Abstract
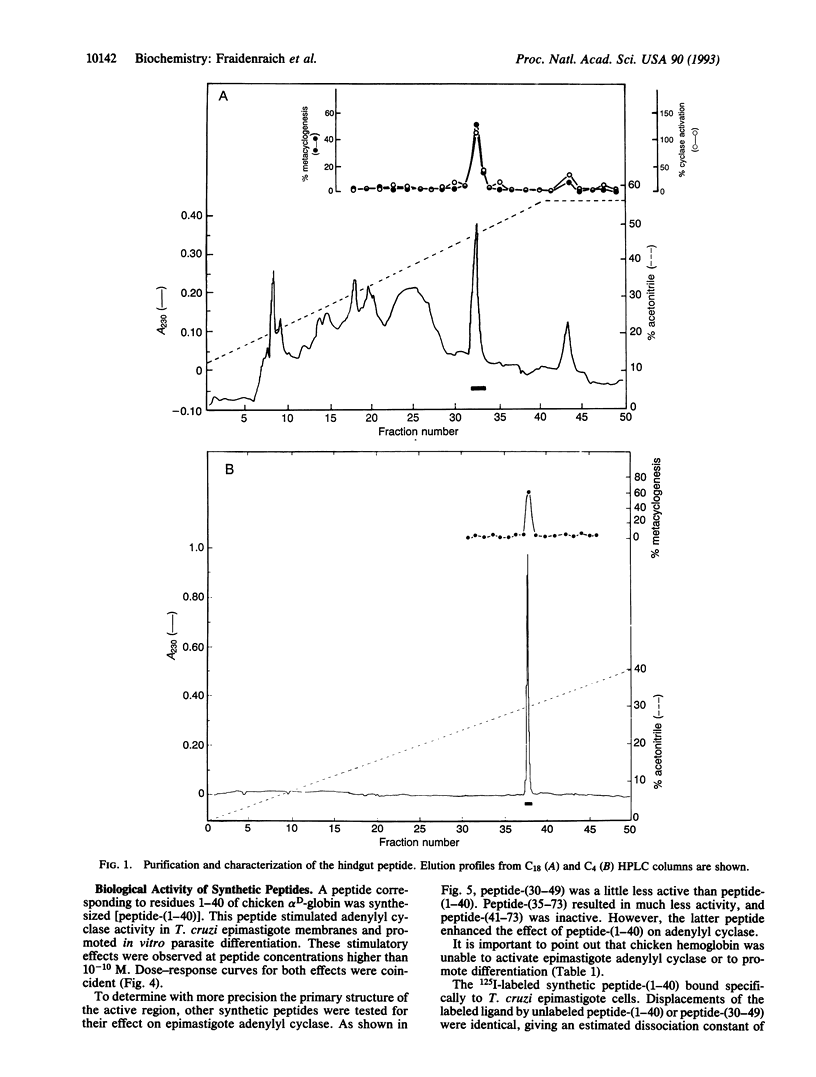
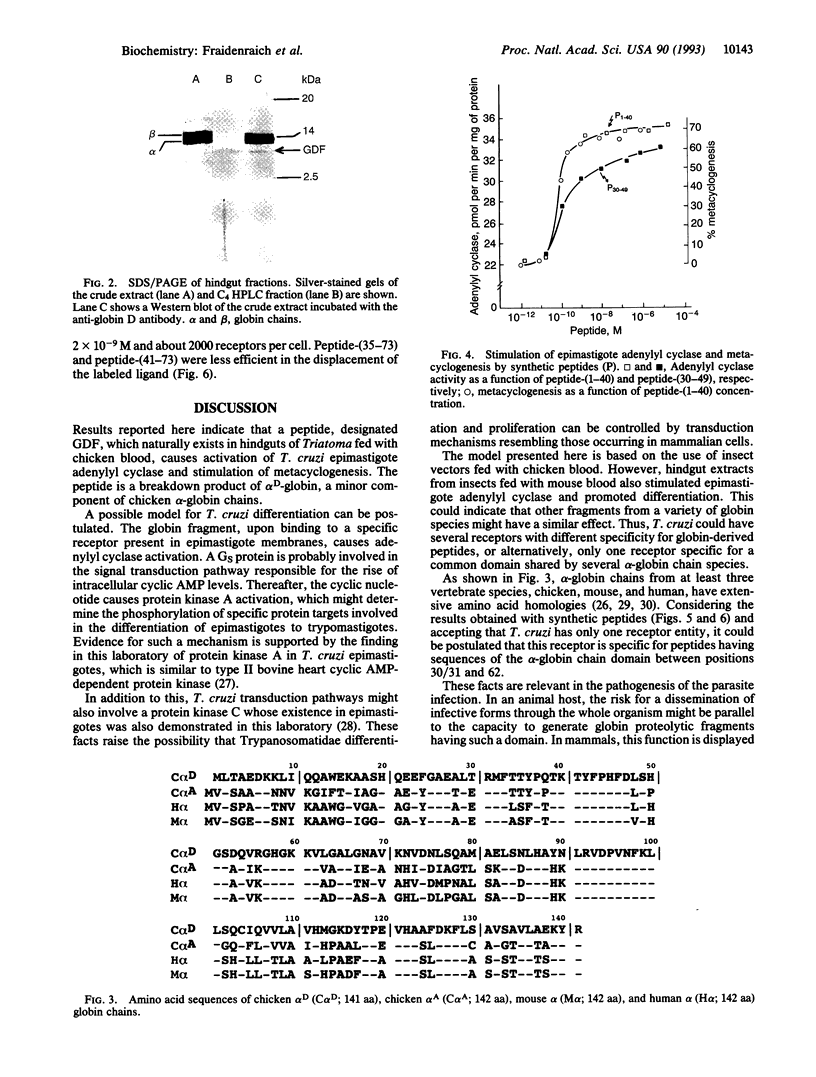
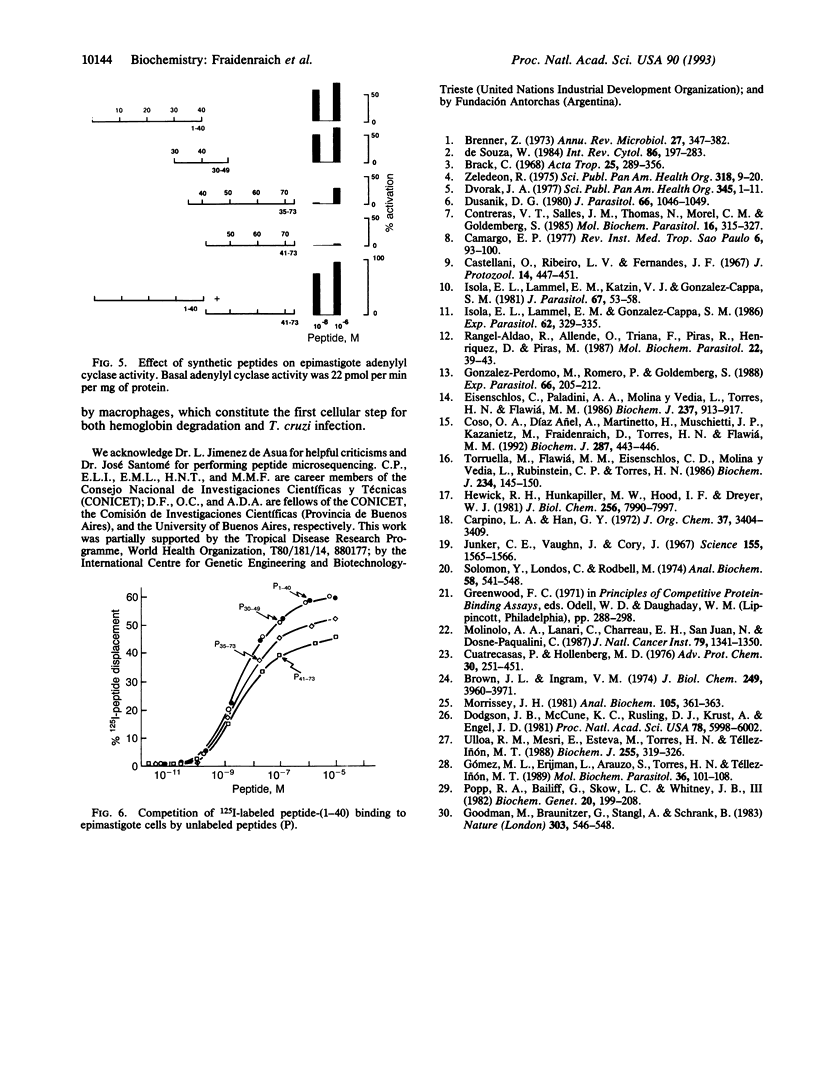
A peptide from hindguts of the Triatoma hematophagous Chagas insect vector activates adenylyl cyclase activity in Trypanosoma cruzi epimastigote membranes and stimulates the in vitro differentiation of epimastigotes to metacyclic trypomastigotes. Hindguts were obtained from insects fed 2 days earlier with chicken blood. Purification was performed by gel filtration and HPLC on C18 and C4 columns. SDS/PAGE of the purified peptide showed a single band of about 10 kDa. The following sequence was determined for the 20 amino-terminal residues of this peptide: H2N-Met-Leu-Thr-Ala-Glu-Asp-Lys-Lys-Leu-Ile-Gln- Gln-Ala-Trp-Glu-Lys-Ala-Ala-Ser-His. This sequence is identical to the amino terminus of chicken alpha D-globin. On a Western blot, the peptide immunoreacted with a polyclonal antibody against chicken globin D. A synthetic peptide corresponding to residues 1-40 of the alpha D-globin amino terminus also stimulated adenylyl cyclase activity and promoted differentiation. This 125I-labeled synthetic peptide bound specifically to T. cruzi epimastigote cells. Activation of epimastigote adenylyl cyclase by the hemoglobin-derived peptide may play an important role in T. cruzi differentiation and consequently in the transmission of Chagas disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brack C. Elektronenmikroskopische Untersuchungen zum Lebenszyklus von Trypanosoma cruzi Unter besonderer