Abstract
Aims
(1) To determine the brain regions activated by dentoalveolar pressure stimulation in persistent dentoalveolar pain disorder (PDAP) patients, and (2) to compare these activation patterns to those seen in pain-free control subjects.
Methods
A total of 13 PDAP patients and 13 matched controls completed the study. Clinical pain characteristics and psychosocial data were collected. Dentoalveolar mechanical pain thresholds were determined with a custom-made device over the painful area for patients and were used as the stimulation level during functional magnetic resonance imaging (fMRI) data acquisition. Control subjects received two stimulation levels over matched locations during fMRI scanning: one determined (as above) that evoked equally subjective pain ratings matching those of patients (subjective-pain match) and another nonpainful stimulation level matching the average stimulus intensity provided to patients (stimulus-intensity match). Clinical and psychosocial data were analyzed using independent samples t tests, Mann-Whitney U test, and Spearman rank-order correlation coefficient. fMRI data were analyzed using validated neuroimaging software and tested using a general linear model.
Results
PDAP patients had greater anxiety (P < .0001) and depression scores (P = .001), more jaw function impairment (P < .0001), and greater social impact (P < .0001) than controls. No significant differences were found for brain activation spatial extent (PDAP × Controls subjective pain: P = .48; PDAP × Controls stimulus intensity: P = .12). Brain activations were significantly increased for PDAP patients compared to control subjects when matched to stimulus intensity in several regions related to the sensory-discriminative and cognitive components of pain perception, including the primary and secondary somatosensory cortices, inferior parietal lobule, insula, premotor cortex, prefrontal cortex, and thalamus. When matched to subjective pain ratings, increased brain activations were still present for PDAP patients compared to controls, although to a lesser extent.
Conclusion
The present results suggest that dentoalveolar pressure is processed differently in the brain of PDAP patients, and the increased activation in several brain areas is consistent with amplified pain processing.
Keywords: atypical odontalgia, functional neuroimaging, neuropathic pain, orofacial pain, sensory testing
Persistent pain in the dentoalveolar regions without evidence of local pathologic and/or inflammatory processes has long puzzled health care providers.1–3 Previously known as phantom tooth pain4 and atypical odontalgia,5 recent efforts to develop diagnostic criteria for persistent dentoalveolar pain disorder (PDAP)6 have suggested that this varied nomenclature likely referred to similar conditions that would fall within such criteria. PDAP is considered a diagnosis by exclusion7 that has a significant impact on quality of life.8 PDAP is characterized by persistent, nonparoxysmal pain over a tooth or at a site formerly occupied by a tooth, and often affects middle-aged women more often than men.9,10 Current estimates are that 1.6% of patients who have undergone root canal therapy fit the PDAP diagnostic criteria11 and that approximately 20 million endodontic procedures are performed in the United States annually.12 Since treatments for this condition are not effective,13 PDAP represents a significant clinical problem.
Although the pathophysiology underlying PDAP remains largely unknown,11 peripheral nerve injury following dental procedures is the most commonly proposed mechanism.9,10 PDAP has been proposed by some to be a neuropathic-type pain10 or even a trigeminal variant of complex regional pain syndrome,13 while others have suggested a psychogenic origin.10 The clinical features of PDAP support a neuropathic pain classification,14–16 including reduced dentoalveolar mechanical pain thresholds17 and results from standardized quantitative sensory testing (QST).18 Investigators have argued that PDAP is not solely due to persistent peripheral input,19 since spontaneous pain was only partially reduced following local anesthetic blocks over the affected intraoral site,20 and that psychosocial factors may contribute to pain expression in PDAP.21 Although multiple factors may contribute to PDAP pathophysiology, ranging from peripheral nerve injury and sensitization to central neural plasticity, little is known about brain mechanisms in PDAP and no neuroimaging studies have yet been reported.10 Neuroimaging methods provide the opportunity to probe anatomical, functional, and chemical brain characteristics of chronic pain patients.22
Evidence for pain amplification in the brain has been reported for chronic pain conditions such as fibromyalgia (FM)23 and idiopathic chronic low-back pain (CLBP),24 but not as yet for PDAP. Both FM and CLBP patients displayed more extensive patterns of brain activation following matching levels of mechanical stimulation than control subjects. In order to evoke comparable brain activation patterns, increased stimulation levels were applied to the control group to match subjective pain ratings between them and FM and CLBP patients.23,24 Although PDAP pain is generally confined to dento alveolar regions, unlike the widespread pain of FM, it is possible that they share common brain mechanisms of pain amplification. This rationale is supported by factors common to both conditions: (1) heterogeneous findings for potentially measurable disease markers, eg, QST; (2) comorbid mood disorders and somatization and other psychosocial problems; and (3) persistent pain. The aims of the present study were (1) to determine the brain regions activated by dentoalveolar pressure stimulation in PDAP patients, and (2) to compare these activation patterns to those seen in pain-free control subjects. The study hypotheses were: (1) brain activations in PDAP patients are greater in magnitude and spatial extent than control subjects following matching stimulus intensity levels, and (2) brain activation patterns are similar for patient and control groups following stimulation levels matched to subjective pain ratings.
Materials and Methods
The University of Minnesota Institutional Review Board approved the study’s experimental protocol, and all subjects provided informed consent prior to their participation and received monetary compensation for participating in the study.
Subjects
PDAP patients were recruited from the University of Minnesota (UMN) Temporomandibular Disorders (TMD) and Orofacial Pain Clinic. Inclusion criteria were presence of intraoral pain that was localized in an endodontically treated tooth/teeth or in a site formerly occupied by a tooth/teeth (dentoalveolar surrounding tissues); was present for more than 6 months; was present for 8 hours or more within a 24-hour period and 15 days or more a month; was nonparoxysmal in character; had no signs of gross pathology present during clinical examination or in available radiographic imaging,6 and was able to be provoked/increased by rubbing the affected area. Control subjects were recruited from the UMN community. The inclusion criterion for age-, sex-, and handedness-matched controls was absence of bodily pains in the previous 6 months (handedness determined from subjects’ self-reporting). Exclusion criteria for both groups were the presence of the following conditions, as determined by history and clinical examination: (1) tooth pathology, sinus infection, trigeminal neuralgia, herpes zoster; (2) history of destructive trigeminal nerve procedures or trauma-associated facial bone fractures within the trigeminal nerve distribution; (3) pregnancy, planning pregnancy, or the potential of being pregnant; and (4) claustrophobia or any other magnetic resonance imaging (MRI)-related contraindication. Telephone or in-person screening was performed to assess subject eligibility, followed by a clinical evaluation to determine if they met the criteria. Thirteen PDAP patients and 13 matched controls completed the study.
Questionnaires
Five questionnaires were used to characterize the study’s subject sample. The short-form McGill Pain Questionnaire (SF-MPQ)25 provided information on the dimensions of clinical pain by adding each item’s score (0 = none, 1 = mild, 2 = moderate, 3 = severe) on 11 sensory and 4 affective items, as well as the overall pain intensity according to a visual analog scale (VAS) ranging from 0 to 100. Chronic pain severity was graded according to the classification given by the Graded Chronic Pain Scale (GCPS),26 where grade 0 = no pain, grade I = low disability/low intensity; grade II = low disability/high intensity; grade III = high disability/moderately limiting; and grade IV = high disability/severely limiting. Characteristic pain intensity (CPI) was determined by averaging the present pain and the worst and average pain in the past 6 months multiplied by 10. Anxiety and depression were assessed using the sum of scores for the respective subscales of the Hospital Anxiety and Depression Scale (HADS) with its four-point Likert scale.27 In the eight-item version of the Jaw Functional Limitation Scale (JFLS-8),28 a 0 to 10 numeric scale anchored by “no limitation” and “severe limitation” is provided for each item; the scores were added to provide a global functional jaw limitation score. Finally, a summary score for the social impact of oral disorders on the subjects’ well-being was derived from the 14-item Oral Health Impact Profile (OHIP-14)29 by summing each response score (never/don’t know = 0; hardly ever = 1; occasionally = 2; fairly often = 3; very often = 4).
