Abstract
Inflammation is an important contributor to the development and progression of human cancers. Inflammatory lipid metabolites, prostaglandins, formed from arachidonic acid by prostaglandin H synthases commonly called cyclooxygenases (COXs) bind to specific receptors that activate signaling pathways driving the development and progression of tumors. Inhibitors of prostaglandin formation, COX inhibitors, or nonsteroidal anti-inflammatory drugs (NSAIDs) are well documented as agents that inhibit tumor growth and with long-term use prevent tumor development. NSAIDs also alter gene expression independent of COX inhibition and these changes in gene expression also appear to contribute to the anti-tumorigenic activity of these drugs. Many NSAIDs, as illustrated by sulindac sulfide, alter gene expressions by altering the expression or phosphorylation status of the transcription factors specificity protein 1 and early growth response-1 with the balance between these two events resulting in increases or decreases in specific target genes. In this review, we have summarized and discussed the various genes altered by this mechanism after NSAID treatment and how these changes in expression relate to the anti-tumorigenic activity. A major focus of the review is on NSAID-activated gene (NAG-1) or growth differentiation factor 15. This unique member of the TGF-β superfamily is highly induced by NSAIDs and numerous drugs and chemicals with anti-tumorigenic activities. Investigations with a transgenic mouse expressing the human NAG-1 suggest it acts to suppress tumor development in several mouse models of cancer. The biochemistry and biology of NAG-1 were discussed as potential contributor to cancer prevention by COX inhibitors.
Keywords: COX inhibitors, NSAIDs, NAG-1, GDF15, Prostaglandins, TGF-β, EGR-1/Sp1
1 Prostaglandins and cancer
Chronic inflammation is clearly associated with an increase in the risk of cancer [1]. One of the strongest associations between chronic inflammation and cancer is the increased risk in individuals with inflammatory bowel diseases. Inflammation also appears to have an important role in the development of other cancers, for example prostate, bladder, and pancreatic cancers. Chronic inflammation causes the upregulation of a number of inflammatory cytokines including IL-1β, IFNγ, and TNFα. The NFκB pathway is upregulated in many chronic inflammatory states, and evidence directly links the NFκB pathway to increased tumor formation and inflammation in experimental mouse models of intestinal cancer [2, 3]. Because NFκB plays a role in cyclooxygenase-2 (COX-2) regulation at the transcriptional level, prostaglandin H synthase or COX-2 expression is increased, and higher levels of inflammatory lipids prostaglandins are formed. Thus, inflammation and enhanced metabolism of arachidonic acid by COXs are linked to higher cancer risk. In many tumors, higher prostaglandin levels are observed. This appears to be due not only to higher COX activity but also to the diminished expression of 15-prostaglandin dehydrogenase (15-PGDH), a prostaglandin degradation enzyme. In many tumors, the expression of 15-PGDH is reduced which provides an additional mechanism by which cancer cells increase the levels of prostaglandins. 15-PGDH expression is silenced epigenetically in tumors by increased methylation of promoter regions in this gene [4], while other reports suggest 15-PGDH may act as a tumor suppressor [5, 6]. The aberrant arachidonic acid metabolism observed in cancer cells causes a high concentration of prostaglandins, in particular prostaglandin E2 (PGE2).
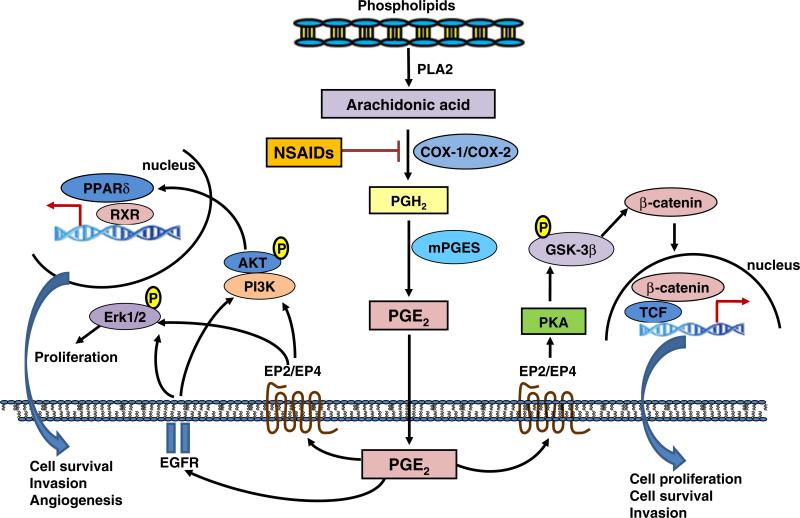
Prostaglandins exert their biological activity by binding a family of receptors, designated EP for PGE2 receptors, FP for PGF2α receptors, TP for thromboxane receptor, FP for PGF2α receptors, and IP for PGI2 receptors [7]. Reports in the literature have suggested roles for each of these families of receptors as positive or negative regulators of tumor growth. Because of the high concentrations of PGE2 in tumors, many investigations have focused on the EP receptors, which have four different receptors, designated as EP1, EP2, EP3, and EP4 [7]. The EP receptor signaling pathways control cell proliferation, invasion, apoptosis, and angiogenesis. Deletion of the EP2 receptor in APC/Min mice substantially reduced polyp formation [8], while deletion of the EP4 receptor has been shown to decrease the formation of aberrant crypt foci in animals treated with the colon carcinogen azoxymethane [9]. EP2 expression is upregulated compared with normal tissues in colorectal [8] and breast [10] cancers. Fiebich et al. found that both EP2 and EP4 mRNA expressions are upregulated in human glioblastoma–astrocytoma U373 MG cells compared to the primary astrocytes [11]. The EP2/4 receptors are G protein-coupled receptors. As illustrated in Fig. 1, PGE2 can activate the protein kinase A (PKA) signaling pathway mediating many of pro-tumorigenic activities [12]. The PKA pathway phosphorylates GSK-3, thereby altering the APC/β-catenin/TCF pathway, which regulates cell proliferation, angiogenesis, and apoptosis [12]. PGE2 also can transactivate the EGF receptor [13], increase amphiregulin [14], enhance the RAS-MAP kinase pathway [15], and transactivate the peroxisome proliferator-activated receptor (PPAR)δ receptor pathway [16] (Fig. 1). Because biological activity is mediated by the receptors changing the expression levels of, for example, the EP2 and EP4 receptors, this activity would have a profound effect on tumor growth.
