Abstract
The Ah receptor (AhR)-responsive CALUX (chemically-activated luciferase expression) cell bioassay is commonly used for rapid screening of samples for the presence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin), dioxin-like compounds, and AhR agonists/antagonists. By increasing the number of AhR DNA recognition sites (dioxin responsive elements), we previously generated a novel third generation (G3) recombinant AhR-responsive mouse CALUX cell line (H1L7.5c3) with significantly enhanced sensitivity and response to DLCs compared to existing AhR-CALUX cell bioassays. However, the elevated background luciferase activity of these cells and the absence of comparable G3 cell lines derived from other species have limited their utility for screening purposes. Here, we describe the development and characterization of species-specific G3 recombinant AhR-responsive CALUX cell lines (rat, human, and guinea pig) that exhibit significantly improved sensitivity and dramatically increased TCDD induction response. The low background luciferase activity, low minimal detection limit (0.1 pM TCDD) and enhanced induction response of the rat G3 cell line (H4L7.5c2) over the H1L7.5c3 mouse G3 cells, identifies them as a more optimal cell line for screening purposes. The utility of the new G3 CALUX cell lines were demonstrated by screening sediment extracts and a small chemical compound library for the presence of AhR agonists. The increased sensitivity and response of these new G3 CALUX cell lines will facilitate species-specific analysis of DLCs and AhR agonists in samples with low levels of contamination and/or in small sample volumes.
INTRODUCTION
The aryl hydrocarbon receptor (AhR) is a chemical-responsive transcription factor that is responsible for mediating the toxic and/or biological effects of a wide range of structurally diverse chemicals.1–3 While many of these AhR-active chemicals are toxic environmental contaminants of widespread concern, including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin), related dioxin-like halogenated aromatic hydrocarbons (HAHs), and numerous polycyclic aromatic hydrocarbons (PAHs), a wide variety of nontoxic synthetic, endogenous, and naturally occurring AhR agonists have also been identified.1–4 New insights into some of the endogenous physiological functions of the AhR has also led to the identification and development of numerous AhR ligands (agonists/antagonists) as potential human therapeutic drugs.5–7 Thus, given the structural diversity and ubiquitous nature of AhR active chemicals and the established potential/ability of different classes of AhR ligands to produce adverse and/or beneficial effects, the detection and characterization of AhR-active chemicals in environmental, biological, food and other matrices to which humans and animals are exposed is necessary.
While instrumental analysis methods are the gold standard for detection and quantitation of selected AhR agonists (i.e. TCDD and related TCDD-like HAHs)8, these methods are inadequate high-throughput screening (HTS) approaches for the detection, identification and characterization of the wide range of structurally diverse AhR activators that may or may not be known.1, 3 Accordingly, numerous AhR-mechanism-based bioassays and bioanalytical methods have been developed, optimized and validated for detection, identification and characterization of AhR active chemicals and determination of total AhR agonist activity in extracts of a wide variety of sample matrices.9, 10 Although analysis of crude extracts of a given sample provides no information as to the identity or potency of the responsible AhR-active chemical(s), when a crude sample extract is first subjected to an appropriate and selective cleanup methodology, these bioassay/bioanalytical methods can be used for the detection and relative quantitation of a specific class of AhR-active chemicals (i.e., TCDD and related TCDD-like HAHs).11–13 The so-called AhR-based Chemically-Activated LUciferase eXpression (CALUX) bioassay is one such cell-based bioassay that has received USEPA certification as a validated and approved method (USEPA Method 4435) for the detection of TCDD and TCDD-like HAHs in selected environmental matrices.14
Beyond their utility as bioassays for the detection and relative quantitation of TCDD-like HAHs in sample extracts, AhR-based bioassays can also be utilized to increase our understanding of the structural diversity of AhR active chemicals and their molecular mechanisms. This is particularly important given the key role that this receptor appears to play in various toxicological, biochemical, physiological and developmental responses.3, 5, 15 However, although there may be similarities across different species in relative responsiveness and rank order potency of some classes of AhR active chemicals (TCDD and TCDD-like HAHs), there exists dramatic species-specific differences in the chemical structures of other AhR-active ligands.16, 17 As such, activation of the AhR by a given chemical in one species does not necessarily predict its ability to activate the AhR or produce an AhR-dependent response in another species.1, 12, 18–20 Thus, optimal utility of AhR-based bioassays for the detection of the full spectrum of AhR active substances (toxic and nontoxic) for different species necessitates the development of a series of sensitive and highly responsive species-specific bioassays (optimally containing a common AhR-responsive reporter system). Using a molecular approach an extremely responsive and highly sensitive recombinant mouse hepatoma CALUX cell bioassay (the so-called third generation (G3) CALUX cell bioassay) containing a stably transfected plasmid (pGudLuc7.5) with a firefly luciferase reporter gene under control of 20 dioxin responsive elements was recently developed.21 Here we describe the development of novel human, rat and guinea pig G3 CALUX cell lines also stably transfected with pGudLuc7.5. Screening analysis with these four G3 CALUX cell lines using sediment extracts and a small chemical compound library not only revealed significant species-specific differences in ligand-dependent responsiveness, but they identified the new stably transfected rat hepatoma G3 CALUX cell line as a more optimal line for the detection of TCDD and TCDD-like HAHs.
MATERIALS AND METHODS
Materials
TCDD was obtained from Dr. Steve Safe (Texas A&M University) and was handled and disposed of in accordance with University of California safety policies. The chemical library of 176 compounds obtained from Dr. Bruce Hammock (University of California, Davis) was described previously.9 Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA), and Geneticin (G418) and Alpha Minimal Essential Medium (α-MEM) were from Invitrogen (San Diego, CA). CALUX cell lines H1L6.1c3 (mouse hepatoma (Hepa1c1c7)), H4L1.1c4 (rat hepatoma (H4IIe)), G16L1.1c8 (guinea pig intestinal adenocarcinoma (GPC-16)), and HG2L6.1c1 (human hepatoma (HepG2)) were previously described.22, 23
Luciferase expression vectors and stable transfection
Construction of the third generation (G3) AhR-responsive luciferase reporter gene plasmid pGudLuc7.5 containing five concatenated 480 bp dioxin responsive domain (DRD)-containing fragments (each with 4 DREs for a total of 20 DREs) was described previously.21 Rat hepatoma (H4IIe), human hepatoma (HepG2), and guinea pig intestinal adenocarcinoma (GPC16) cells (all obtained from ATCC) were stably cotransfected with pGudLuc7.5 and pSV2neo using Lipofectamine 2000 (Invitrogen) following manufacturer’s procedures. After 24 hour of growth in nonselective medium, cells were split 1 to 10 and replated into selective medium containing G418 (400 mg/L for rat H4IIe and human HepG2 cells and 200 mg/L for guinea pig GPC16 cells). After 2–4 weeks of growth in selective medium, individual cell colonies were identified, cloned and their TCDD-inducible luciferase activity determined.