Dentoalveolar Stimulus Device
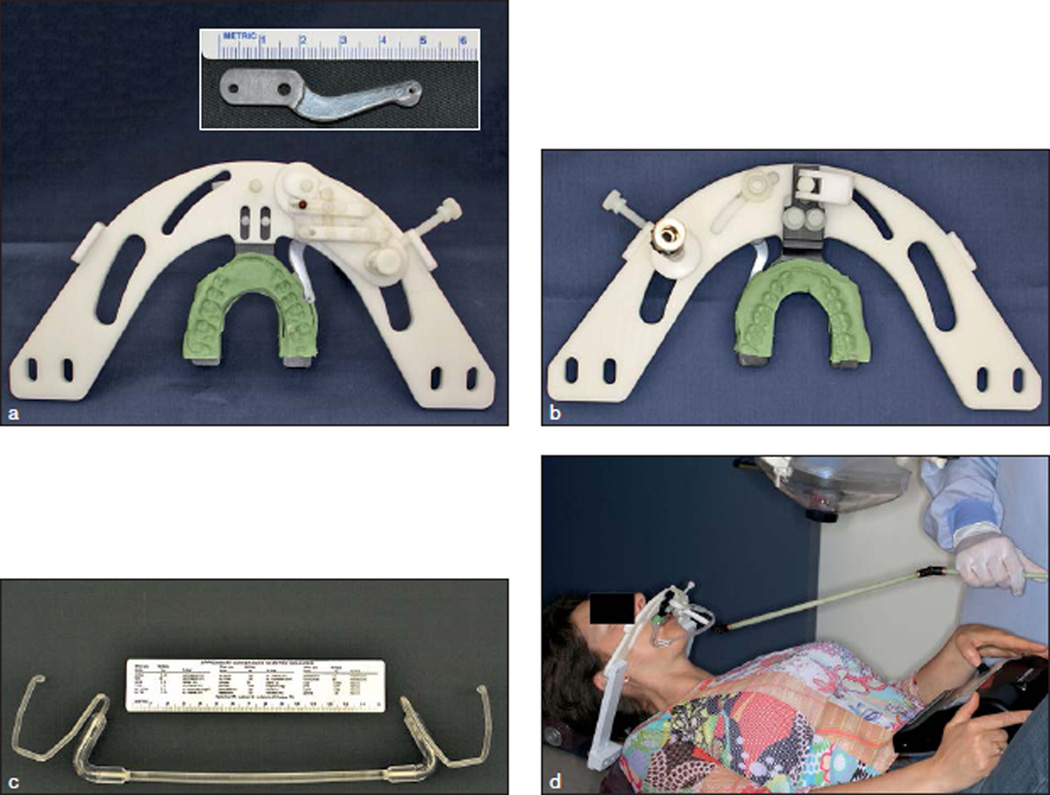
An MRI-compatible device was used to deliver dentoalveolar pressure stimulation.17 This mechanical device was manually controlled by an operator and delivered a range of nonpainful to painful pressure to the buccal dentoalveolar tissues and could be directed at all intra-oral quadrants by an intraoral plastic probe (Fig 1 inset). Pressure intensity was determined by the number of ⅛-inch (3.175 mm) elastic bands used in the linkage supporting the intraoral probe (range: 1 to 8) (Fig 1a). The contacting surface area (“head”) of the intraoral probe was approximately 2 mm2, and its excursion length over the dentoalveolar tissues during stimulation was approximately 5 to 7 mm. This design allowed threshold stimuli to be delivered without occluding blood flow or causing tissue injury. A bar with an individualized bite impression firmly attached to the supporting frame provided a biting surface for subjects (Figs 1a and 1b). A custom-made plastic lip and cheek retractor provided adequate exposure of the dentoalveolar tissues of interest and allowed the intraoral probe to touch only those tissues and not the labial commissure, lips, or internal mucosa of the cheeks (Fig 1c). The device was compatible for use in a dental chair (Fig 1d) and inside an MRI scanner with adequate attachments for each setting.
Fig 1.
Stimulus device and dental chair-side use. (a) Top and (b) bottom views of the stimulus device fully assembled, and closer view of the intraoral probe (inset); (c) custom-made lip and cheek retractor (d) Subject rating dentoalveolar stimulation using COVAS in the dental chair. (Figs 1a to 1c reproduced from Moana-Filho et al17 with permission from BioMed Central.)
Experimental Protocol
Initial visit
This visit consisted of explaining the experimental protocol to the subject, clinically evaluating, completing questionnaires, and establishing dentoalveolar mechanical pain thresholds. A bite imprint over the device’s bar was made with silicone-based putty material (Express bite, 3M ESPE) and used to ensure the stimulus was provided to the same location across visits. Stimulation with minimal pressure was performed while real-time pain ratings were collected using a computerized pain scale (COVAS) (Medoc Ltd) of 0 to 10 (“no pain” and “worst imaginable pain” anchors, respectively) (Fig 1d). Stimulation in PDAP patients was done over the buccal gingiva of the pain site, and locations used for control subjects were matched to the same intraoral quadrant as for patients. Stimulation consisted of repeated excursions of the intraoral probe head over the buccal dentoalveolar tissues at a force level dictated by the number of ⅛-inch elastic bands used in the linkage system (dynamic mechanical) at approximately 1 Hz frequency. The target subjective pain level was a rating of 3 to 5 on the COVAS during 30 seconds of dynamic mechanical stimulation. The stimulation was repeated as needed, adjusting the number of elastic band(s) accordingly until the target pain level was reached. The number of elastic band(s) found to elicit the target pain rating was used during the neuroimaging session.
Neuroimaging visit
Subjects returned within 5 to 7 days from the initial visit for the neuroimaging session at the Center for Magnetic Resonance Research at the UMN. Explanation of the MRI scanning session was given and its potential risks were discussed prior to imaging.
A 3-tesla Siemens Trio MRI scanner with a circularly polarized radiofrequency transmit/receive head coil was used. The subject was placed in the MRI scanner bed and fitted with the stimulus device to reach the targeted dentoalveolar location. Subjects were instructed to bite lightly on the bite bar during image acquisition. The intraoral probe head was positioned about 1 mm away from the stimulation site. Each subject received 5 to 10 seconds of dentoalveolar stimulation to confirm the location and pressure level prior to image acquisition were the same as those established during the initial visit for the PDAP patients and control subjects (subjective pain match). For controls only, an additional nonpainful stimulation level was delivered using the average number of ⅛-inch elastic bands found to elicit the target subjective pain ratings in PDAP patients in the authors’ previous study,17 thus matching the stimulus intensity used for patients (stimulus intensity match).