Fig. 1.
NSAIDs inhibit PGE2-induced tumorigenesis through targeting multiple downstream signaling pathways of PGE2. PGE2 exerts its biological activity by binding to EP receptors EP1, EP2, EP3, and EP4. The EP receptor signaling pathways control cell proliferation, invasion, apoptosis, and angiogenesis. The EP2/4 receptors are G protein-coupled receptors. PGE2 can activate the PKA signaling pathway mediating many of pro-tumorigenic activities. The PKA pathway phosphorylates GSK-3, thereby altering the APC/β-catenin/TCF pathway, which regulates cell proliferation, angiogenesis, and apoptosis. PGE2 can also transactivate the EGF receptor and activate MAP kinase ERK1/2 pathway that induce proliferation and/or PI3K-ATK signaling pathway and thus transactivate the PPARδ cascade and induce transcriptional regulation of genes promoting cell survival, invasion and angiogenesis
COX inhibitors are well-established chemopreventative drugs. Numerous epidemiological, clinical, laboratory, and animal and cell culture studies confirm that the use of COX inhibitors or nonsteroidal anti-inflammatory drugs (NSAIDs) is effective at inhibiting the incidence and mortality of colorectal cancer [17, 18]. In addition to colorectal cancer, NSAIDs have also been associated with a reduced risk of other cancers, for example breast, esophageal, stomach, bladder, ovary, and lung cancers [19–21]. Despite the extensive studies on the effectiveness of using NSAIDs as chemopreventative agents, the molecular mechanisms underlying the chemopreventative effects of NSAIDs are not well understood. The anti-inflammatory properties of COX inhibitors are clearly dependent on the reduction in the levels of prostaglandins. The cancer-preventative activity of NSAIDs has generally been attributed to the inhibition of COX-1/COX-2 activity and prostaglandin production. However, this concept is challenged by the fact that very high doses of COX inhibitors are frequently required to exhibit tumor inhibitory effects but only low doses are required for an inhibition of prostaglandin formation [22]. Therefore, COX-independent mechanisms may be involved, and these COX-independent effects may contribute to the chemopreventative activity of NSAIDs [22]. NSAIDs inhibit the growth of colon cancer cell lines that do not express COX-1 or COX-2 [23] and inhibit the growth of mouse embryo fibroblasts null for both COX-1 and COX-2 genes [24]. Chiu et al. reported that the suppression of polyp growth by sulindac in the APC/Min mouse is independent of prostaglandin biosynthesis [25]. Studies from this laboratory and other investigators suggest that NSAID primarily induce apoptosis independent of COX activity [26].