Chemical treatment and luciferase analysis
In the screening and characterization studies, 75,000 cells were plated into each well of a white, clear-bottomed 96-well tissue culture plates in 100 μL α-MEM containing 10% FBS and allowed to attach for 24 hours. Cells were incubated with carrier solvent DMSO (1% final concentration) or the indicated concentration of TCDD (in DMSO) for 24 hours at 37°C, after which cells were lysed and luciferase activity in each well measured using an Orion microplate luminometer.24
Chemical compound library
The chemical library used for screening contained 176 compounds in DMSO9, and cells were incubated with each chemical at a final incubation concentration of 10 μM for 24 h. For comparative studies, luciferase induction values were normalized to maximal luciferase activity induced by 1 nM TCDD (set at 100%).
Sediment samples
Thirty sediment samples were collected by the National Oceanic and Atmospheric Administration from the Great Lakes and their river tributaries.25 Approximately 10 g of wet mass (9.5–10.5g) of each sediment was removed from a thawed and homogenized sediment sample and mixed with anhydrous sodium sulfate (~28 g) in an 8 oz mortar bowl. The sample was ground to dryness and transferred to a 33 mL Accelerated Solvent Extraction (ASE; Dionex Inc.) cell and capped. Extraction was completed under pressure with dichloromethane and acetone (50:50 (v/v)). For sediment samples undergoing biological analysis, the resulting ~40 mL extract was filtered through phase separation filter paper (Whatman SP1) and sodium sulfate into evaporation vessels and solvent volumes were reduced to ~0.5 mL under a gentle nitrogen stream (Zymark TurboVap system). Evaporation tubes were rinsed with acetone (~5 mL) and reduced under nitrogen and this solvent exchange was carried out twice. Final evaporation volumes approached dryness (~200 μL), and the resulting sample extract was transferred to a graduated amber glass vial (2 mL) and brought to 1 mL with 75:25 DMSO:acetone (v/v) and diluted using 75:25 DMSO:acetone (v/v) such that the final test concentrations were between 0.001–10 mg sediment equivalents/mL. Extracted samples were capped with Teflon-lined septa caps and stored at 4°C in the dark until analysis. The ability of an aliquot of the sediment extract (1 μL) to induce luciferase activity in each cell line was determined as described above. For sediment samples undergoing chemical analysis, after the ASE, samples were subjected to Gel Permeation Chromatography and Solid Phase Extraction and solvent exchanged into hexane for chemical analysis by GC/MS as described previously.25 Method blanks, spiked blanks and standard reference materials (NIST 1944) were included in all chemical analyses to ensure chemical data quality.25
Statistical and potency calculations
Luciferase activity (RLU) in lysates of cells incubated with solvent or method blank samples was subtracted from luciferase activity in chemical/extract-treated cells to obtain final induced luciferase activity. Half-maximal induction concentrations (EC50) with each chemical or extract were determined as previously described.24 Bioanalytical equivalency (BEQ) values were obtained as described previously24 and expressed as the average BEQ value (ng TCDD equivalents/g wet weight of original sediment) for each sediment sample extract obtained from triplicate experiments. The minimal detection limit (MDL) for TCDD (i.e., activity that was significantly above background) was determined using Student’s t-test (2-tailed, Type 2).
RESULTS AND DISCUSSION
Numerous AhR mechanism-based bioassays have been developed for chemical screening purposes and each have advantages and limitations with regards to their utility and applications.22, 26 The mouse hepatoma AhR-CALUX (H1L6.1c3) cell bioassay was approved by the USEPA for detection of TCDD and related TCDD-like chemicals in environmental extracts (Method 4435).14 However, the MDL of this bioassay was not low enough to analyze samples with extremely low levels of contamination by TCDD-like chemicals and/or those with limited sample volumes. Attempts to address these limitations led to the development of the enhanced recombinant mouse hepatoma G3 CALUX cell line with dramatically improved sensitivity and response.21 However, the relatively high background luciferase activity of this new CALUX cell line has been somewhat problematic for low-level analysis, and analogous G3 CALUX cell lines from other species are lacking for species-specific analysis of AhR active chemicals. Here we describe the development and application of improved rat, human and guinea pig AhR-responsive G3 CALUX cell lines.
Generation and testing of the species-specific G3 CALUX cell lines
Rat hepatoma (H4IIe), human hepatoma (HepG2) and guinea pig intestinal carcinoma (GPC16) cells were stably cotransfected with pGudLuc7.5 and pSV2neo, and from this transfection 95 H4L7.5, 34 G16L7.5, and 53 HG2L7.5 individual cell clones were isolated. The optimal clone for each stably transfected cell line was selected for low background luciferase activity and high magnitude of luciferase induction in response to TCDD. The TCDD-induced and background luciferase activity of the selected rat, human and guinea pig G3 CALUX cell lines (H4L7.5c2, G16L7.5c1, and HG2L7.5c1, respectively) and previously generated mouse hepatoma G3 CALUX cell line H1L7.5c3 are shown in Supplemental Figure S1. These results reveal that the new rat hepatoma (H4L7.5c2) cell had the most dramatic induction response to TCDD of all G3 cell lines. The luciferase activity of the human hepatoma G3 CALUX cell line HG2L7.5c1 was somewhat comparable to the response observed with the mouse H1L7.5c3 cells, and the induction response in the guinea pig G3 CALUX cell line G16L7.5c1 was significantly lower than all other cell lines examined.