T1-weighted magnetization prepared rapid gradient echo (MPRAGE) anatomical images (repetition time [TR] = 2,530 ms, echo time [TE] = 3.68 ms, flip angle = 7 degrees, 224 axial slices, matrix size = 256 × 256, voxel size = 0.96 × 0.96 × 1 mm3) were acquired first. All functional runs were T2*-weighted echo-planar imaging sensitive to the blood oxygenation level dependent (BOLD) signal (TR = 3,000 ms, TE = 30 ms, flip angle = 90 degrees, 36 axial slices, interleaved acquisition, 70 volumes, matrix size = 64 × 64, voxel size = 3 × 3 × 5.375 mm3). An operator inside the MRI scanner room manually triggered dentoalveolar dynamic mechanical stimulation in a blocked design fashion (30 seconds initial baseline, 3 ON blocks of 30 seconds intermingled with 30 seconds OFF blocks, total scan time for each functional run = 210 seconds), as previously described.17 Starting time for functional runs was signaled visually by an operator in the control room to the second operator inside the magnet room, and timing of stimulus delivery was determined using a digital stopwatch by the latter. At the end of the imaging session, the subject was removed from the MRI scanner and a brief inspection of intraoral tissues took place. Four to 6 functional runs were collected from PDAP patients, while controls had 8 to 12 functional runs, 4 to 6 for each of the two stimulus levels provided to controls (subjective-pain and stimulus-intensity match). Variation in the number of functional runs was due to limited scanner availability; however, the mean number of functional runs between PDAP patients and control subjects under the subjective pain match (independent samples t test, 2-tailed, P = .23) or stimulus-intensity match (P = .19) conditions were not significantly different. Image data from all functional runs for each subject were averaged before group analysis (see below).
Neuroimaging Data Processing and Analysis
Data processing and analysis were carried out using FEAT 6.00 and other tools part of the FMRIB’s (Functional MRI of the Brain) Software Library (FSL) 5.0.5 software package30,31 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Non-brain structures were removed from anatomical images prior to processing. Visual inspection of functional images in cine mode was used to detect gross head movements (> 3 mm in any direction), as well as absolute (motion detected in volumes of each functional time series by using the middle volume as reference) and relative (reference volume is the next neighboring volume in the time series) motions as calculated by the “MCFLIRT” tool prior to correction. Preprocessing steps for functional data included discarding of the first three volumes to ensure that subsequent volumes had MRI signals at a longitudinal magnetization steady state, head motion correction, non-brain structures removal, spatial smoothing with a 5-mm full-width-at-half-maximum kernel, grand mean intensity normalization, and temporal high-pass filtering (cutoff 60 seconds). Image registration for each subject was done in three stages: (1) functional to anatomical images co-registration using a boundary-based registration method; (2) normalization of the anatomical image to the Montreal Neurological Institute (MNI) 152 brain at 1 mm3 resolution using linear registration (FMRIB’s Linear Image Registration Tool [FLIRT]); and (3) refined normalization using FMRIB’s Nonlinear Image Registration Tool (FNIRT) with a 10-mm warp resolution.
First-level analysis (functional run level) was carried out using FMRIB’s Improved Linear Model (FILM) with local autocorrelation correction. Explanatory variable (EV) for dentoalveolar pressure stimulation was modeled with a square waveform and convolved using a double-gamma hemodynamic response function (HRF), along with a separate temporal derivative EV of stimulation timing. Motion effects were removed using additional regressors of non-interest: (1) motion parameters derived from the preprocessing motion correction step and (2) volumes with excessive residual image intensity changes as identified jointly by two metrics, framewise displacement and the derivative of the root mean square variance over voxels.32 Importantly, this modeling of the HRF takes into account ongoing brain processes (cognitive, emotional, sensory, self-reflection, spontaneous pain in PDAP patients) and removes them as nuisance regressors, thus being only sensitive to brain activations evoked by the dentoalveolar mechanical stimulation. Second-level analysis (subject level) averaged all functional runs for each subject by using a fixed-effects model and prethreshold masking with the MNI 152 brain binary mask. Cluster-based threshold correction for multiple comparisons was carried out with a z statistic threshold of 2.3 to define contiguous clusters and cluster probability threshold (P value) of .05. Third-level analysis (group level) was done with both fixed- and mixed-effects models, also using the MNI 152 brain as a prethreshold binary mask and using a cluster-based threshold correction adopting a cluster threshold of z = 2.3 and P value < .01 and P value < .05 for between-group comparisons and for group mean activations, respectively.
The mean brain activation spatial extent was determined from second-level analysis output and was defined as the total number of voxels contained within clusters of activation for each subject. Mean brain activation spatial extent was compared between PDAP and control subjects for both subjective pain and stimulus intensity match conditions. Correlation analysis between brain activation spatial extent and clinical characteristics was done for PDAP patients only. Third-level analysis results included mean group activations for PDAP and control subjects for both conditions (subjective pain and stimulus intensity match) and also between-group comparisons. All between-group comparisons used contrast masking to restrict the comparisons to locations with positive mean group activation (z > 0). Thresholded z statistic maps were overlaid on the MNI 152 brain in order to determine anatomical location of brain activations using the “FSLview” display tool and its brain atlases (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). A whole-brain approach was used for brain activations detection instead of a focused region of interest analysis, as there were no previous studies with PDAP patients that could support a prior hypothesis on which brain regions would be likely to be activated following dentoalveolar mechanical stimulation.
Statistical Analyses
Data sets were assessed for normality by using descriptive statistics and the Kolmogorov-Smirnov statistic with Lilliefors significance correction. For those data sets fitting the assumptions of parametric tests, a t test for independent samples compared group differences for PDAP and control subjects under subjective pain and stimulus intensity match conditions. The Mann-Whitney U test for differences between independent groups and the Spearman rank-order correlation coefficient for correlations were used to assess nonparametric data sets. Pearson’s correlation coefficient as a measure of effect size33 was used to quantify the magnitude of the observed brain activation spatial extent following dentoalveolar pressure stimulation. Results are reported as mean ± standard deviation (SD) unless otherwise noted. Statistical tests were two-tailed and used a significance level of P < .05, unless otherwise noted. All statistical procedures were done using the statistical software package PASW Statistics 18.0 (SPSS Inc).
Results
Characteristics of Subjects
A total of 16 PDAP patients were recruited. However, two were excluded because their pain did not increase after local stimulation (light rubbing with gloved fingertip) and no longer fit inclusion criteria, whereas one patient had extreme sensitivity in the affected area and could not tolerate even minimum pressure on the affected area. Of the 17 control subjects assessed for eligibility, one was excluded for claustrophobia, one discontinued after the initial visit, one experienced hypersensitivity to the dentoalveolar stimulus and declined to continue, and for one neuro-imaging data could not be retrieved for analysis.
Thirteen PDAP patients (mean age: 54.7 ± 9.7 years; 11 females, 11 right-handed) and 13 age-, sex-, and handedness-matched control subjects (mean age: 53.3 ± 10.6 years, 11 females, 12 right-handed) completed the study (Table 1). Dentoalveolar stimulus location was matched across the groups. The mean number of ⅛-inch elastic bands used to stimulate PDAP patients was 2 (± 1), while control subjects in the subjective pain match condition had a mean of 7 (± 1). All control subjects under the stimulus-intensity match condition were stimulated with two ⅛-inch elastic bands (Table 1). Three PDAP patients had a clinical diagnosis of TMD, and another patient reported a previous diagnosis of migraine headache. No PDAP patient reported current widespread pain or a previous diagnosis of FM. No present or past comorbid pain conditions were reported for control subjects.
Table 1.
Summary Characteristics of Subjects
| N | Mean age ± S D (y) |
Sex | Handedness* | Dentoalveolar stimulus location (intraoral quadrant) |
Number of ⅛-inch elastic bands used for stimulation (1–8); mean ± SD |
|||
|---|---|---|---|---|---|---|---|---|
| Females | Males | Right | Left | |||||
| PDAP | 13 | 54.7 ± 9.7 | 11 | 2 | 11 | 2 | UR = 5; UL = 4 | 2 ± 1 |
| LR = 3; LL = 1 | ||||||||
| Controls | 13 | 53.3 ± 10.6 | 11 | 2 | 12 | 1 | UR = 6; UL = 4 | Subjective pain match = 7 ± 1 |
| LR = 3; LL = 1 | Stimulus intensity match = 2 ± 0 | |||||||
Self-report.