2 15-Lipoxygenase and cancer
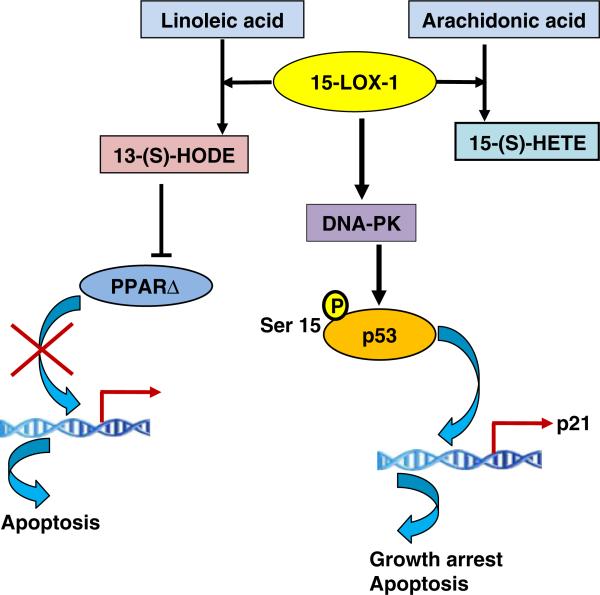
NSAIDs are very effective inhibitors of COX activity, and the inhibition of COX activity makes the substrate arachidonic acid available for metabolism by other enzymes and may cause a shift in the arachidonic acid metabolite profile from prostaglandins to lipoxygenase (LOX)-derived hydroxylated lipids. 5-LOX, 12-LOX, 15-LOX-1, and 15-LOX-2 are reported to have some influence on tumor development. This laboratory has investigated 15-LOX-1 as related to colorectal cancer. 15-LOX-1 is present in human colorectal cancer cells [27] and converts arachidonic acid to 15-hydroxyeicosatetraenoic acid (15-HETE) and linoleic acid to 13-hydroxyoctadecadienoic acid (13-HODE). 15-LOX-1 in human colorectal cells has an anti-tumorigenic activity [28]. The investigations of Shureiqi and his colleagues suggest that 15-LOX-1 plays an anti-tumorigenic role in human colorectal cancer [29]. The expression of 15-LOX-1 is lower in human colorectal tumors as compared to normal tissue, and as a result, the levels of the major 15-LOX-1 metabolite, 13-HODE, are lower in colorectal tumors [30]. 13-HODE is reported to downregulate PPARδ in human colorectal cancer cells [31] (Fig. 2). The overexpression of 15-LOX-1 in colorectal cells stimulates the phosphorylation of the tumor suppressor gene p53 at Ser15, which results in increased expression of many downstream target genes [32]. The growth inhibitory effects of 15-LOX-1 were p53 dependent. However, the 15-LOX-1 metabolites failed to induce phosphorylation of p53, and a 15-LOX-1 inhibitor did not inhibit the phosphorylation of p53 [32]. Because the active enzyme 15-LOX-1, but not its metabolites, activates p53, a possible explanation is the direct interaction of 15-LOX-1 protein with the DNA-PK kinase responsible for the increase in phosphorylation of p53 [33]. A mutant 15-LOX-1 devoid of enzymatic activity was created by replacing a key histidine with a leucine. Both the mutant and the wild-type protein increased p53 activation, suggesting that activation was independent of enzymatic activity [34]. 15-LOX-1 and DNA-PK co-immunoprecipitated and the kinase activity of the DNA-PK immunoprecipitates was three to four times higher in the presence of 15-LOX-1, supporting the hypothesis that 15-LOX-1 directly increases its kinase activity [34]. As illustrated in Fig. 2, this investigation elucidated a novel mechanism for the activation of p53 and provided a rational explanation for the tumor suppressor activity of 15- LOX-1. Thus, lipoxygenases, as illustrated by 15-LOX-1, appear also to have a role in the reduction of tumors by COX inhibitors.
Fig. 2.
Mechanisms of anti-tumorigenic activity of 15-LOX-1. 15-LOX-1 converts arachidonic acid to 15-HETE and linoleic acid to 13-HODE. 13-HODE can downregulate PPARΔ in human colorectal cancer cells and induce apoptosis through inhibiting transcription. 15-LOX-1 also directly binds to DNA-PK kinase and thus stimulates the phosphorylation of the tumor suppressor gene p53 at Ser15, which results in increased expression of many downstream target genes that are involved in growth arrest and apoptosis
3 COX inhibitors alter protein expression involved in tumorigenesis/inflammation
Considerable research is currently directed towards the development of molecular target-based interventions, and a number of anti-tumorigenic and anti-inflammatory compounds have been developed and tested for their effects. In this regard, NSAIDs, phytochemicals, and PPARγ ligands have a potent preventive and therapeutic value with regards to cancer, obesity, and inflammatory diseases. Many drugs and chemicals are reported to alter gene expression. The analysis of gene expression changes observed after treating cells with chemical agents reveals a large number of genes related to growth control, and the development or suppression of tumorigenesis is either increased or suppressed. We hypothesized that COX inhibitors would change gene expression and these changes in expression could contribute to the ability of these drugs to prevent cancer development. Subtractive hybridization and micro array analysis identified a large number of genes whose expression was increased or decreased by COX inhibitors [23]. We have primarily focused on one gene, nonsteroidal anti-inflammatory drug-activated gene (NAG-1), for further investigation but have also examined other proteins associated with tumor formation. NSAIDs modulate many regulatory proteins including transcription factors, enzymes, cytokines, and growth factors. In the last decade, considerable research was published on transcription regulation by anticancer compounds, which revealed that alterations of transcription machinery affect many genes in the anti-tumorigenesis pathways. Some of the molecular targets of NSAIDs in the transcription machinery are presented below under different headings. In this section, we will focus on discussing NSAID targets that are altered independent of COX inhibition.
3.1 EGR-1
Early growth response gene-1 is an inducible zinc finger transcription factor and an immediate early gene induced by stress or injury, mitogens, growth factors, cytokines, hypoxia, and differentiation factors [35]. The expression of early growth response-1 (EGR-1) in cancer can have a dual effect; it can cause either promotion or inhibition of cell growth, which depends on cell type and environment. However, EGR-1 has been shown to act as a tumor suppressor gene in colorectal cancer, and its loss can lead to progression of cancer in colorectal tumorigenesis [36]. It exhibits its pro-apoptotic function by directly binding and/or controlling to p53 [37], NAG-1[38], and PTEN promoters [39]. We have previously shown that some NSAIDs can increase EGR-1 expression, followed by enhancing tumor suppressor protein NAG-1 expression in human colorectal cancer cells [38]. Although the molecular mechanism of how NSAIDs increase EGR-1 expression remains to be elucidated, EGR-1 induction by NSAIDs likely affects many genes involved in pro-apoptosis pathways. EGR-1 induction may play a role in not only NSAID-induced apoptosis, but also in other anticancer compounds such as green tea [40], resveratrol [41], and PI3K inhibitor [42].