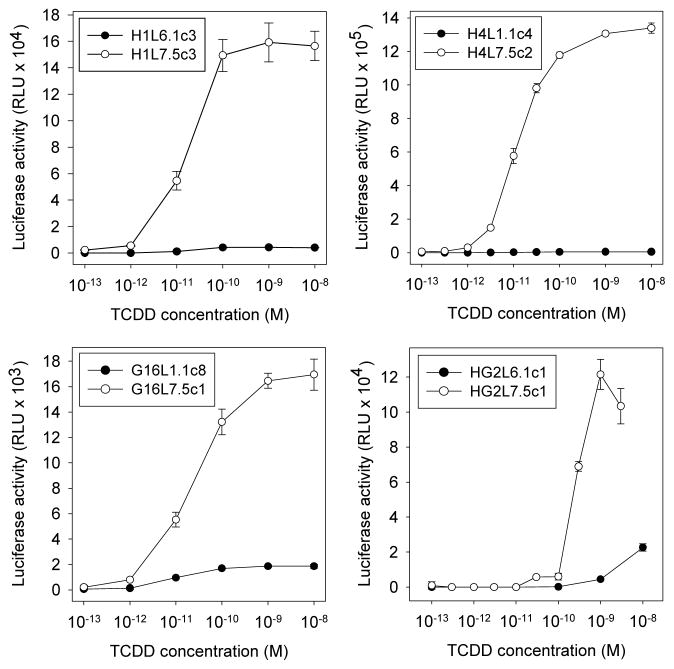
To evaluate the relative responsiveness of each of the new species-specific G3 CALUX cell lines we examined the concentration-dependent ability of TCDD to induce luciferase in each cell line (Figure 1) and compared these responses to those of earlier versions of CALUX cell lines prepared in the same parental cell lines (H1L6.1c3, H4L1.1c2, G16L1.1c8, HG2L6.1c1).27 The absolute luciferase activity induction response in each of the newly generated G3 CALUX cell lines is dramatically greater than that produced in all earlier CALUX cell lines (Figure 1), consistent with our previous observations using the mouse hepatoma G3 CALUX cells.21 Comparison of the concentration response curves of luciferase activities normalized to the maximal luciferase activity obtained with TCDD in each of the cell lines, reveals that the overall shape of the concentration response curves and the relative potency (EC50) were very similar for the mouse, rat and guinea pig G3 and previous CALUX cell lines (Table 1, Supplemental Figure S2), consistent with a common AhR-dependent mechanism of action. However, the EC50 for TCDD in the human G3 CALUX cell line (HG2L7.5c1) was approximately 10-fold lower than that in the previous HepG2-based CALUX cell line HG2L6.1c1 (Table 1, Supplemental Figure S2). While the mechanism for the increased potency to TCDD in the human G3 CALUX cells is unknown, the human HG2L7.5c1 cell line exhibited ~10-fold less relative potency to TCDD when compared to the mouse, rat, and guinea pig cell lines, presumably due to the 10-fold lower affinity of the human AhR for TCDD.16, 28 The mouse H1L7.5c3, rat H4L7.5c2 and human HG2L7.5c1 G3 CALUX cells had a 10-fold lower MDL than earlier versions of CALUX cells, with the MDL for TCDD in rat and mouse G3 CALUX cells having the lowest published MDL for cell-based bioassays for TCDD (0.1 pM TCDD (Table 1)). The lack of significant difference in the MDL for TCDD between the two generations of guinea pig CALUX cell lines may result from the relatively low induction response and low concentration of AhR present in this cell line, but this remains to be confirmed.
Figure 1.
G3 CALUX cell lines (stably transfected with pGudLuc7.5 construct) have dramatically increased concentration-dependent response to TCDD compared to their respective previous generation CALUX cell lines. Recombinant mouse hepatoma (H1L6.1c3, H1L7.5c3), rat hepatoma (H4L1.1c4, H4L7.5c2), guinea pig intestinal adenocarcinoma (G16L1.1c8, G16L7.5c1), and human hepatoma (HG2L6.1c1, HG2L7.5c1) cells were incubated for 24 h with increasing concentrations of TCDD and luciferase activity (relative light units (RLUs)) in cell lysates was measured as described under Materials and Methods. Luciferase activity induced by DMSO/TCDD (1 nM) was 7,200 ± 610/170,000 ± 15,000, 8,300 ± 370/1,300,000 ± 40,000, 2,200 ± 240/19,000 ± 590, 8,600 ± 1,700/130,000 ± 8,600 RLU in H1L7.5c3, H4L7.5c2, G16L7.5c1, and HG2L7.5c1 cells, respectively. Results are representative of n ≥ 8 individual experiments and represent the mean ± SD of triplicate determinations after subtraction of the luciferase activity obtained in cells exposed to DMSO.
Table 1.
Comparison of the EC50 and minimal detection limits (MDL) for luciferase induction from TCDD concentration response analysis in CALUX cell lines from various species.
| Cell line | Reporter Vector | Cell clone | Luciferase Induction
|
|
|---|---|---|---|---|
| EC50 (M)a | MDL (M) | |||
| Mouse | ||||
| Hepa1c1c7 | pGudLuc6.1 | H1L6.1c3 | 3.7 ± 0.7 × 10−11 | 1.0 × 10−12 |
| pGudLuc7.5 | H1L7.5c3 | 1.6 ± 0.4 × 10−11 | 1.0 × 10−13 | |
| Rat | ||||
| H4IIE | pGudLuc1.1 | H4L1.1c4 | 3.1 ± 0.7 × 10−11 | 1.0 × 10−12 |
| pGudLuc7.5 | H4L7.5c2 | 4.3 ± 0.6 × 10−11 | 1.0 × 10−13 | |
| Guinea pig | ||||
| GPC16 | pGudLuc1.1 | G16L1.1c8 | 1.7 ± 0.5 × 10−11 | 1.0 × 10−12 |
| pGudLuc7.5 | G16L7.5c1 | 2.5 ± 0.9 × 10−11 | 1.0 × 10−12 | |
| Human | ||||
| HepG2 | pGudLuc6.1 | HG2L6.1c1 | 2.2 ± 0.2 × 10−9 | 1.0 × 10−10 |
| pGudLuc7.5 | HG2L7.5c1 | 2.2 ± 0.3 × 10−10 | 3.0 × 10−11 | |
values represent the mean ± SD of 4–12 replicate analyses.
Optimization of the rat hepatoma G3 CALUX cell line, H4L7.5c2
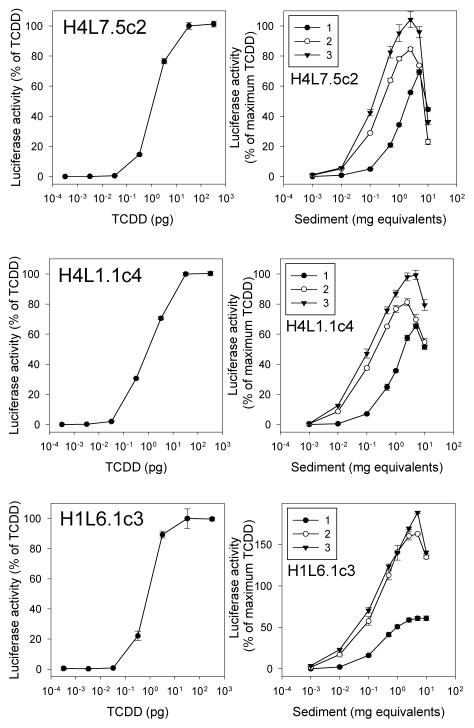
One goal of developing the species-specific G3 CALUX cell lines was to develop an improved G3 CALUX cell line that would be more optimal for screening purposes than that of the recently developed mouse hepatoma G3 CALUX cell line H1L7.5c3, which has high background luciferase activity.21 The rat hepatoma G3 CALUX cell line (H4L7.5c2) was found to be an improvement as it had a more consistent response to TCDD than H1L7.5c3 cells, relatively lower background luciferase activity (compare 8,000 RLU in H4L7.5c2 cells to 100,000 RLU in H1L7.5c3 cells) and a higher fold induction response (Supplemental Figure S3)). Additional time course experiments revealed significant levels of TCDD-induced luciferase activity in H4L7.5c2 cells by 2 hours of incubation (p < 0.05), with maximal luciferase activity reached at least by 24 hours and maintained for up to 48 hours of incubation (Supplemental Figure S4). While 24 hours of incubation was selected as the recommended exposure time for bioassay analysis, shorter incubation periods can be used for detection of metabolically labile or unstable AhR agonists (i.e. PAHs).29–31
Using optimal assay conditions, we compared the assay parameters derived from concentration response studies with H4L7.5c2 cells to those of other commonly used rat and mouse luciferase CALUX cell bioassays and a rat cell ethoxyresorufin-O-deethylase (EROD) bioassay.21, 22, 27, 32, 33 While the EC50 values for TCDD were comparable between the five luciferase bioassays (which is not surprising given that the TCDD binding affinity is comparable for the AhRs in these cells), the MDL for TCDD in the G3 CALUX H4L7.5c2 cell line was lower than any other CALUX-type cell bioassay22 aside from our previously described H1L7.5c3 G3 CALUX cell line (Supplemental Table S1).