UR = upper right; UL = upper left; LR = lower right; LL = lower left.
The data from the questionnaires are summarized in Table 2. Dentoalveolar pain duration for PDAP patients averaged 8 years (± 6.5), ranging from 0.6 to 20 years. Patients had significantly higher scores in all questionnaires compared to control subjects (Table 2), as shown by the SF-MPQ VAS mean score as well as by sensory and affective components scores. The CPI averaged 55.5 (± 25.6) for the PDAP group and 0.6 (± 1.9) for control subjects. Five PDAP patients were graded as “low disability/low intensity” pain (grade I) for chronic pain severity, four as “low disability/high intensity” (grade II), and two had a grade of “high disability/moderately limiting” (grade III). Scores for anxiety and depression (HADS) for PDAP patients were significantly different than those of control subjects. Global functional jaw limitation derived from JFLS-8 showed moderate limitation for PDAP patients and no limitation for control subjects. The social impact of oral disorders as measured by the OHIP-14 was moderate for PDAP subjects and minimal for control subjects.
Table 2.
Pain and Functional Characteristics of Subjects
| PDAP | Controls | P value† | |
|---|---|---|---|
| McGill-SF | |||
| VAS (0–100) | 41.5 ± 21.2 | 0.3 ± 0.9* | < .0001 |
| Sensory (0–33) | 8.7 ± 6.2 | 0 ± 0* | < .0001 |
| Affective (0–12) | 2.8 ± 3.4 | 0 ± 0* | < .0001 |
|
Dentoalveolar pain duration (years since onset) |
8.0 ± 6.5 | - | - |
| GCPS | |||
| CPI (0–100) | 55.5 ± 25.6** | 0.6 ± 1.9* | < .0001 |
| III = 2 | |||
| Chronic pain severity | II = 4** | 0 = 12* | - |
| (Grade 0-IV) | I = 5 | ||
| HADS | |||
| Anxiety (0–21) | 10.1 ± 5.3 | 2.4 ± 1.4* | < .0001 |
| Depression (0–21) | 5.5 ± 3.7 | 1.3 ± 1.1* | .001 |
| JFLS-8 | |||
| (0–80) | 20.5 ± 14.1*** | 0.1 ± 0.3* | < .0001 |
| OHIP-14 | |||
| (0–56) | 25.7 ± 12.1** | 2.2 ± 4.2 | < .0001 |
Mean ± SD, except GCPS chronic pain severity.
Missing respondents: = 3;
= 2;
= 1.
Mann-Whitney U test, 2-tailed, Exact test.
McGill-SF = short-form McGill pain questionnaire; JFLS = Jaw Functional Limitation Scale; GCPS = Graded Chronic Pain Scale; CPI = Characteristic Pain Intensity; HADS = Hospital Anxiety and Depression Scale; OHIP = Oral Health Impact Profile.
Neuroimaging Data
The subjective-pain match condition included all 13 control subjects and 13 PDAP patients, whereas there were only 12 control subjects for the stimulus-intensity match condition since one control subject discontinued the study.
The mean absolute head motion was 0.19 mm (± 0.13) for PDAP patients and 0.14 mm (± 0.05) for control subjects in the subjective-pain match condition, whereas in the stimulus-intensity match it was 0.12 mm (± 0.06). There were no significant head-motion differences between PDAP and control subjects under either condition (independent samples t test, 2-tailed, .075 < P < .212).
Brain activation spatial extent included all voxels within clusters significantly activated as detected in the subject-level analysis. No significant differences were found between PDAP patients and control subjects for stimulus-intensity (P = .123) or subjective-pain (P = .479) match conditions (Table 3). A moderate effect size was found for the former (r = −0.31), while the latter only showed a small effect size (r = −0.14). No significant correlations between spatial extent and clinical characteristics of PDAP patients were found.
Table 3.
Comparison of Extent of Brain Activation
| Unpaired |
P value* |
||||
|---|---|---|---|---|---|
| PDAP (n = 13) |
Control subjective- pain match (n = 13) |
Control stimulus- intensity match (n = 12) |
PDAP × Control subjective-pain match |
PDAP × Control stimulus-intensity match |
|
| Median activation extent (voxels)† | 20,841 | 15,176 | 7,442 | .479 (U = 70.5, z = −0.72, r = −0.14) |
.123 (U = 49.5, z = −1.553, r = −0.31) |
Mann-Whitney U test, 2-tailed, Exact test.
Voxel size = 1 mm3.
z = U test statistic z-score; r = effect size estimate.
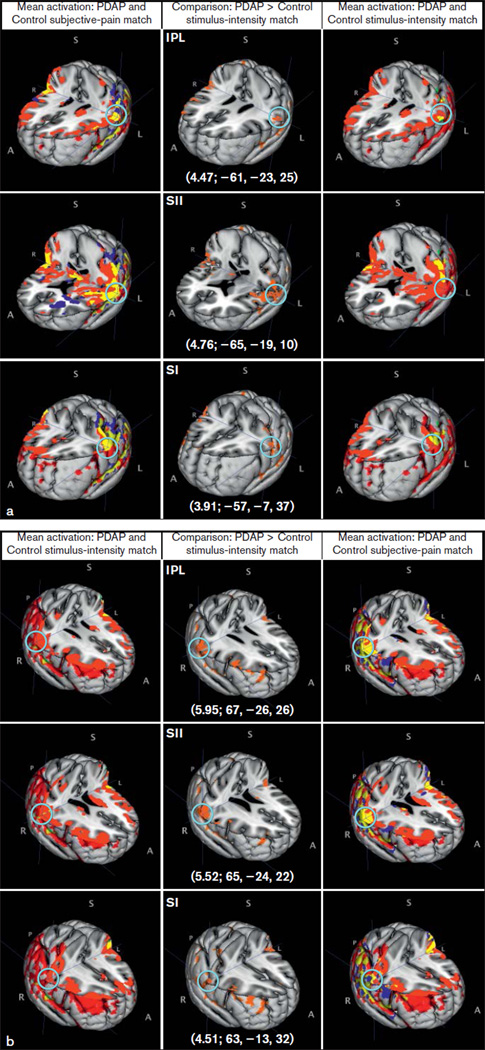
Significant between-group brain activation differences between PDAP patients and control subjects in both match conditions were found using a fixed-effects model (Table 4). Under the stimulus-intensity match condition, PDAP patients had greater brain activation in several brain regions compared to control subjects. Two major activation clusters were seen and included regions related to the sensory-discriminative aspect of pain processing (primary [SI] and secondary [SII] somatosensory cortices) and somatosensory integrative cortical areas (inferior parietal lobule [IPL]) bilaterally (Fig 2). Other cortical areas of activation that may be involved in pain perception included the insula and premotor cortex bilaterally (Fig 3), right middle frontal gyrus, frontal orbital cortex, and superior parietal lobule; subcortical areas included the right thalamus, right cerebellum, and left caudate nucleus (Fig 3). Of interest, several areas showing significant differences between control subjects and PDAP patients under the stimulus-intensity match condition (SI, SII, IPL, premotor cortex, insula, thalamus) also displayed overlapping of group mean brain activations under the subjective-pain match condition (Figs 2 and 3, yellow-colored locations highlighted by light-blue circle). When matched to subjective pain ratings, PDAP patients also exhibited greater activation compared to control subjects although in a less extensive region, which included SII, premotor cortex, insula, inferior frontal gyrus, and middle frontal gyrus—all on the right side—and the left frontal orbital cortex (Table 4). Control subjects had no brain regions with significantly greater activation relative to PDAP patients under the stimulus intensity or subjective-pain match conditions.