3.2 Specificity protein
Specificity proteins are another zinc-finger transcription factor that binds to the GC box in the promoter of many genes. There are four subtypes: specificity protein (Sp)1, Sp2, Sp3, and Sp4. Among those, Sp1 controls housekeeping genes, but recent data suggest that Sp1 is also involved in the regulation of tumorigenesis [43]. For many human cancers, Sp protein overexpression is a negative prognostic factor for survival and, not surprisingly, these transcription factors contribute to the proliferative and metastatic tumor phenotype. Recent reports from Dr. Safe's group suggest that decreased Sp1 protein phosphorylation and decreased pSp1/Sp1 ratios play a role in celecoxib-induced downregulation of VEGF [44]. These observations suggest that the anti-angiogenic activity of celecoxib in pancreatic cancer cells is linked to targeted dephosphorylation of Sp1. In colon cancer, the growth inhibitory effects of NSAIDs are accompanied by downregulation of activity in Sp1 and Sp4 but not Sp3 proteins [45]. It was also shown that the decreased Sp1/Sp4 by COX-2 inhibitors in colon cancer cells was COX-2 independent and due to activation of proteosomes that specifically target degradation of Sp1 and Sp4 [45].
3.3 ESE-1
Epithelial-specific ETS-1 (ESE-1) protein belongs to the ETS family of transcription factors and is also identified as ERT [46], ELF3 [47], and ESX [48]. ESE-1 proteins are constitutively expressed in many types of epithelia, including lung and intestine [49], and regulate terminal differentiation of the epidermis [49, 50]. ESE-1 have multiple functions in the transcriptional regulation of genes involved in epithelial differentiation and development of cancer [51], depending on cell context, and exact molecular mechanisms and their transcriptional targets need to be defined in cancer cells. We have shown that knockdown of ESE-1 by RNA interference inhibited NSAID-induced cell death (caspase 3/7 enzyme activity and PARP cleavage), which is associated with decreased expression of EGR-1 [52]. Furthermore, nuclear ESE-1 translocation by NSAIDs and binding of ESE-1 in the EGR-1 promoter nicely corresponded to NSAID-induced activation of EGR-1 gene expression at the transcriptional level (Fig. 3). These data suggest expression of a sequence pathway ESE/EGR-1/NAG-1 by NSAID in colorectal cancer cells.
Fig. 3.
Transcriptional regulation of NAG-1 gene by NSAIDs. NSAIDs such as sulidac sulfide activate NAG-1 transcription through regulating its transcriptional factor EGR-1. NSAIDs first induce the translocation of ESE-1 into nuclear which then bind to ESE-1 binding site on the EGR-1 promoter and thus induce EGR-1 production. EGR-1 then binds to EGR-1 binding site in the NAG-1 promoter which overlaps with a SP1 binding site. Both SP-1 and EGR-1 regulate NAG-1 expression transcriptionally. The transcriptional activity of NAG-1 depends on the balance of EGR-1 and Sp1 transcription factors
3.4 NAG-1 expression
Our investigation on the regulation of NAG-1 expression revealed that both transcriptional and post-transcription mechanisms are operative. Some drugs and chemicals increase expression by increasing the stability of NAG-1 mRNA while many chemopreventive drugs and chemicals act via several transcription factors increasing RNA expression and hence NAG-1 protein. Transcriptional regulation appears complex, but it is clear that NAG-1 is an important downstream target of three tumor suppressor pathways, p53, EGR-1, and GSK-3β, suggesting NAG-1 maybe a key mediator for these tumor suppression genes [53, 54]. The COX inhibitor sulindac sulfide induces NAG-1 expression at the transcriptional level via EGR-1 transcription factors [38, 55]. The expression of EGR-1 appears to be mediated by increased expression of ESE-1 [52]. Interestingly, the EGR-1 binding site in the NAG-1 promoter overlaps with a Sp1 site. The basal expression of NAG-1 is regulated by the Sp1 sites in the promoter. As shown in Fig. 3, the transcriptional activity of NAG-1 depends on the balance of EGR-1 and Sp1 transcription factors. The expression of Sp1 is not altered by sulindac sulfide, whereas EGR-1 expression is increased [38].