Screening sediment samples using G3 CALUX cells
CALUX bioassays are used for screening a wide variety of environmental, biological, food, feed and commercial and consumer products for the presence of TCDD-like chemicals and AhR activators.22, 34–38 One application of our newly developed G3 CALUX cell lines is to examine species-specificity of chemicals (as pure compounds or present in known or unknown mixtures). While species-specific CALUX AhR bioassays are available, the majority of the validated (and commonly used) cell lines contain different luciferase reporter plasmids, complicating clear interpretation of results from common samples analyzed between cell lines.22, 23, 27 Accordingly, the G3 CALUX cell lines developed here contain the identical pGudLuc7.5 construct, eliminating one variable between these different cell lines and allows a more accurate comparison of species differences in response to test chemicals.
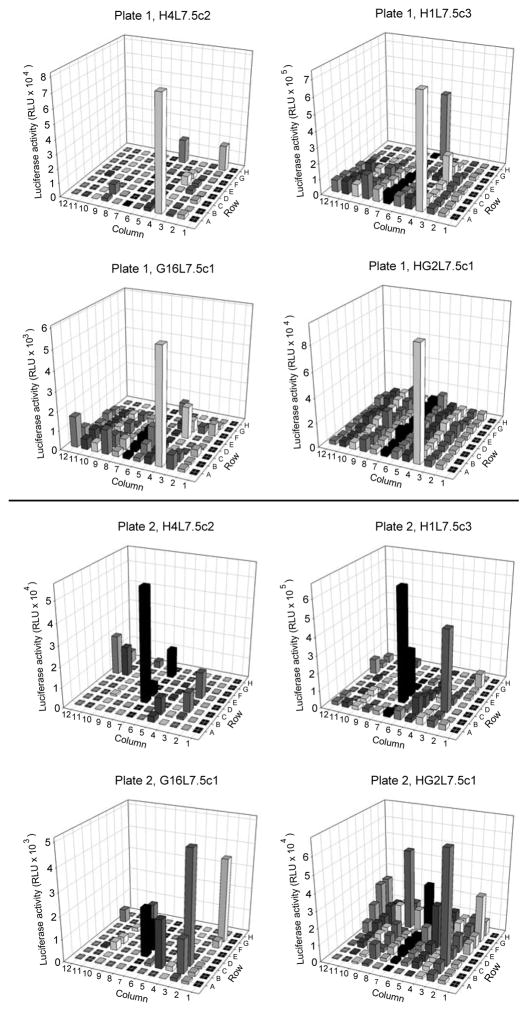
The utility of the four G3 CALUX cells for detection of species-specific AhR agonists in complex samples was first examined by screening thirty sediment extracts collected from Lake Erie and Lake Michigan (Figure 2). Interestingly, the new rat H4L7.5c2 G3 CALUX cells had the lowest background luciferase activity with the DMSO solvent blank (plate position 1A) and the method blanks (plate positions 1B–1E) of all G3 cell lines. Significant differences in overall relative response are observed with G3 CALUX cell lines from different species, with guinea pig G3 CALUX cells (G16L7.5c1) exhibiting substantial superinduction of luciferase activity in response to many extracts. The significant differences in overall induction by the various sediments between the G3 CALUX cell lines can result from numerous factors, including (but not limited to): differences in ligand binding specificity and affinity of the AhR from different species1–3; differences in metabolism in each cell line, which can enhance or reduce overall response; and differences in other cellular factors and/or cell signaling pathways in each cell line that can be differentially affected by chemicals in the extract to modulate AhR responsiveness and response.3
Figure 2.
Screening of sediment extracts in H4L7.5c2 (rat), H1L7.5c3 (mouse), G16L7.5c1 (guinea pig), and HG2L7.5c1 (human) G3 CALUX cell lines. Extracts from thirty sediment samples were screened for AhR agonist activity at a final amount of 10 mg sediment equivalent. Cells were incubated for 24 h, lysed, and luciferase levels measured as described in Materials and Methods. Luciferase activity is expressed as a percent of maximal luciferase induction observed with 1 nM TCDD in each cell line. Each bar represents the average of triplicate determinations. All blanks and sample extracts are in the same position in each plate with each cell line. Activity of DMSO (plate position 1A) and method blanks (plate positions 1B–1E) are included in each plate. Luciferase activity of DMSO/TCDD-treated H4L7.5c2, H1L7.5c3, G16L7.5c1, and HG2L7.5c1 cells was 2,600 ± 680/690,000 ± 29,000, 71,000 ± 1,100/590,000 ± 19,000, 460 ± 91/5,500 ± 420, and 6,100 ± 1,500/30,000 ± 1,800 RLU, respectively.
To further examine the utility of the new rat G3 CALUX bioassay for detection of AhR active chemicals in complex mixtures, three of the sediment extracts which induced luciferase activity in the H4L7.5c2 rat cell line were selected for concentration-response analysis in the G3 rat hepatoma H4L7.5c2 cells (Figure 3A, B), rat hepatoma H4L1.1c4 (Figure 3C, D), and mouse hepatoma H1L6.1c3 (Figure 3E, F) CALUX cells. Extracts were serially diluted and incubated with each of the cell lines at relative concentrations of 0.001–10 mg of sediment equivalents per well for 24 hours and luciferase activity determined. Clear differences between the relative potency of the three sediment sample extracts to induce luciferase activity were observed in the CALUX cell lines, when results were normalized to maximal activity induced by TCDD (Figure 3B, D, F; Table 2). Sediment 1 (collected from East Basin of Lake Erie) was the least potent of the three sample extracts and produced the lowest magnitude of luciferase induction in all cell lines. The normalized induction responses obtained with the rat H4L7.5c2 cells were similar to those obtained with the well-established H4L1.1c4 and H1L6.1c3 cell lines (Figure 3), although superinduction responses were observed in H1L6.1c3 cells with sediments 2 and 3 (collected from the bay of Lake Michigan and a tributary to Lake Michigan, respectively) in the H1L6.1c3 cells. While the reason for superinduction in the H1L6.1c3 cells (and G16L7.5c1 cells (Supplemental Figure S5)) is unclear, superinduction has been previously observed in these cell lines and several mechanistic explanations have been proposed.24 The reason for the decline in luciferase activity at high sediment extract concentrations (Figure 3B, D, F) is currently unknown but does not appear to be due to cell toxicity based on visual inspection of the cells prior to lysis. EC50 values for each sediment sample, with superinduction results normalized as previously described24, were similar between the three CALUX cell lines (and nearly identical between the two rat cell lines), resulting in comparable calculated TCDD bioanalytical equivalent (BEQ) values for those sediments (Table 2). BEQ values were slightly higher for Sediment 3 compared to Sediment 2 for all three CALUX cell lines, and TCDD BEQs per g sediment were significantly greater for Sediments 2 and 3 compared to that of Sediment 1 (Table 2). While the CALUX induction potency trend for these three sediment extracts in the rat and mouse CALUX cell lines is consistent with the increased levels of PCBs, PAHs and DDTs in the sediments (Supplemental Table S2), the identity of the chemical(s) responsible for the induction response remains to be determined.