Table 4.
Between-Group Brain Activation Comparisons
| Brain region | Side | Cluster size (voxels)* |
Thresholded z-score (FE) |
Unthresholded z-score (ME) |
MNI coordinates (mm) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| PDAP > Control stimulus-intensity match | |||||||
| Inferior parietal lobule | Right | 11,491 | 5.95 | 2.28 | 67 | −26 | 26 |
| SII | Right | - | 5.52 | 2.11 | 65 | −24 | 22 |
| SI | Right | - | 4.51 | 2.41 | 63 | −13 | 32 |
| SII | Left | 10,304 | 4.76 | 1.51 | −65 | −19 | 10 |
| Inferior parietal lobule | Left | - | 4.47 | 1.96 | −61 | −23 | 25 |
| SI | Left | - | 3.91 | 2.39 | −57 | −7 | 37 |
| Premotor cortex | Left | - | 2.99 | 2.04 | −48 | −6 | 57 |
| Cerebellum | Right | 628 | 3.95 | 2.19 | 20 | −66 | −19 |
| Insula | Right | 479 | 5.07 | 2.05 | 43 | −3 | −12 |
| Thalamus | Right | 380 | 3.1 | 1.34 | 12 | −16 | 9 |
| Middle frontal gyrus (BA 9) | Right | 312 | 4.17 | 2.09 | 43 | 44 | 33 |
| Middle frontal gyrus (BA 10) | Right | 305 | 3.87 | 2.67 | 43 | 52 | 19 |
| Insula | Left | 291 | 4.04 | 2.75 | −37 | −5 | −5 |
| Caudate | Left | 211 | 3.68 | 2.20 | −16 | 4 | 19 |
| Frontal orbital cortex | Left | 177 | 3.69 | 2.40 | −40 | 30 | −7 |
| Premotor cortex | Right | 114 | 3.38 | 1.27 | 47 | −15 | 61 |
| Superior parietal lobe | Right | 109 | 3.51 | 2.17 | 36 | −44 | 45 |
| PDAP > Control subjective-pain match | |||||||
| SII | Right | 11,621 | 4.19 | 1.87 | 47 | −7 | 6 |
| Premotor cortex | Right | 11,365 | 4.19 | 2.18 | 52 | 9 | 44 |
| Inferior frontal gyrus (pars opercularis) | Right | - | 3.81 | 1.80 | 51 | 22 | 26 |
| Middle frontal gyrus (BA 9) | Right | 908 | 3.82 | 2.25 | 41 | 45 | 34 |
| Insula | Right | 838 | 3.98 | 2.37 | 40 | 3 | −8 |
| Medial frontal gyrus (BA 8) | Right | 469 | 3.65 | 2.14 | 4 | 49 | 41 |
| Middle frontal gyrus | Right | 248 | 4.05 | 3.70 | 51 | 39 | 16 |
| Frontal orbital cortex | Left | 103 | 3.59 | 2.96 | −38 | 30 | −7 |
Voxel size = 1 mm3. Reported only clusters with > 100 voxels.
MNI = Montreal Neurological Institute; FE = fixed-effects; ME = mixed-effects; SI = primary somatosensory cortex; SII = secondary somatosensory cortex; BA = Brodmann area; Shadowed rows: maximum activation within the cluster. White rows: local maxima within the cluster listed above it.
Fig 2.
Brain activations (a, left-sided; b, right-sided) within major clusters found using a fixed-effects model. Middle column shows comparison between PDAP patients and controls under stimulus-intensity match condition with the brain area with peak activity noted, followed by its z-score and MNI coordinates (z-score; X, Y, Z). A light-blue circle shows the peak activity voxel corresponding to the reported z-score. IPL = inferior parietal lobule; SI = primary somatosensory cortex; SII = secondary somatosensory cortex; A = anterior; S = superior; I = inferior; P = posterior; R = right; L = left. Red = PDAP; green = controls stimulus-intensity match; blue = controls subjective-pain match; yellow = overlap.
Fig 3.
Additional significant brain activations (a, left-sided; b, right-sided) found using a fixed-effects model. Middle column shows comparison between PDAP patients and controls under stimulus-intensity match condition with the brain area with peak activity noted, followed by its z-score and MNI coordinates (z-score; X, Y, Z). A light-blue circle shows the peak activity voxel corresponding to the reported z-score. PM = premotor cortex; Ins = insula, Thal = thalamus; Caud = Caudate nucleus. A = anterior; S = superior; I = inferior; P = posterior; R = right; L = left. Red = PDAP; green = controls stimulus-intensity match; blue = controls subjective-pain match; yellow = overlap.
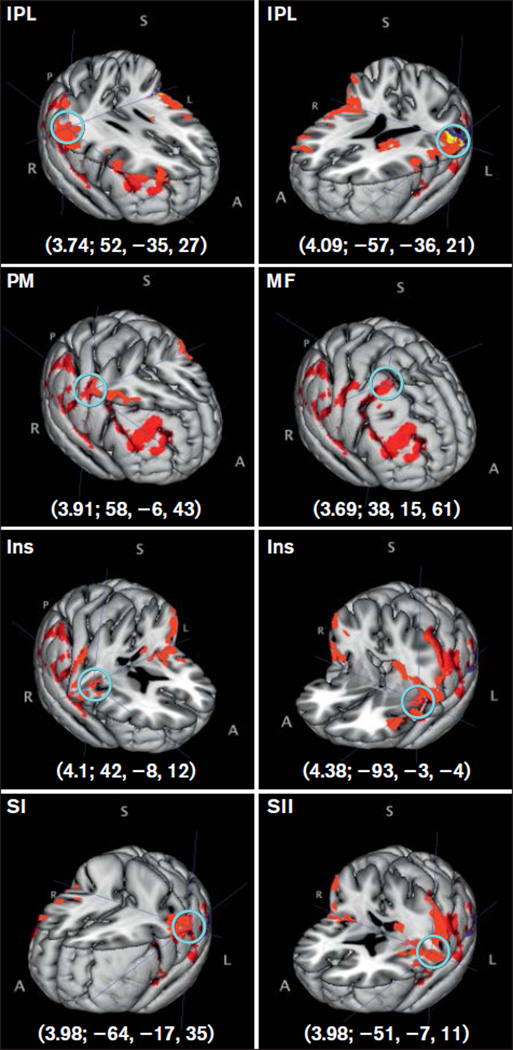
No significant group differences were found when a mixed-effects model was used; unthresholded z scores from this analysis in the same MNI coordinates from the fixed-effects model results are shown in Table 4. Group mean activations using a mixed-effects model were found for PDAP patients and control subjects under the subjective-pain match condition (Table 5) but not for the stimulus-intensity match. Activations in the insula and IPL bilaterally, left SI, left SII, right premotor cortex, and right middle frontal gyrus were found for PDAP patients, while only the left IPL was found active for control subjects under the subjective-pain match condition (Fig 4).
Table 5.