3.5 EP4 expression
Because the biological effects of prostaglandin PGE2 are mediated by a family of receptors, one would expect changes in the expression of the receptors would have a profound effect on inflammatory response and cancer development. Considerable evidence with knockout mice confirmed this hypothesis. Initial examination of the promoter regions of human EP2 and EP4 receptors suggested the EP4 receptor has EGR-1/Sp1 sites, and indeed incubation of glioblastoma cells expressing these receptors with some COX inhibitors and troglitazone reduced the expression of EP4 but not EP2 [56]. TGZ and sulindac sulfide first increased the expression of the EP4 receptor then suppressed the EP4 expression [56]. The human EP4 promoter region contains two Sp1 sites [57] overlapping with an EGR-1 site [56], and mutations of these sites in luciferase promoter studies confirmed that Sp1/EGR-1 sites are important in the regulation of EP4 expression and the response to sulindac sulfide treatment. In addition, the ChIP assay and expression studies with EGR-1 and Sp1 proteins confirmed that the EGR-1 sites are involved in the increased expression of EP4, while Sp1 sites are important for sulindac sulfide suppression of EP4 expression [56, 58]. Phosphorylated threonine residues in Sp1 activated by sulindac sulfide-induced Erk were detected. The ChIP assay experiment revealed that sulindac sulfide decreases DNA-binding activity of responsible Sp1 binding sites in the human EP4 promoter [56, 58]. Taken together, these data suggest that phosphorylation of Sp1 is critical and results in a decrease in Sp1 DNA binding, and hence suppression of transcription activation of target genes like EP4 is observed. Thus, on longer treatment with sulindac sulfide, activation of Erk kinase is observed resulting in phosphorylation of threonine resides in Sp1. Phosphorylation of Sp1 critically alters the binding to DNA, and a decrease in Sp1 DNA binding can occur. Furthermore, the phosphorylated Sp1 can effectively block the binding of either EGR-1 or Sp1 to the Egr-1/Sp1 binding sites on the NAG-1 promoter, and hence suppression of transcription activation of target genes is observed [56, 58]. Figure 4 summarizes the transcriptional regulation of the EP4 receptor by two mechanisms and shows that COX inhibitors, as illustrated by sulindac sulfide, can either increase (a) or decrease (b) gene expression by altering the balance and phosphorylation status of the EGR-1 and Sp1 transcription factors. The suppression of EP4 expression by sulindac sulfide represents a new and unique mechanism for the inhibition of tumor growth by COX inhibitors.
Fig. 4.
Dual regulation of EP4 receptor transcription by NSAIDs. The promoter region of EP4 receptor contains both EGR-1 and Sp-1 binding sites. EGR-1 sites are involved in the increased expression of EP4, while Sp-1 sites are important for sulindac sulfide suppression of EP4 expression. NSAIDs such as sulidac sulfide first increases the expression of EP4 receptor by activating EGR-1 transcription which then bind to EGR-1 binding site on EP4 receptor promoter and thus cause EP4 upregulation (a). However, prolonged incubation with sulindac sulfide then suppresses EP4 expression through the activation of MEK1/2-ERR1/2 pathway which results in phosphorylation of threonine resides in Sp-1. Phosphorylation of Sp-1 decreases in Sp-1 binding to its binding site on EP4 receptor promoter. Furthermore, the phosphorylated Sp-1 can effectively block the binding of EGR-1 to its binding site on the EP4 receptor promoter, and hence suppression of transcription activation of EP4 receptor (b)
3.6 ATF3
ATF3, a member of the ATF/cAMP response element binding family of bZIP transcription factors, is characterized as a stress inducible and/or adaptive response gene [59]. Much controversy exists as to the physiological role ATF3 has in tumorigenesis, and ATF3 is demonstrated to be a positive or negative effector in tumor progression. Recently, a dichotomous role was reported for ATF3 in cancer development; the authors concluded its role as a tumor suppressor or oncogene is largely dependent on cellular context and extent of malignancy [60]. However, several lines of evidence suggest ATF3 may function as a tumor suppressor. First, ATF3 expression is markedly reduced in cancer tissues when compared to normal adjacent tissue [61]. Secondly, ATF3 overexpression is demonstrated to elicit a number of cellular responses, including induction of cell cycle arrest and inhibition of proliferation [62], induction of apoptosis in vitro and in vivo [42, 59, 63], inhibition of invasion [64–66], and retardation of tumor formation in vivo [59, 66]. ATF3 is reported to mediate or enhance induction of apoptosis by NSAIDs [66], and recent data suggest that ATF2 controls NSAID-induced ATF3 expression in human colorectal cancer cells. This pathway is mediated through the phosphorylation of ATF2, which is mediated by p38 mitogen-activated protein kinase (MAPK)-, JNK-, and extracellular signal-regulated kinase (ERK)-dependent pathways [67].
3.7 β-catenin signaling
A molecular linkage between NSAIDs and the β-catenin pathway has been suggested since the first evidence was reported by McEntee et al. [68]. Since most colorectal cancer exhibits β-catenin activity, inhibition of this pathway may prove beneficial in cancer prevention and/or suppression. In this regard, several NSAIDs were shown to inhibit β-catenin translocation in colorectal cancer cells [69, 70]. Furthermore, recent data using ibuprofen suggest that ibuprofen inhibits β-catenin activity as well as NF-κB activity, providing a novel mechanism of NSAID in the β-catenin pathway in sporadic colorectal cancer [71]. Since those NSAIDs inhibit the β-catenin signaling pathway and several genes are known to be involved in the β-catenin pathways, it is necessary to know the detailed mechanisms of how NSAIDs inhibit the β-catenin signaling, and thereby enhance anti-tumorigenesis. In addition, NSAIDs have been known to affect many other targets, depending on cell types and content. Figure 5 summarizes the effects of NSAIDs on a number of proteins or signaling pathways independent of the inhibition of COX activity.