Berückischtigung der Entwicklungsformen im Ubertrager Rhodnium prolixus. Acta Trop. 1968;25(4):289–356. [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Ingram V. M. Structural studies on chick embryonic hemoglobins. J Biol Chem. 1974 Jun 25;249(12):3960–3972. [PubMed] [Google Scholar]
- Castellani O., Ribeiro L. V., Fernandes J. F. Differentiation of Trypanosoma cruzi in culture. J Protozool. 1967 Aug;14(3):447–451. doi: 10.1111/j.1550-7408.1967.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Contreras V. T., Salles J. M., Thomas N., Morel C. M., Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985 Sep;16(3):315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- Coso O. A., Díaz Añel A., Martinetto H., Muschietti J. P., Kazanietz M., Fraidenraich D., Torres H. N., Flawia M. M. Characterization of a Gi-protein from Trypanosoma cruzi epimastigote membranes. Biochem J. 1992 Oct 15;287(Pt 2):443–446. doi: 10.1042/bj2870443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., McCune K. C., Rusling D. J., Krust A., Engel J. D. Adult chicken alpha-globin genes alpha A and alpha D: no anemic shock alpha-globin exists in domestic chickens. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5998–6002. doi: 10.1073/pnas.78.10.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusanic D. G. In vitro production of metacyclic trypomastigotes of Trypanosoma cruzi. J Parasitol. 1980 Dec;66(6):1046–1049. [PubMed] [Google Scholar]