Group Mean Brain Activations (Mixed-Effects Model)
| Brain region | Side | Cluster size (voxels)* |
Thresholded z-score |
MNI coordinates(mm) | PDAP > Control stimulus-intensity match unthresholded z-score |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| PDAP | |||||||
| Insula | Left | 28,972 | 4.38 | −39 | −3 | −4 | 2.59 |
| Inferior parietal lobule | Left | - | 4.09 | −57 | −36 | 21 | 2.32 |
| SI | Left | - | 3.98 | −64 | −17 | 35 | 2.61 |
| SII | Left | - | 3.98 | −51 | −7 | 11 | 2.22 |
| Insula | Right | 14,459 | 4.1 | 42 | −8 | 12 | 2.54 |
| Premotor cortex | Right | - | 3.91 | 58 | −6 | 43 | 2.82 |
| Inferior parietal lobule | Right | - | 3.74 | 52 | −35 | 27 | 2.67 |
| Middle frontal gyrus | Right | 6,735 | 3.69 | 38 | 15 | 61 | 2.74 |
| Middle frontal gyrus (BA 9) | Right | - | 3.58 | 41 | 44 | 35 | 2.45 |
| Medial frontal gyrus (BA 6) | Right | - | 3.46 | 46 | 6 | 44 | 2.23 |
| Control subjective- pain match | |||||||
| Inferior parietal lobule | Left | 2,889 | 3.87 | −48 | −41 | 30 | - |
Voxel size = 1 mm3. All clusters found reported.
MNI = Montreal Neurological Institute; SI = primary somatosensory cortex; SII = secondary somatosensory cortex; BA = Brodmann area.
Shaded rows: maximum activation within cluster. White rows: local maxima within the cluster listed above it.
Fig 4.
Mean group brain activations found using a mixed-effects model. Brain area activated is noted, followed by its z-score and MNI coordinates (z-score; X, Y, Z). A light-blue circle shows the peak activity voxel corresponding to the reported z-score. IPL = inferior parietal lobule; Ins = insula; PM = premotor cortex; MF = middle frontal gyrus; SI = primary somatosensory cortex; SII = secondary somatosensory cortex. A = anterior; S = superior; I = inferior; P = posterior; R = right; L = left. Red = PDAP; blue = controls subjective-pain match; yellow = overlap.
Discussion
This study provides initial neuroimaging evidence in PDAP patients of amplified brain processing of dentoalveolar pressure stimulation. These results suggest that amplification of pain processing observed in select brain centers may underlie PDAP pathophysiology.
Characteristics of Subjects
The case definition used for PDAP and the sample characteristics such as age and pain duration were similar to those of previous studies.15,16,19–21 One inclusion criterion used here that has not been well described previously is pain increase as a consequence of local provocation of the affected area. This was anecdotally observed by clinicians within the UMN TMD and Orofacial Pain Clinic and in a previous study,17 with other groups concurring to this via questionnaire data34 or testing for dynamic mechanical allodynia,15 while others included local hyperesthesia in their criteria.35,36 PDAP patients showed moderate clinical pain, as identified by VAS and CPI scores, and significantly different sensory and affective components compared to controls. Chronic pain severity was mostly within the “low-disability” range for cases, which corresponded to the moderate levels of PDAP continuous pain previously reported.21 Assessment of anxiety produced a score of “possible”27 for PDAP patients, but depression did not; however, both scores were significantly higher compared to those of control subjects. Jaw function limitation related to PDAP pain was significantly higher than for control subjects, in agreement with previous findings.21 Perceived impact of PDAP pain on the individual’s well-being was moderate, as measured by OHIP-14, and researchers using different quality of life measurement instruments reported similar findings.21
Brain Activations
The spatial extent of brain activation, ie, the total number of voxels within activation clusters, was not different for PDAP patients and control subjects under either match condition, although a moderate effect size difference was found under the stimulus-intensity match condition. Extraoral noxious heat stimulation in burning mouth syndrome (BMS) patients has revealed a reduced brain activation spatial extent for cases compared to control subjects, and this was attributed to diminished inhibitory controls of sensory input.37 It is possible that although sharing similar neural pathways, PDAP and BMS recruit different supraspinal mechanisms. Alternatively, differences in stimulus modality and location may have played a role, since PDAP patients received mechanical stimulation directly over the affected painful site, whereas a heat stimulus was applied to a distant site from the affected area in BMS patients.37
These results support the primary hypothesis that brain activations following dentoalveolar pressure stimulation are significantly greater in PDAP patients compared to control subjects when matched to the same stimulus intensity (evoking little to no pain). Contrary to the second hypothesis that brain activation patterns are similar for patient and control groups following stimulation levels matched to subjective pain ratings, control subjects still had several brain areas with less activation than PDAP patients even when matched to subjective pain ratings. It is interesting that the brain regions with significant differences between the two groups when matched to stimulus intensity corresponded to those found by Gracely and collaborators in FM patients.23 Thus, despite major differences regarding the spatial extent of the pain in PDAP (localized) and FM (widespread), these two conditions may share similar brain mechanisms of abnormal somatosensory processing of mechanical stimulation.
PDAP patients showed greater brain activations compared to control subjects in regions related to somatosensory processing38 (thalamus, SI, SII, posterior parietal cortex, IPL) when matched to stimulus intensity. Other regions associated with nociceptive processing that displayed differences in activation between the two groups were the insula and the pre-motor and prefrontal cortical areas. Each of these brain regions has been implicated in the sensory-discriminative or cognitive-attentional aspects of pain perception.39,40 Surprisingly, brain activation in the anterior cingulate cortex (ACC), an area typically associated with the affective component of pain among other functions, revealed no significant differences between PDAP patients and control subjects. This was unexpected, given the study’s findings of greater anxiety, moderate jaw function impairment, and greater social impact of oral disorders in patients. In addition, these results, based on the areas of group brain activations for PDAP patients and control subjects under subjective-pain match using a mixed-effects model, support the notion of differential processing of sensory-discriminative and cognitive, but not affective, dentoalveolar noxious pressure signals in PDAP.
Neuroimaging provides a suitable, noninvasive approach to assess central neural processing in chronic pain patients that may aid in unraveling structural, functional, and biochemical brain changes associated with human chronic pain states.22,41 The clinical features of PDAP suggest that neuroimaging may provide new information that would otherwise be difficult to obtain. First, PDAP likely has both peripheral and central components, since peripheral input barrage is not sufficient to explain PDAP symptoms, given that local anesthesia provided only partial relief for PDAP spontaneous pain compared to placebo.20 Second, somatosensory abnormalities consistent with central sensitization have been reported in PDAP,15–18 but the mechanisms related to those abnormalities are not fully understood. Third, anecdotal evidence from several PDAP patients seen at the UMN TMD and Orofacial Pain Clinic showed that the pain can spread from a localized dentoalveolar region to neighboring tooth/teeth and even to the opposite dentoalveolar arch over time. Thus, despite being a topic of clinical investigation for several decades with numerous techniques including thermography,42 somatic and/or sympathetic blocks,20,43,44 psychological questionnaires,21,45–49 psychophysics,47,48 blink reflex,50 topical and/or intravenous pharmacologic agents,19,51 and QST,15,16,18 the causal and perpetuating mechanisms underlying PDAP remain elusive notwithstanding all the knowledge gained to date.
The results reported here bring a different perspective on the pathophysiology of PDAP. The most accepted theory by investigators in the field is deafferentation following procedures such as root canal treatment, tooth extraction, or other surgical procedures.10 Although preclinical data in cats52,53 and rats54 support functional alterations in trigeminal brainstem neurons following tooth pulp extirpation, evidence from primates, including humans, is lacking. Dental procedures preceding PDAP onset have been anecdotally described, with reported occurrence of 54%55 up to 83%.21 By contrast, other studies have reported that 24%55 to 64%56 of the patients with pain did not have a preceding dental procedure. The evidence of increased brain activation in areas associated with sensory-discriminative and cognitive aspects of pain processing (eg, thalamus, SI, SII, insula, prefrontal cortex) as well as an integrative cortical area related to somatosensation (IPL)38,57,58 support the hypothesis of altered central neural mechanisms in PDAP pain. Although these findings cannot exclude peripheral factors, they provide initial evidence of dysregulation of intraoral somatosensory stimulus processing in PDAP and thus warrant further investigation.