Fig. 5.
Potential pathways affected by NSAIDs in a COX-independent manner. COX inhibitors stimulate the expression of Egr-1 via ESE-1 and thus increase the expression of NAG-1. Also, COX inhibitors can alter Sp-1 by either decreasing Sp-1 expression or changing the phosphorylation status which alters the binding of this transcription factor to promoter region-binding sites. Likewise, NSAIDs can alter the expression of ATF3 that appears to act as a tumor suppressor. Also shown in this figure are proteins/pathways increased or inhibited by individual COX inhibitors. Thus, COX inhibitors appear to have many effects on cells and in animal models in addition to their well-documented inhibition of prostaglandin formation
4 NAG-1/GDF15
We identified NAG-1 as a divergent member of the TGF-β superfamily by PCR-based subtractive hybridization from an NSAID-induced library in COX-negative cells [23]. NAG-1 has also been identified by other groups using a variety of different cloning strategies, for example, macrophage inhibitory cytokine-1 [72], placental transformation growth factor-β [54], prostate-derived factor [73], growth differentiation factor 15 (GDF15) [74], and placental bone morphogenetic protein [75]. Experimental evidence suggests that NAG-1 may share at least some of the common functions of TGF-β superfamily cytokines. For instance, NAG-1 expression reduces TNF-α secretion in macrophages [72], and ectopic expression of NAG-1 causes cell growth arrest as assessed by soft agar and the cloning efficiency assay [23, 53]. Further, overexpression of NAG-1 in colon, glioblastoma, and prostate cancer cells inhibits tumor formation in the nude mouse model [23, 76]. Similarly, TGF-β1 induces apoptosis and cell growth arrest in epithelial cells, and TGF-β1 knockout mice die of widespread inflammation. Overall, NAG-1 will be a novel molecular target protein to examine anti-inflammatory and/or anticancer activity; in fact, NAG-1 is a protein that is highly induced by several chemopreventive compounds such as NSAIDs, phytochemicals, and PPARγ ligands.
4.1 Biochemistry
NAG-1 is a divergent member of the TGF-β superfamily with a peptide sequence that is most similar to the bone morphogenic protein genes. The human NAG-1 locus has been mapped by fluorescence in situ hybridization to 19p12.1-13.1 [77]. The NAG-1 protein is encoded by two exons, the 309 bp exon I contains a 71 bp 5′ untranslated region (UTR) and a 238 bp coding region, and 647 bp exon II contains a 3′ UTR. The gene contains a single 1,820-bp intron [77]. The NAG-1 pro-domain consists of 167 amino acids and contains an N-linked glycosylation site at amino acid position 70 [78]. Proteolytic cleavage of the protein at the amino acid target sequence RRAR results in the release of a 112 amino acid C-terminal mature region. This mature region is highly glycolysated and shares very little of its identity with other TGF-β superfamily proteins. The mature NAG-1 has seven cysteine residues with six cysteines likely forming a cysteine knot, a key structural characteristic of the members of the TGF-β superfamily. The seventh cysteine forms a disulfide linkage to a second molecule of NAG-1 forming a homodimer that is secreted. The secreted dimer is present in the blood of humans and secreted into the media of cultured cells expressing NAG-1. There is some evidence for the presence of the pro-form of NAG-1 as well as the pro-peptide in the media of cultured cells (Fig. 6). NAG-1 appears to be a divergent member of the bone morphogenetic protein subfamily and is most like GDF-8 or myostatin based on molecular modeling. TGF-β members bind to form a complex between type I and type II receptors. Seven type I and five type II receptors have been identified for the TGF-β superfamily, but the specific receptor for NAG-1 has not been identified. Recently, it has been reported that the pro-domain of NAG-1 selectively binds to an extracellular matrix [79]. Therefore, both the mature domain and pro-domain of NAG-1 are likely to play central roles in modulating the biological activity of NAG-1. Although the impact that this effect has on patient outcome and/or function of NAG-1 is unknown, these observations are strong evidence that NAG-1 represents a multifunctional cytokine.
Fig. 6.
