercise stress. Circ Res. 1967;20:154–160. [Google Scholar]
- 9.Roughton FJW, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special references to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11:290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 10.Vracko R, Thorning D, Huang TW. Basal lamina of alveolar epithelium and capillaries: quantitative changes with aging and in diabetes mellitus. Am Rev Respir Dis. 1979;120:973–983. doi: 10.1164/arrd.1979.120.5.973. [DOI] [PubMed] [Google Scholar]
- 11.Weynand B, Honckheere A, Frans A, Rarier J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration. 1999;66:12–13. doi: 10.1159/000029331. [DOI] [PubMed] [Google Scholar]
- 12.Popov D, Hasu M, Costache G, Stern D, Simionescu M. Capillary and aortic endothelia interact in situ with nonenzymatically glycated albumin and develop specific alterations in early experimental diabetes. Acta Diabetol. 1997;34:285–293. doi: 10.1007/s005920050090. [DOI] [PubMed] [Google Scholar]
- 13.Niranjan V, McBrayer DG, Ramirez LC, Raskin P, Hsia CCW. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am J Med. 1997;103:504–513. doi: 10.1016/s0002-9343(97)00251-9. [DOI] [PubMed] [Google Scholar]
- 14.Van Gent R, Brackel HJL, de Vroede M, van der Ent CK. Lung function abnormalities in children with type I diabetes. Respir Med. 2002;96:976–978. doi: 10.1053/rmed.2002.1402. [DOI] [PubMed] [Google Scholar]
- 15.Cazzato S, Bernardi F, Salardi S, Tassinari D, Corsini I, Luca R, Cicognani A, Cacciari E. Lung function in children with diabetes mellitus. Pediatr Pulmonol. 2004;37:17–23. doi: 10.1002/ppul.10399. [DOI] [PubMed] [Google Scholar]
- 16.Villa MP, Montesano M, Barreto M, Pagani J, Stegagno M, Multari G, Ronchetti R. Diffusing capacity for carbon monoxide in children with type 1 diabetes. Diabetologia. 2004;47:1931–35. doi: 10.1007/s00125-004-1548-7. [DOI] [PubMed] [Google Scholar]
- 17.Sandler M, Bunn AE, Stewart R. Pulmonary function in young insulin-dependent diabetic subjects. Chest. 1986;90:670–675. doi: 10.1378/chest.90.5.670. [DOI] [PubMed] [Google Scholar]
- 18.Gusso S, Pinto TE, Baldi JC, Robinson E, Cutfield WS, Hofman PL. Diastolic function is reduced in adolescents with type 1 diabetes in response to exercise. Diabetes Care. 2012;35:2089–2094. doi: 10.2337/dc11-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsia CCW, McBrayer DG, Ramanathan M. Reference values of pulmonary diffusing capacity during exercise by a rebreathing technique. Am J Respir Crit Care Med. 1995;152:658–665. doi: 10.1164/ajrccm.152.2.7633723. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RL, Jr, Spicer WS, Bishop JM, Forster RE. Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol. 1960;15:893–902. doi: 10.1152/jappl.1960.15.5.893. [DOI] [PubMed] [Google Scholar]
- 21.Miller JM, Johnson RL., Jr Effect of lung inflation on pulmonary diffusing capacity at rest and exercise. J Clin Invest. 1966;45:493–499. doi: 10.1172/JCI105363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepard RH, Varnauskas E, Martin HB, White HA, Permutt S, Cotes JE, Riley RL. Relationship between cardiac output and apparent diffusing capacity of the lung in normal men during treadmill exercise. J Appl Physiol. 1958;13:205–210. doi: 10.1152/jappl.1958.13.2.205. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SJC, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 24.Crouse SF, Coast JR, Oden GL. Clinical Exercise Physiology Laboratory Manual. 2. Kendall Hunt Publishing Company; Dubuque: 2011. [Google Scholar]