Figure 3.
Sediment extracts induce luciferase in a dilution-dependent manner in rat hepatoma G3 CALUX cells and previous generation mouse and rat hepatoma CALUX cells. Rat H4L7.5c2 G3 CALUX cells, rat H4L1.1c4 CALUX cells, or mouse H1L6.1c3 CALUX cells were incubated with TCDD or three different sediment extracts, and luciferase activity in cell lysates was measured as described under Materials and Methods. The sediment extracts studies correspond to those in positions 5A, 5C and 6E of the plates presented in Figure 2. Luciferase activity was expressed as a percent of maximum TCDD induction and values represent the mean ± SD of triplicate determinations after subtraction of the luciferase activity obtained in cells exposed to DMSO (for TCDD) or method blanks (for sediments). These data are representative of results from three separate experiments.
Table 2.
EC50 and bioanalytical equivalents (BEQs) values from concentration-/dilution-response analysis of sediment extracts in the H4L7.5c2 G3 cell line and more commonly used H4L1.1c4 and H1L6.1c3 CALUX cell lines.
| Cell line | Sample | EC50 (pg TCDD or mg sediment) | TCDD BEQs (ng eqv/g)a |
|---|---|---|---|
| H4L7.5c2 | TCDD | 1.4 ± 0.05 | 1 |
| Sediment 1 | 1.9 ± 0.10 | 0.76 ± 0.18 | |
| Sediment 2 | 0.26 ± 0.09 | 5.5 ± 1.5 | |
| Sediment 3 | 0.17 ± 0.03 | 8.3 ± 1.3 | |
| H4L1.1c4 | TCDD | 0.99 ± 0.03 | 1 |
| Sediment 1 | 2.1 ± 0.53 | 0.49 ± 0.13 | |
| Sediment 2 | 0.27 ± 0.05 | 3.7 ± 0.4 | |
| Sediment 3 | 0.17 ± 0.10 | 6.2 ± 0.87 | |
| H1L6.1c3 | TCDD | 0.97 ± 0.22 | 1 |
| Sediment 1 | 0.80 ± 0.26 | 1.3 ± 0.77 | |
| Sediment 2 | 0.20 ± 0.01 | 4.2 ± 0.24 | |
| Sediment 3 | 0.22 ± 0.03 | 5.3 ± 0.64 |
BEQ values are expressed as ng TCDD equivalents per g sediment and represent the mean ± SD of BEQ values from three separate experiments.
Analysis of complex mixtures of sample extracts provides an additional avenue in which to examine species differences in AhR-responsiveness. Induction of luciferase reporter gene activity by sediment extracts 1–3 were also examined in mouse, guinea pig, and human G3 CALUX cell lines (Supplemental Figure S5). Like that of the mouse H1L6.1c3 cells (but not the mouse H1L7.5c3 cells), sediment sample extracts 2 and 3 resulted in superinduction of luciferase reporter gene activity in the guinea pig G3 CALUX cell line (consistent with the results in Supplemental Figure S5), while inducing poorly in the human G3 CALUX cell line. The ability of the extracts to superinduce luciferase activity in the mouse H1L6.1c3 cells (Figure 3F), but not the H1L7.5c3 cells (Supplemental Figure S5B), suggests that the superinduction response is not simply mediated directly via the AhR or a cellular factor in the mouse hepatoma (Hepa1c1c7) cells, but by a factor selectively enhancing the induction response from the earlier version of the luciferase reporter gene plasmid (i.e. pGudLuc6.1) present in the H1L6.1c3 cells. Superinduction of AhR-dependent gene expression has been previously reported with other chemicals and sample extracts.21, 24, 39 The low level of luciferase induction (relative to TCDD) observed in HG2L7.5c1 cells likely results from the 10-fold lower affinity of the human AhR for ligands compared to that of rodent species.16, 28
Screening a chemical compound library using G3 CALUX cells
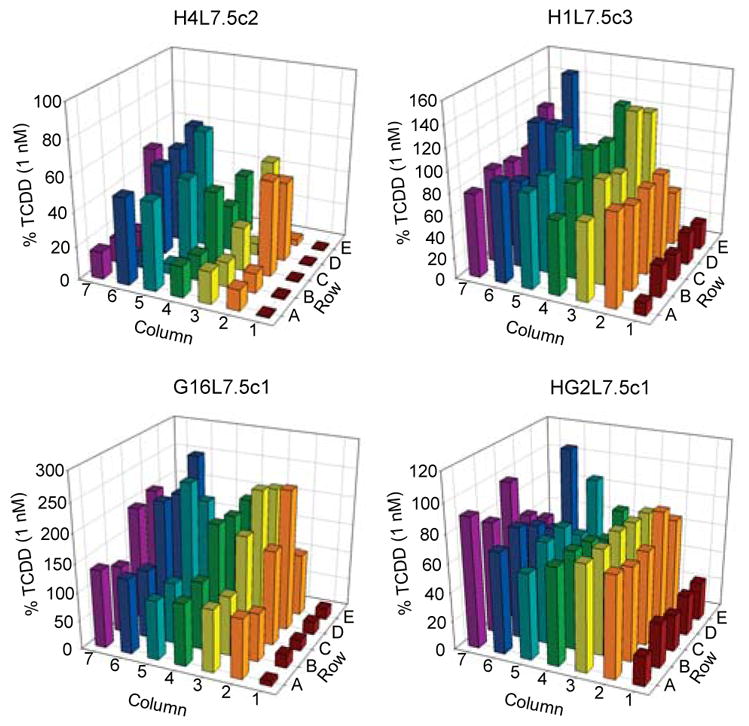
CALUX cell lines are also used to identify and characterize pure chemicals as AhR agonists/antagonists and have revealed some species differences in AhR ligand specificity/selectivity.9, 29, 40 The most commonly used CALUX and CALUX-type AhR bioassays typically do not contain identical luciferase reporters and this can complicate interpretations of agonist/antagonist characterization because of the potential effect of other cellular factors on different plasmids. The G3 CALUX cell lines described here contain the same luciferase reporter construct, and their greatly amplified response and enhanced sensitivity over commonly used CALUX and CALUX-type AhR bioassays allows for improved species-specific comparisons of large sample sets via HTS. The species-specificity of our G3 CALUX cell lines for AhR agonists was examined by comparing the luciferase induction response of the four G3 CALUX cell lines to a chemical library consisting of 176 pure pesticides and industrial chemicals contained in two 96-well plates.9 The chemicals present in the test library and their plate positions are indicated in Supplemental Table S3. The rat H4L7.5c2 G3 CALUX cells responded to the fewest number of library chemicals, while the human HG2L7.5c1 G3 cells were activated by the greatest number of library chemicals (Figure 4). Carbaryl (position A4 on plate 1) induced significant luciferase activity in all four G3 CALUX cell lines consistent with its documented activity as an AhR agonist41, whereas chloranocryl (position H6 on plate 1) induced high luciferase activity in the mouse H1L7.5c3 cell line but to a much lesser extent in the other three G3 CALUX cell lines. Diuron (location C7 on plate 2), a previously identified AhR activator42, induced the highest luciferase activity in the mouse and rat G3 CALUX cells and to a lesser extent in the guinea pig CALUX cells; little induction was observed in human G3 CALUX cells. In contrast, dichlone (location C3 on plate 2) induced highly in the mouse, guinea pig, and human G3 CALUX cells, while only weakly inducing in rat G3 CALUX cells (Figure 4). Dichlone was