Different forms of NAG-1 in cell before secretion and after secretion. The full length of NAG-1 is composed of the pro-peptide and the mature form of NAG-1. After cleavage at the consensus RXXR site, the mature form of NAG-1 is secreted out of the cell as a homodimer. New evidence suggests that the pro-form or the pro-peptide of NAG-1 is also secreted out of cell. However, it is not clear whether the pro-peptide is still bound to the mature form of NAG-1 after secretion
4.2 NAG-1 and cancer
The role of NAG-1 is highly controversial because both the inhibition of cancer development and the promotion of cancer progression have been reported. NAG-1 appears to modulate or regulate both negatively and positively, cell proliferation, apoptosis, differentiation, invasion, and metastasis and appears to be dependent on the tumor type, the stage of the tumor, and likely other unidentified factors. Based upon our investigations with the NAG-1 over-expressing transgenic mouse and other in vitro studies with mouse xenographs or growth of cancer cells on soft agar, NAG-1 appears to act anti-tumorigenic in the early stages of cancer. With retroviral or conventional expression systems, the overexpression of the full-length coding region of NAG-1 in colorectal, breast, prostate, and glioblastoma cancer cells inhibits tumor growth in mouse xenografts. The growth of these cells on soft agar is also suppressed, which supports the hypothesis that NAG-1 has an anti-tumorigenic activity. The most compelling data come from studies with a transgenic mouse expressing human NAG-1 ubiquitously. To investigate if NAG-1 alters intestinal cancer, NAG-1 Tg mice were treated with azoxymethane, a known intestinal carcinogen. The NAG-1 mice showed smaller numbers of foci in their intestinal tract compared to the wild-type mice, suggesting that NAG-1expression inhibits tumor growth [80]. Mice carrying the ApcMin mutation on a C57BL/6 background spontaneously develop intestinal polyps. This mouse has been frequently used to investigate intestinal cancer and has been a model to determine if drugs can inhibit colorectal tumor growth. The NAG-1 Tg mice were crossed with ApcMin mice. ApcMin/+/NAG± showed a ~50% reduction in the number of small intestine polyp and 40% reduction in tumor load [80]. Thus, expression of human NAG-1 inhibits the development of chemically and genetically induced neoplasia in the intestinal tract suggesting it functions as a tumor suppressor gene in vivo. Recently, we have used the NAG-1 Tg mouse to investigate if NAG-1 expression can alter the development of pulmonary tumors. NAG-1 transgenic mice on an FVB background (NAG-1Tg+/FVB) was first developed for use in this study. After treatment with urethane, the NAG-1Tg+/FVB mice had decreased number and reduced size of urethane-induced tumors compared to control littermates [81]. Urethane-induced pulmonary adenomas and adenocarcinomas were observed in control mice, but only adenomas were observed in NAG-1Tg+/FVB mice suggesting also inhibition of tumor progression. NAG-1 protein inhibits urethane-induced tumor formation, probably mediated by the p38 MAPK pathway [81]. Based on these investigations, one can conclude that NAG-1 has an anti-tumorigenic activity at the early stage of tumor growth.
As cancer progresses, NAG-1 appears to act to promote tumor growth, progression, migration invasion, and metastasis. Several studies have observed major upregulation of NAG-1 mRNA and protein in cancer biopsies [82, 83], whereas a number of studies have described an anti-tumorigenic function for NAG-1, by which it induces apoptosis and may negatively affect tumor growth [23, 80, 84–87]. The overexpression of NAG-1 or the addition of recombinant NAG-1 (mature form) to cells appears to activate the ERK-1/2 pathway, resulting in increased expression of plasminogen activator in gastric cells, the activation of the EGF receptor in gastric and breast cells, and an increase in the invasion of these cells [88]. The overexpression of NAG-1 in prostate cancer PC-3 cells has been shown to increase migration and invasion of these cells [83]. The authors also found that NAG-1 expression increases metastases to distant organs in PC-3 orthotopic prostate model with the nude mouse [83]. Chen et al. found that NAG-1 promotes cell proliferation of LNCaP cells through Erk activation [89]. In contrast, NAG-1 reduces colorectal cancer as investigated in NAG-Tg mice and NAG-1 KO mice [80, 90, 91]. NAG-1 seems to work as a pro-tumorigenic protein in prostate cancer cells, whereas NAG-1 works as an anti-tumorigenic protein in colorectal cancer cells [83, 92]. In fact, there are other examples of dual biological functions of proteins in the prostate versus colon. EGR-1, for example, has been shown to be associated with pro-tumorigenic activity in prostate cancer [93], whereas EGR-1 acts like a tumor suppressor protein in other cancers [94]. 15-LOX1 is another example [30, 95]. Some possible explanations of the contradictory activity in vitro include the different functions of NAG-1 in the different cancer types and the contribution of a NAG-1 binding protein or receptor in different cells. In addition, NAG-1 is biosynthesized as a pro-protein and cleaved to a mature dimer that is secreted. Thus, the full-length, the pro-peptide, and the mature form exit within the cells and there is some evidence for the secretion of the pro-peptide in addition to the secretion of the mature form [96]. For example, Kim et al. reported that NAG-1 treatment increases ERK1/2 pathways, thereby enhancing pro-tumorigenic activity of NAG-1 in gastric cancer cells [88]. In this report, the authors used a recombinant NAG-1 purified from the cell lysates, which ectopically expressed only the mature region of NAG-1 [88]. In contrast, studies with urethane-induced pulmonary tumorigenesis in NAG-1 transgenic mice in which the full-length of NAG-1 is expressed showed ERK1/2 expression was not altered but did show p38 MAPK inhibition [90]. In this study, urethane-induced pulmonary tumorigenesis was reduced in NAG-1 transgenic mice [90]. These findings may illustrate the importance of understanding how NAG-1 is processed from cytoplasm into extracellular matrix. Investigation into the detailed mechanism by which NAG-1 is processed during the maturation is necessary. An additional aspect is the translocation of NAG-1 inside cells, since the movement of NAG-1 in the cells has not been characterized. NAG-1 is a protein forming in a vesicle and translocating to the cell membrane. Once quantitative and comprehensive assessment of NAG-1 movements is established, we could evaluate the influence of NAG-1 inducers, including chemopreventative compounds on NAG-1's movement in the cells. Overall, NAG-1 represents a new molecular target of cancer and inflammation in in vitro and in vivo studies. NAG-1 seems to be a pivotal protein that controls colorectal cancer, colorectal inflammation, and lung tumorigenesis in vivo. Thus, the fact that NAG-1 shows dual function in carcinogenesis is not surprising. Further study on the molecular mechanisms by which NAG-1 interacts with other proteins in the cells and extracellular matrix, and how NAG-1 affects its biological activity is needed.