- 25.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 26.Tamhane RM, Johnson RL, Jr, Hsia CCW. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120:1850–1856. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 27.Fuso L, Cotroneo P, Basso S, De Rosa M, Manto A, Ghirlanda G, Pistelli R. Postural variation of pulmonary diffusing capacity in insulin-dependent diabetes mellitus. Chest. 1996;110:1009–1013. doi: 10.1378/chest.110.4.1009. [DOI] [PubMed] [Google Scholar]
- 28.Innocenti F, Fabbri A, Anichini R, Tuci S, Pettina G, Vannucci F, De Giorgio LA, Seghieri G. Indications of reduced pulmonary function in type 1 (insulin-dependent) diabetes mellitus. Diabetes Res Clin Pract. 1994;25:161–168. doi: 10.1016/0168-8227(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 29.Strojek K, Ziora D, Sroczynski JW, Oklek K. Pulmonary complications of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1992;35:1173–1176. doi: 10.1007/BF00401373. [DOI] [PubMed] [Google Scholar]
- 30.Scaramuzza AE, Morelli M, Rizzi M, Borgonovo S, de Palma A, Mameli C, Giani E, Beretta S, Zuccotti GV. Impaired diffusing capacity for carbon monoxide in children with type 1 diabetes: is this the first sign of long-term complications? Acta Diabetol. 2012;49:159–164. doi: 10.1007/s00592-011-0353-2. [DOI] [PubMed] [Google Scholar]
- 31.Schnack Ch, Festa A, Schwarzmaier-D’Assie A, Haber P, Schernthaner G. Pulmonary dysfunction in type 1 diabetes in relation to metabolic long-term control and to incipient diabetic nephropathy. Nephron. 1996;74:395–400. doi: 10.1159/000189342. [DOI] [PubMed] [Google Scholar]
- 32.Weir DC, Jennings PE, Hendy MS, Barnett AH, Sherwood Burge P. Transfer factor for carbon monoxide in patients with diabetes with and without microangiopathy. Thorax. 1988;43:725–726. doi: 10.1136/thx.43.9.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheatley CM, Baldi JC, Cassuto NA, Foxx-Lupo WT, Snyder EM. Glycemic control influences lung membrane diffusion and oxygen saturation in exercise-trained subjects with type 1 diabetes. Eur J Appl Physiol. 2011;111:567–578. doi: 10.1007/s00421-010-1663-8. [DOI] [PubMed] [Google Scholar]
- 34.The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 35.Ljubic S, Metelko Z, Car N, Roglic G, Drazic Z. Reduction of diffusion capacity for carbon monoxide in diabetic patients. Chest. 1998;114:1033–1035. doi: 10.1378/chest.114.4.1033. [DOI] [PubMed] [Google Scholar]
- 36.Brownlee M. Banting Lecture 2004: The pathobiology of diabetic complications, a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 37.Lewis BM, Lin T, Noe FE, Komisaruk R. The measurement of pulmonary capillary blood volume and pulmonary membrane diffusing capacity in normal subjects; the effects of exercise and position. J Clin Invest. 1958;37:1061–1070. doi: 10.1172/JCI103687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartzstein RM, Parker MJ. Respiratory Physiology A Clinical Approach. Lippincott, Williams, & Wilkins; 2006. p. 103. [Google Scholar]
- 39.Torre-Bueno JR, Wagner PD, Saltzman HA, Gale GE, Moon RE. Diffusion limitation in normal humans during exercise at sea level and simulated altitude. J Appl Physiol. 1985;58:989–995. doi: 10.1152/jappl.1985.58.3.989. [DOI] [PubMed] [Google Scholar]
- 40.Chance WW, Rhee C, Yilmaz C, Dane DM, Pruneda L, Rasin P, Hsia CCW. Diminished alveolar microvascular reserves in type 2 diabetes reflect systemic microangiopathy. Diabetes Care. 2008;31:1596–1601. doi: 10.2337/dc07-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]