This study had some limitations. First, it was planned as an exploratory study with a limited sample size even though it is not far from sample sizes described in recent neuroimaging studies of orofacial pain conditions.59–61 This may explain why differences in brain activation spatial extent did not reach significance between PDAP patients and controls matched to stimulus intensity, as well as why only a fixed-effects model (within-subject variability) showed significant differences in brain activations between groups. Mixed-effects analysis results that use subject-to-subject variation (cross-subject variability) as a measure of variance were also reported, which allows inferences based on it to be extrapolated to the population level.62 By reporting both fixed- and mixed-effects analyses’ results, it is expected that this will provide a comprehensive perspective of the results presented as well as their limitations. Second, a multiple-comparison correction method was used (cluster-based thresholding) that is sensitive to activations and for activations across neighboring voxels; however, it lacks spatial specificity with large clusters63 that may have an impact on correctly identifying the specific activated brain regions. Third, the threshold used to identify clusters of activation (P < .01) has been considered not as stringent by some authors63; however, after repeating the fixed-effects analyses with the recommended threshold by those authors (P < .001), no major changes in the brain activations were detected. The threshold applied here was in agreement with the current standard used in many neuroimaging studies of P < .05 for cluster-based threshold.60,64–66 Finally, this study matched PDAP patients and control subjects on several aspects, including stimulus location. While this reduced the differences in dentoalveolar stimulation at the group level, it also resulted in subjects within each group being stimulated in different intraoral quadrants. This prevented group comparisons of: (1) somatotopic features of dentoalveolar stimulus; (2) laterality of brain activated areas; and (3) potential differential activity in trigemino-thalamo-cortical pathways.
Conclusions
PDAP patients had greater brain activation in regions related to the sensory-discriminative and cognitive components of pain perception when stimulated with matched dentoalveolar pressure intensity applied to control subjects. When matched to subjective pain ratings, PDAP patients continued to display greater brain activations than control subjects, albeit the activations were less extensive. Application of other stimulus modalities (eg, thermal), experimental interventions (eg, local anesthesia of the pain site), use of additional neuroimaging modalities (eg, resting state, diffusion weighted), as well as use of longitudinal study designs may further clarify pathophysiologic brain mechanisms underlying PDAP.
Acknowledgments
The authors would like to thank Richard H. Gracely, PhD, for his valuable comments during the preparation of this manuscript. This research was supported by the following National Institutes of Health grants: K12-RR023247, P41-RR008079, and P30-NS057091.
Footnotes
None of the authors have any conflicts of interest to disclose.
Contributor Information
Estephan J. Moana-Filho, Clinical Assistant Professor, Division of TMD and Orofacial Pain, School of Dentistry, University of Minnesota, Minneapolis, Minnesota, USA.
David A. Bereiter, Professor, Division of Basic Sciences, Department of Diagnostic and Biological Sciences, School of Dentistry, University of Minnesota, Minneapolis, Minnesota, USA.
Donald R. Nixdorf, Associate Professor, Division of TMD and Orofacial Pain, School of Dentistry, Adjunct Assistant Professor, Department of Neurology, Medical School, University of Minnesota, Research Investigator, HealthPartners Institute for Education, and Research, Minneapolis, Minnesota, USA.
References
- 1.Glaser MA. Applied neuralgia, so called: A critical analysis of one hundred and forty-three cases. Arch Neurol Psychiatr. 1928;20:537–558. [Google Scholar]
- 2.McElin TW, Horton BT. Atypical facial pain: A statistical consideration of 65 cases. Ann Intern Med. 1947;27:749–768. doi: 10.7326/0003-4819-27-5-749. [DOI] [PubMed] [Google Scholar]
- 3.Hunter J. A Practical Treatise on the Diseases of the Teeth; Intended as a Supplement to the Natural History of Those Parts. 6. London: J. Johnson; 1778. p. 128. iv. [Google Scholar]
- 4.Marbach JJ. Phantom tooth pain. J Endod. 1978;4:362–372. doi: 10.1016/S0099-2399(78)80211-8. [DOI] [PubMed] [Google Scholar]
- 5.Rees RT, Harris M. Atypical odontalgia. Br J Oral Surg. 1979;16:212–218. doi: 10.1016/0007-117x(79)90027-1. [DOI] [PubMed] [Google Scholar]
- 6.Nixdorf DR, Drangsholt MT, Ettlin DA, et al. Classifying orofacial pains: A new proposal of taxonomy based on ontology. J Oral Rehabil. 2012;39:161–169. doi: 10.1111/j.1365-2842.2011.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durham J, Exley C, John MT, Nixdorf DR. Persistent dentoalveolar pain: The patient’s experience. J Orofac Pain. 2013;27:6–13. doi: 10.11607/jop.1022. [DOI] [PubMed] [Google Scholar]
- 8.Durham J, Nixdorf DR. Healthcare pathway and biopsychosocial impact of persistent dentoalveolar pain disorder: A qualitative study. Int Endod J. 2014;47:1151–1159. doi: 10.1111/iej.12263. [DOI] [PubMed] [Google Scholar]
- 9.Melis M, Lobo SL, Ceneviz C, et al. Atypical odontalgia: A review of the literature. Headache. 2003;43:1060–1074. doi: 10.1046/j.1526-4610.2003.03207.x. [DOI] [PubMed] [Google Scholar]
- 10.Baad-Hansen L. Atypical odontalgia—Pathophysiology and clinical management. J Oral Rehabil. 2008;35:1–11. doi: 10.1111/j.1365-2842.2007.01813.x. [DOI] [PubMed] [Google Scholar]
- 11.Nixdorf DR, Moana-Filho EJ. Persistent dento-alveolar pain disorder (PDAP): Working towards a better understanding. Rev Pain. 2011;5:18–27. doi: 10.1177/204946371100500404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Dental Association. 2005–06 Survey of Dental Services Rendered. Chicago: American Dental Association; 2007. [Google Scholar]
- 13.Lewis MA, Sankar V, De Laat A, Benoliel R. Management of neuropathic orofacial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(suppl S32):e1–e24. doi: 10.1016/j.tripleo.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Baad-Hansen L, Pigg M, Ivanovic SE, et al. Chairside intraoral qualitative somatosensory testing: Reliability and comparison between patients with atypical odontalgia and healthy controls. J Orofac Pain. 2013;27:165–170. doi: 10.11607/jop.1062. [DOI] [PubMed] [Google Scholar]
- 15.List T, Leijon G, Svensson P. Somatosensory abnormalities in atypical odontalgia: A case-control study. Pain. 2008;139:333–341. doi: 10.1016/j.pain.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Zagury JG, Eliav E, Heir GM, et al. Prolonged gingival cold allodynia: A novel finding in patients with atypical odontalgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:312–319. doi: 10.1016/j.tripleo.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Moana-Filho EJ, Nixdorf DR, Bereiter DA, John MT, Harel N. Evaluation of a magnetic resonance-compatible dentoalveolar tactile stimulus device. BMC Neurosci. 2010;11:142. doi: 10.1186/1471-2202-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baad-Hansen L, Pigg M, Ivanovic SE, et al. Intraoral somato-sensory abnormalities in patients with atypical odontalgia— A controlled multicenter quantitative sensory testing study. Pain. 2013;154:1287–1294. doi: 10.1016/j.pain.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baad-Hansen L, Juhl GI, Jensen TS, Brandsborg B, Svensson P. Differential effect of intravenous S-ketamine and fentanyl on atypical odontalgia and capsaicin-evoked pain. Pain. 2007;129:46–54. doi: 10.1016/j.pain.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 20.List T, Leijon G, Helkimo M, Oster A, Svensson P. Effect of local anesthesia on atypical odontalgia—A randomized controlled trial. Pain. 2006;122:306–314. doi: 10.1016/j.pain.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 21.List T, Leijon G, Helkimo M, Oster A, Dworkin SF, Svensson P. Clinical findings and psychosocial factors in patients with atypical odontalgia: A case-control study. J Orofac Pain. 2007;21:89–98. [PubMed] [Google Scholar]