- Eisenschlos C. D., Paladini A. A., Molina y Vedia L., Torres H. N., Flawiá M. M. Evidence for the existence of an Ns-type regulatory protein in Trypanosoma cruzi membranes. Biochem J. 1986 Aug 1;237(3):913–917. doi: 10.1042/bj2370913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales-Perdomo M., Romero P., Goldenberg S. Cyclic AMP and adenylate cyclase activators stimulate Trypanosoma cruzi differentiation. Exp Parasitol. 1988 Aug;66(2):205–212. doi: 10.1016/0014-4894(88)90092-6. [DOI] [PubMed] [Google Scholar]
- Goodman M., Braunitzer G., Stangl A., Schrank B. Evidence on human origins from haemoglobins of African apes. Nature. 1983 Jun 9;303(5917):546–548. doi: 10.1038/303546a0. [DOI] [PubMed] [Google Scholar]
- Gómez M. L., Erijman L., Arauzo S., Torres H. N., Téllez-Iñn M. T. Protein kinase C in Trypanosoma cruzi epimastigote forms: partial purification and characterization. Mol Biochem Parasitol. 1989 Sep;36(2):101–108. doi: 10.1016/0166-6851(89)90182-5. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Isola E. L., Lammel E. M., González Cappa S. M. Trypanosoma cruzi: differentiation after interaction of epimastigotes and Triatoma infestans intestinal homogenate. Exp Parasitol. 1986 Dec;62(3):329–335. doi: 10.1016/0014-4894(86)90039-1. [DOI] [PubMed] [Google Scholar]
- Molinolo A. A., Lanari C., Charreau E. H., Sanjuan N., Pasqualini C. D. Mouse mammary tumors induced by medroxyprogesterone acetate: immunohistochemistry and hormonal receptors. J Natl Cancer Inst. 1987 Dec;79(6):1341–1350. [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G., Skow L. C., Whitney J. B., 3rd The primary structure of genetic variants of mouse hemoglobin. Biochem Genet. 1982 Feb;20(1-2):199–208. doi: 10.1007/BF00484946. [DOI] [PubMed] [Google Scholar]