previously identified from chemical library screening in mouse H1L6.1c3 cells as an AhR agonist.9 2,3,4,6-tetrachlorophenol (location G2 on plate 2) activated luciferase to the greatest extent in the guinea pig CALUX cell line whereas 1-nitro naphthalene (location G9 on plate 2) induced the most strongly in the human CALUX cell line and not at all in the rat CALUX cell line (Figure 4). These results demonstrate the utility of the G3 CALUX cell lines as a screening approach to identify novel species-specific AhR agonists in chemical libraries. By examining the ability of the test chemicals to inhibit luciferase induction by a known AhR agonist such as TCDD or β-naphthoflavone, species-specific AhR antagonists in the chemical library can also be identified.43 Although the AhR ligand binding domain is fairly well conserved, numerous amino acid differences in the ligand binding domain between different species has been shown to greatly impact affinity and specificity of an AhR for a given ligand, and clearly contribute to some of the apparent species differences observed for selected ligands in these and other studies.43–46
Figure 4.
Screening results of a chemical library of common pesticides and industrial chemicals in H4L7.5c2 (rat), H1L7.5c3 (mouse) G16L7.5c1 (guinea pig), and HG2L7.5c1 (human) G3 CALUX cells. Chemicals in the library were screened for AhR agonist activity at a final concentration of 10 μM. Cells were incubated with the indicated chemical for 24 hours, cells lysed, and luciferase levels measured and analyzed as described in Materials and Methods. Luciferase activity is expressed as relative light units (RLU) after subtraction of background luciferase activity in each cell line. Luciferase activity induced by 1 nM TCDD in the H4L7.5c2, H1L7.5c3, G16L7.5c1, and HG2L7.5c1 cell lines was 1,100,000 ± 83,000, 970,000 ± 71,000, 6,800 ± 540, and 92,000 ± 16,000, respectively. Each bar represents the mean of triplicate determinations. The specific chemicals in the library are presented in Supplemental Table S3.
In addition to evaluating species-specific response of the G3 cell lines to a chemical library, each cell line was incubated with increasing concentrations of selected HAHs (Supplemental Figure S6) or PAHs (Supplemental Figure S7) and luciferase activity determined. These results also revealed some significant chemical-selective differences in both the relative potency and efficacy of selected chemicals as activators of AhR-dependent luciferase gene expression in these cell lines. For example, human AhR appears to be poorly activated by the AhR active PCBs 3,3′,4,4′-tetrachlorobiphenyl (PCB77) and 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) and unaffected by 2,3,3′,4,4′,5-hexachlorobiphenyl (PCB157), whereas PCB157 can stimulate AhR-dependent luciferase gene expression in rat, mouse and guinea pig G3 CALUX cells (Supplemental Figure S6). These observations are consistent with recent studies that reported human AhR as significantly less responsive than rodent AhR to PCB77 and PCB126.47, 48 In addition, while 2,3,4,7,8-pentachlorodibenzofuran (23478PCDF) was nearly equipotent to TCDD in mouse, guinea pig and human G3 CALUX cells, but it was more than 10-fold less potent than TCDD in rat G3 CALUX cells. Similar species differences in the relative potency and efficacy of select PAHs was also apparent (Supplemental Figure S7), with human G3 CALUX cells typically exhibiting much lower sensitivity and/or responsiveness, likely resulting from the lower AhR ligand binding affinity of the human AhR compared to that of other species. Together, these results demonstrate the utility of these novel species-specific G3 CALUX cell lines to begin to identify apparent species differences in AhR responsiveness of chemicals and to utilize these cell lines, in combination with AhR ligand and DNA binding analyses, for in-depth mechanistic studies of these species differences.
Our G3 CALUX cell lines have both enhanced sensitivity and responsiveness which make them more useful for monitoring of TCDD-like compounds and other AhR-active substances than previously described AhR-based CALUX and CALUX-type cell lines. Their increased sensitivity and responsiveness allows detection of AhR agonists at ultra-low concentrations and/or in very small amounts of sample or sample extract. In fact, the enhanced response in the G3 CALUX cells has made them particularly useful for HTS purposes using 384 and 1536 well plates, because significantly fewer cells are needed to obtain a measured response. Overall, the availability of these new G3 CALUX cell lines and their ease of use and availability make possible the HTS of AhR agonists/antagonists for identification and characterization of species-specific differences in AhR responsiveness, an area in which little progress has been made.
Supplementary Material
Acknowledgments
This work was supported by grants to M.S.D from the National Institutes of Environmental Health Sciences (R01ES007685 and P42ES04699 (Superfund Research Grant)), grants to J.C.B. from the University of California, Davis (Jastro-Shields) and a National Institutes of Environmental Health Sciences training grant (T32 ES007058-33), the California Agricultural Experiment Station and the American taxpayers. T.T. was supported in part by Health and Labour Sciences Research Grants and a Japan Food Hygiene Association Grant for Promoted Project of Research on Risk of Chemical Substances.