- 22.Borsook D, Sava S, Becerra L. The pain imaging revolution: Advancing pain into the 21st century. The Neuroscientist. 2010;16:171–185. doi: 10.1177/1073858409349902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 24.Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 25.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 26.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohrbach R, Larsson P, List T. The jaw functional limitation scale: Development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain. 2008;22:219–230. [PubMed] [Google Scholar]
- 29.Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. 1997;25:284–290. doi: 10.1111/j.1600-0528.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):208S–219S. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Field A. Discovering Statistics Using IBM SPSS Statistics. London, UK: Sage; 2013. [Google Scholar]
- 34.Klasser GD, Kugelmann AM, Villines D, Johnson BR. The prevalence of persistent pain after nonsurgical root canal treatment. Quintessence Int. 2011;42:259–269. [PubMed] [Google Scholar]
- 35.Graff-Radford SB, Solberg WK. Atypical odontalgia. J Craniomandib Disord. 1992;6:260–265. [PubMed] [Google Scholar]
- 36.Oshima K, Ishii T, Ogura Y, Aoyama Y, Katsuumi I. Clinical investigation of patients who develop neuropathic tooth pain after endodontic procedures. J Endod. 2009;35:958–961. doi: 10.1016/j.joen.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Albuquerque RJ, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: An fMRI study. Pain. 2006;122:223–234. doi: 10.1016/j.pain.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 38.Kandel ER. Principles of Neural Science. New York: McGraw-Hill; 2012. [Google Scholar]
- 39.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 41.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: Is chronic pain a disease? J Pain. 2009;10:1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Graff-Radford SB, Ketelaer MC, Gratt BM, Solberg WK. Thermographic assessment of neuropathic facial pain. J Orofac Pain. 1995;9:138–146. [PubMed] [Google Scholar]
- 43.Graff-Radford SB, Solberg WK. Differential Neural Blockade in Atypical Odontalgia—Somatic vs. Sympathetic. Cephalalgia. 1991;11:289–291. [Google Scholar]
- 44.Vickers ER, Cousins MJ, Walker S, Chisholm K. Analysis of 50 patients with atypical odontalgia. A preliminary report on pharmacological procedures for diagnosis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:24–32. doi: 10.1016/s1079-2104(98)90393-6. [DOI] [PubMed] [Google Scholar]
- 45.Graff-Radford SB, Solberg WK. Is atypical odontalgia a psychological problem? Oral Surg Oral Med Oral Pathol. 1993;75:579–582. doi: 10.1016/0030-4220(93)90228-v. [DOI] [PubMed] [Google Scholar]
- 46.Marbach JJ. Is phantom tooth pain a deafferentation (neuropathic) syndrome? Part II: Psychosocial considerations. Oral Surg Oral Med Oral Pathol. 1993;75:225–232. doi: 10.1016/0030-4220(93)90098-o. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs R, Wu CH, Goossens K, et al. A case-control study on the psychophysical and psychological characteristics of the phantom tooth phenomenon. Clin Oral Investig. 2002;6:58–64. doi: 10.1007/s00784-001-0149-9. [DOI] [PubMed] [Google Scholar]
- 48.Tassinari G, Migliorini A, Girardini F, Luzzani A. Reference fields in phantom tooth pain as a marker for remapping in the facial territory. Funct Neurol. 2002;17:121–127. [PubMed] [Google Scholar]
- 49.Baad-Hansen L, Leijon G, Svensson P, List T. Comparison of clinical findings and psychosocial factors in patients with atypical odontalgia and temporomandibular disorders. J Orofac Pain. 2008;22:7–14. [PubMed] [Google Scholar]
- 50.Baad-Hansen L, List T, Kaube H, Jensen TS, Svensson P. Blink reflexes in patients with atypical odontalgia and matched healthy controls. Exp Brain Res. 2006;172:498–506. doi: 10.1007/s00221-006-0358-1. [DOI] [PubMed] [Google Scholar]
- 51.Baad-Hansen L, List T, Jensen TS, Svensson P. Increased pain sensitivity to intraoral capsaicin in patients with atypical odon-talgia. J Orofac Pain. 2006;20:107–114. [PubMed] [Google Scholar]
- 52.Hu JW, Dostrovsky JO, Lenz YE, Ball GJ, Sessle BJ. Tooth pulp deafferentation is associated with functional alterations in the properties of neurons in the trigeminal spinal tract nucleus. J Neurophysiol. 1986;56:1650–1668. doi: 10.1152/jn.1986.56.6.1650. [DOI] [PubMed] [Google Scholar]
- 53.Hu JW, Sessle BJ. Effects of tooth pulp deafferentation on nociceptive and nonnociceptive neurons of the feline trigeminal subnucleus caudalis (medullary dorsal horn) J Neurophysiol. 1989;61:1197–1206. doi: 10.1152/jn.1989.61.6.1197. [DOI] [PubMed] [Google Scholar]
- 54.Kwan CL, Hu JW, Sessle BJ. Effects of tooth pulp deafferentation on brainstem neurons of the rat trigeminal subnucleus oralis. Somatosens Mot Res. 1993;10:115–131. doi: 10.3109/08990229309028828. [DOI] [PubMed] [Google Scholar]
- 55.Takenoshita M, Sato T, Kato Y, et al. Psychiatric diagnoses in patients with burning mouth syndrome and atypical odontalgia referred from psychiatric to dental facilities. Neuropsychiatr Dis Treat. 2010;6:699–705. doi: 10.2147/NDT.S12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ram S, Teruel A, Kumar SK, Clark G. Clinical characteristics and diagnosis of atypical odontalgia: Implications for dentists. J Am Dent Assoc. 2009;140:223–228. doi: 10.14219/jada.archive.2009.0136. [DOI] [PubMed] [Google Scholar]
- 57.Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 58.Caspers S, Schleicher A, Bacha-Trams M, Palomero-Gallagher N, Amunts K, Zilles K. Organization of the human inferior parietal lobule based on receptor architectonics. Cereb Cortex. 2013;23:615–628. doi: 10.1093/cercor/bhs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moayedi M, Weissman-Fogel I, Salomons TV, et al. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. Pain. 2012;153:1467–1477. doi: 10.1016/j.pain.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152:384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 61.Lickteig R, Lotze M, Kordass B. Successful therapy for temporomandibular pain alters anterior insula and cerebellar representations of occlusion. Cephalalgia. 2013;33:1248–1257. doi: 10.1177/0333102413491028. [DOI] [PubMed] [Google Scholar]
- 62.Monti MM. Statistical Analysis of fMRI Time-Series: A Critical Review of the GLM Approach. Front Hum Neurosci. 2011;5:28. doi: 10.3389/fnhum.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He SS, Li F, Song F, et al. Spontaneous neural activity alterations in temporomandibular disorders: A cross-sectional and longitudinal resting-state functional magnetic resonance imaging study. Neuroscience. 2014;278:1–10. doi: 10.1016/j.neuroscience.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 65.Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: Is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci. 2012;32:14874–14884. doi: 10.1523/JNEUROSCI.1733-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Youssef AM, Gustin SM, Nash PG, et al. Differential brain activity in subjects with painful trigeminal neuropathy and painful temporomandibular disorder. Pain. 2014;155:467–475. doi: 10.1016/j.pain.2013.11.008. [DOI] [PubMed] [Google Scholar]