- Rangel-Aldao R., Allende O., Triana F., Piras R., Henriquez D., Piras M. Possible role of cAMP in the differentiation of Trypanosoma cruzi. Mol Biochem Parasitol. 1987 Jan 2;22(1):39–43. doi: 10.1016/0166-6851(87)90067-3. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Torruella M., Flawiá M. M., Eisenschlos C., Molina y Vedia L., Rubinstein C. P., Torres H. N. Trypanosoma cruzi adenylate cyclase activity. Purification and characterization. Biochem J. 1986 Feb 15;234(1):145–150. doi: 10.1042/bj2340145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa R. M., Mesri E., Esteva M., Torres H. N., Téllez-Iñn M. T. Cyclic AMP-dependent protein kinase activity in Trypanosoma cruzi. Biochem J. 1988 Oct 1;255(1):319–326. [PMC free article] [PubMed] [Google Scholar]
- Yunker C. E., Vaughn J. L., Cory J. Adaptation of an insect cell line (Grace's Antheraea cells) to medium free of insect hemolymph. Science. 1967 Mar 24;155(3769):1565–1566. doi: 10.1126/science.155.3769.1565. [DOI] [PubMed] [Google Scholar]
- de Isola E. L., Lammel E. M., Katzin V. J., Gonzalez Cappa S. M. Influence of organ extracts of Triatoma infestans on differentiation of Trypanosoma cruzi. J Parasitol. 1981 Feb;67(1):53–58. [PubMed] [Google Scholar]
- de Souza W. Cell biology of Trypanosoma cruzi. Int Rev Cytol. 1984;86:197–283. doi: 10.1016/s0074-7696(08)60180-1. [DOI] [PubMed] [Google Scholar]