ABBREVIATIONS
- AhR
aryl hydrocarbon receptor
- TCDD
2,7,7,8,-tetrachlorodibenzo-p-dioxin
- PAH
polycyclic aromatic hydrocarbon
- HAH
halogenated aromatic hydrocarbon
- CALUX
chemically activated luciferase expression
- DRE
dioxin response element
- DRD
dioxin response domain
- EC50
effective concentration 50%
- HTS
high throughput screening
Footnotes
Supporting information includes: Tables of 1) commonly used rat and mouse HTS bioassays, 2) chemical classes from sediment extracts, and 3) a list of the chemicals screened in Figure 4 and their position on the microplates; Figures 1 and 3) DMSO- and TCDD-induced luciferase activity in the G3 CALUX cell lines, 2) normalization of luciferase activity from Figure 1, 4) the time-course of TCDD induced luciferase activity in rat H4L7.5c2 cells, 5) luciferase activity of Sediment extracts 1–3 in the mouse, guinea pig and human G3 CALUX cell lines; and, 6 and 7) HAH and PAH concentration response analysis in G3 CALUX cell lines, respectively. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
References
- 1.DeGroot D, He G, Fraccalvieri D, Bonati L, Pandini A, Denison M. AhR ligands: promiscuity in binding and diversity in response. In: Pohjanvirta R, editor. The Ah receptor in biology and toxicology. 1. John Wiley and Sons, Inc; 2011. pp. 63–91. [Google Scholar]
- 2.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Ann Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 3.Denison MS, Soshilov AA, He GC, DeGroot DE, Zhao B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeuken A, Keser BJ, Khan E, Brouwer A, Koeman J, Denison MS. Activation of the Ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J Agric Food Chem. 2003;51(18):5478–87. doi: 10.1021/jf030252u. [DOI] [PubMed] [Google Scholar]
- 5.Safe S, Lee SO, Jin UH. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci. 2013;135(1):1–16. doi: 10.1093/toxsci/kft128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prud’homme GJ, Glinka Y, Toulina A, Ace O, Subramaniam V, Jothy S. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS One. 2010;5(11):e13831. doi: 10.1371/journal.pone.0013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr Rev. 2013;71(6):353–69. doi: 10.1111/nure.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EPA. Appendix A to Part 136: Methods for inorganic chemical analysis of municipal and industrial wastewater. Method 613-2,3,7,8-tetrachlorodibenzo-p-dioxin. Federal Register. 1984:1–20. [Google Scholar]
- 9.Morisseau C, Merzlikin O, Lin A, He GC, Feng W, Padilla I, Denison MS, Pessah IN, Hammock BD. Toxicology in the fast lane: Application of high-throughput bioassays to detect modulation of key enzymes and receptors. Environ Health Perspect. 2009;117(12):1867–1872. doi: 10.1289/ehp.0900834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy SR, Sanborn JR, Hammock BD, Denison MS. Development of a green fluorescent protein-based cell Bioassay for the rapid and inexpensive detection and characterization of Ah receptor agonists. Toxicol Sci. 2002;65(2):200–210. doi: 10.1093/toxsci/65.2.200. [DOI] [PubMed] [Google Scholar]
- 11.Windal I, Denison MS, Birnbaum LS, Van Wouwe N, Baeyens W, Goeyens L. Chemically activated luciferase gene expression (CALUX) cell bioassay analysis for the estimation of dioxin-like activity: Critical parameters of the CALUX procedure that impact assay results. Environ Sci Technol. 2005;39(19):7357–7364. doi: 10.1021/es0504993. [DOI] [PubMed] [Google Scholar]
- 12.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DJ, Orelien J, Gordon JD, Chu AC, Chu MD, Nakamura M, Handa H, Kayama F, Denison MS, Clark GC. Mathematical model developed for environmental samples: Prediction of GC/MS dioxin TEQ from XDS-CALUX bioassay data. Environ Sci Technol. 2007;41(12):4354–4360. doi: 10.1021/es062602+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EPA. Method 4435: Method for toxic equivalents (TEQs) determinations for dioxin-like chemical activity with the CALUX bioassay. 2008:1–58. [Google Scholar]
- 15.Denison MS, Heath-Pagliuso S. The Ah receptor: A regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61(5):557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- 16.Mexia N, Gaitanis G, Velegraki A, Soshilov A, Denison MS, Magiatis P. Pityriazepin and other potent AhR ligands isolated from Malassezia furfur yeast. Arch Biochem Biophys. 2015 doi: 10.1016/j.abb.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard TD, Murray IA, Bisson WH, Lahoti TS, Gowda K, Amin SG, Patterson AD, Perdew GH. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015;5:12689. doi: 10.1038/srep12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safe S. Polychlorinated-biphenyls (PCBs), dibenzo-para-dioxins (PCDDs), dibenzofurans (PCDFs), and related-compounds-environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Crit Rev Toxicol. 1990;21(1):51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- 19.Aarts J, Denison MS, Cox MA, Schalk MAC, Garrison PM, Tullis K, Dehaan LHJ, Brouwer A. Species-specific antagonism of Ah receptor action by 2,2′,5,5′-tetrachlorobiphenyl and 2,2′,3,3′,4,4′-hexachlorobiphenyl. Eur J Pharmacol. 1995;293(4):463–474. doi: 10.1016/0926-6917(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 20.Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141(1–2):131–60. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 21.He GC, Tsutsumi T, Zhao B, Baston DS, Zhao J, Heath-Pagliuso S, Denison MS. Third-generation Ah receptor-responsive luciferase reporter plasmids: Amplification of dioxin-responsive elements dramatically increases CALUX bioassay sensitivity and responsiveness. Toxicol Sci. 2011;123(2):511–522. doi: 10.1093/toxsci/kfr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denison MS, Zhao B, Baston DS, Clark GC, Murata H, Han D. Recombinant cell bioassay systems for the detection and relative quantitation of halogenated dioxins and related chemicals. Talanta. 2004;63(5):1123–1133. doi: 10.1016/j.talanta.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Garrison PM, Tullis K, Aarts J, Brouwer A, Giesy JP, Denison MS. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam Appl Toxicol. 1996;30(2):194–203. doi: 10.1006/faat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 24.Baston DS, Denison MS. Considerations for potency equivalent calculations in the Ah receptor-based CALUX bioassay: Normalization of superinduction results for improved sample potency estimation. Talanta. 2011;83(5):1415–1421. doi: 10.1016/j.talanta.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balthis L, Hyland J, Cooksey C, Wirth E, Fulton M, Moore J, Hurley D. NOAA Technical Memorandum NOS NCCOS. Vol. 150. NOAA Center for Coastal Environmental Health and Biomolecular Research; Charleston, SC: 2012. Support for Integrated Ecosystem Assessments of NOAA’s National Estuarine Research Reserve System (NERRS): Assessment of Ecological Condition and Stressor Impacts in Subtidal Waters of the Sapelo Island National Estuarine Research Reserve. [Google Scholar]
- 26.Behnisch PA, Hosoe K, Sakai S. Bioanalytical screening methods for dioxins and dioxin-like compounds a review of bioassay/biomarker technology. Environ Int. 2001;27(5):413–39. doi: 10.1016/s0160-4120(01)00028-9. [DOI] [PubMed] [Google Scholar]
- 27.Han D, Nagy SR, Denison MS. Comparison of recombinant cell bioassays for the detection of Ah receptor agonists. Biofactors. 2004;20(1):11–22. doi: 10.1002/biof.5520200102. [DOI] [PubMed] [Google Scholar]
- 28.Flaveny C, Reen RK, Kusnadi A, Perdew GH. The mouse and human Ah receptor differ in recognition of LXXLL motifs. Arch Biochem Biophys. 2008;471(2):215–223. doi: 10.1016/j.abb.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidel SD, Li V, Winter GM, Rogers WJ, Martinez EI, Denison MS. Ah receptor-based chemical screening bioassays: Application and limitations for the detection of Ah receptor agonists. Toxicol Sci. 2000;55(1):107–115. doi: 10.1093/toxsci/55.1.107. [DOI] [PubMed] [Google Scholar]
- 30.Machala M, Vondracek J, Blaha L, Ciganek M, Neca J. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mut Res-Gen Toxicol Environ Mutagen. 2001;497(1–2):49–62. doi: 10.1016/s1383-5718(01)00240-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhao B, Bohonowych JE, Timme-Laragy A, Jung D, Affatato AA, Rice RH, Di Giulio RT, Denison MS. Common commercial and consumer products contain activators of the aryl hydrocarbon (dioxin) receptor. PLoS One. 2013;8(2):e56860. doi: 10.1371/journal.pone.0056860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett KL, Gardinali PR, Sericano JL, Wade TL, Safe SH. Characterization of the H4IIE rat hepatoma cell bioassay for evaluation of environmental samples containing polynuclear aromatic hydrocarbons (PAHs) Arch Environ Contam Toxicol. 1997;32(4):442–448. doi: 10.1007/s002449900211. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson JT, Aarts J, Brouwer A, Froese KL, Denison MS, Giesy JP. Comparison of Ah receptor-mediated luciferase and ethoxyresorufin-O-deethylase induction in H4IIE cells: Implications for their use as bioanalytical tools for the detection of polyhalogenated aromatic hydrocarbons. Toxicol Appl Pharmacol. 1996;137(2):316–325. doi: 10.1006/taap.1996.0086. [DOI] [PubMed] [Google Scholar]
- 34.Fries GF. A review of the significance of animal food products as potential pathways of human exposures to dioxins. J An Sci. 1995;73(6):1639–1650. doi: 10.2527/1995.7361639x. [DOI] [PubMed] [Google Scholar]
- 35.Behnisch PA, Hosoe K, Brouwer A, Sakai S. Screening of dioxin-like toxicity equivalents for various matrices with wildtype and recombinant rat hepatoma H4IIE cells. Toxicol Sci. 2002;69(1):125–130. doi: 10.1093/toxsci/69.1.125. [DOI] [PubMed] [Google Scholar]
- 36.Murk AJ, Legler J, Denison MS, Giesy JP, van de Guchte C, Brouwer A. Chemical-activated luciferase gene expression (CALUX): a novel in vitro bioassay for Ah receptor active compounds in sediments and pore water. Fundam Appl Toxicol. 1996;33(1):149–60. doi: 10.1006/faat.1996.0152. [DOI] [PubMed] [Google Scholar]
- 37.Klein GP, Hodge EM, Diamond ML, Yip A, Dann T, Stem G, Denison MS, Harper PA. Gas-phase ambient air contaminants exhibit significant dioxin-like and estrogen-like activity in vitro. Environ Health Perspect. 2006;114(5):697–703. doi: 10.1289/ehp.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao B, Baston DS, Khan E, Sorrentino C, Denison MS. Enhancing the response of CALUX and CAFLUX cell bioassays for quantitative detection of dioxin-like compounds. Science China-Chemistry. 2010;53(5):1010–1016. doi: 10.1007/s11426-010-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kärenlampi SO, Tuomi K, Korkalainen M, Raunio H. 2-(4′-chlorophenyl)benzothiazole is a potent inducer of cytochrome P450IA1 in a human and a mouse cell line. Anomalous correlation between protein and mRNA induction. Eur J Biochem. 1989;181(1):143–8. doi: 10.1111/j.1432-1033.1989.tb14705.x. [DOI] [PubMed] [Google Scholar]
- 40.Hu WY, Sorrentino C, Denison MS, Kolaja K, Fielden MR. Induction of Cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol Pharmacol. 2007;71(6):1475–1486. doi: 10.1124/mol.106.032748. [DOI] [PubMed] [Google Scholar]
- 41.Denison MS, Phelan D, Winter GM, Ziccardi MH. Carbaryl, a carbamate insecticide, is a ligand for the hepatic Ah (dioxin) receptor. Toxicol Appl Pharmacol. 1998;152(2):406–14. doi: 10.1006/taap.1998.9999. [DOI] [PubMed] [Google Scholar]
- 42.Zhao B, Baston DS, Hammock B, Denison MS. Interaction of diuron and related substituted phenylureas with the Ah receptor pathway. J Biochem Mol Toxicol. 2006;20(3):103–113. doi: 10.1002/jbt.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Head JA, Hahn ME, Kennedy SW. Key amino acids in the aryl hydrocarbon receptor predict dioxin sensitivity in avian species. Environ Sci Technol. 2008;42(19):7535–7541. doi: 10.1021/es801082a. [DOI] [PubMed] [Google Scholar]
- 44.Karchner SI, Franks DG, Kennedy SW, Hahn ME. The molecular basis for differential dioxin sensitivity in birds: Role of the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2006;103(16):6252–6257. doi: 10.1073/pnas.0509950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandini A, Soshilov AA, Song YJ, Zhao J, Bonati L, Denison MS. Detection of the TCDD binding-fingerprint within the Ah receptor ligand binding domain by structurally driven mutagenesis and functional analysis. Biochem. 2009;48(25):5972–5983. doi: 10.1021/bi900259z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soshilov AA, Denison MS. Ligand promiscuity of aryl hydrocarbon receptor agonists and antagonists revealed by site-directed mutagenesis. Mol Cell Biol. 2014;34(9):1707–1719. doi: 10.1128/MCB.01183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsson M, van den Berg M, Brenerová P, van Duursen MB, van Ede KI, Lohr C, Luecke-Johansson S, Machala M, Neser S, Pěnčíková K, Poellinger L, Schrenk D, Strapáčová S, Vondráček J, Andersson PL. Consensus toxicity factors for polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls combining in silico models and extensive in vitro screening of AhR-mediated effects in human and rodent cells. Chem Res Toxicol. 2015 doi: 10.1021/tx500434j. [DOI] [PubMed] [Google Scholar]
- 48.Sutter CH, Bodreddigari S, Sutter TR, Carlson EA, Silkworth JB. Analysis of the CYP1A1 mRNA dose-response in human keratinocytes indicates that relative potencies of dioxins, furans, and PCBs are species and congener specific. Toxicol Sci. 2010;118(2):704–15. doi: 10.1093/toxsci/kfq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.