NAG-1 is expressed quite widely, but under resting conditions, the placenta and prostate are the tissues that express large quantities of NAG-1, while the colon expresses modest levels of NAG-1 [97]. In humans, NAG-1 appears to be expressed predominantly in epithelial cells, while in mice the expression appears to be mainly in hepatic tissue and fat [98]. The plasma levels of the secreted mature protein are greatly elevated in patients with a number of cancers including colorectal, breast, prostate, and pancreatic cancer. The normal concentrations in healthy adult individuals are reported as 450±50 pg/ml [99]. Measurement of the secreted NAG-1 in the blood has been proposed as a diagnostic marker for several types of cancer [100] and a measure of cancer progression [101, 102]. NAG-1 serum levels are also proposed as markers for cardiovascular incidences [103, 104], and a recent paper suggests NAG-1 is a marker for “all-cause mortality” [105]. Yet, during pregnancy, the serum levels of NAG-1 are high, increasing with the length of the pregnancy; in fact, levels as high as ~10–20 ng/ml have been reported for the third trimester [106]. In beta thalassemia, a mean concentration of NAG-1 in the blood as high as 66±10 ng/ml has been reported [99]. The concentrations of NAG-1 in beta thalassemia appear to correlate with erythropoiesis. The increased number of erythroblasts observed in beta thalassemia is responsible for the highly secreted NAG-1 levels observed [99]. Thus, for many diseases and conditions, high NAG-1 blood concentrations are observed. We suspect that for some cancers, higher NAG-1 concentrations in the blood are only a reflection of tumor size and not an indicator that NAG-1's acting to enhance tumor growth as is frequently assumed. However, the increase in NAG-1in some tumors appears to contribute to the increase in metastatic potential as reported for prostate cancer [83].
4.3 NAG-1 polymorphisms and prostate cancer
Like TGF-β, genetic polymorphisms of NAG-1 have been described [107]. Three nonsynonymous single nucleotide polymorphisms exist in the gene that cause amino acid changes in the coding region, including a common C to G (exon 2+2423) substitution (histidine to aspartic acid) at codon 202 of the precursor protein which is commonly called H6D because the amino change is at position 6 of the mature GDF15/NAG-1 protein (rs1058587) [107]. A large study of 1,340 prostate cancer cases and 765 controls in Sweden suggested the G allele (the H6D GDF15/NAG-1) is associated with decreased risk of developing prostate cancer [108]. A second large (819) case–control (731) study in Australia had similar findings, although these findings were not statistically significant [109]. However, the results from this study also suggest a higher mortality rate from prostate cancer for patients carrying the G allele relative to men with the CC genotype [109]. Similarly, a case–control study (506 controls and 506 cases) in the USA found that the G allele is marginally associated with a lower prostate cancer incidence although statistically insignificant [110]. The G allele frequency in the study population from the above three studies are 28.8%/27.7% (controls/cases, Swedish), 26.5%/24.3% (Australian), and 27.2%/26.5% (USA), respectively [111]. Other than the study in prostate cancer, only one study from Brown et al. examined the association of H6D NAG-1 with colorectal cancer, in which the H6D NAG-1 is associated with increased risk of colorectal cancer metastasis, but not associated with cancer risk [111]. A few studies also examined the association of other NAG-1 single-nucleotide polymorphisms (SNPs) with prostate cancer risk and mortality. However, these data in general did not support an association such as the H6D NAG-1. Collectively, the role of NAG-1 polymorphisms in human prostate cancer is not clear at present. More studies, especially animal studies, are needed to elucidate the role of H6D NAG-1 or other SNPs of NAG-1 in prostate cancer.
4.4 Epigenetic regulation of NAG-1
The expression of NAG-1 in tumors compared to normal tissue is not clear. There are many contradictory results reported in the literature. The promoter of NAG-1 has several CpG islands but whether the protein is epigenetically silenced by methylation or histone modification has not been investigated. Glioblastoma is the most common central nervous system tumor and is associated with a high morbidity and mortality. It is widely accepted that the histone deacetylase inhibitor trichostatin A (TSA) alters chromatin structure. We investigated if histone acetylation would alter the expression of NAG-1. TSA upregulates expression and acts synergistically with 5-AZA-dC to induce NAG-1 expression. TSA induces NAG-1 promoter activity as well as NAG-1 mRNA and protein expression [112]. SiRNA experiments link NAG-1 expression to apoptosis induced by TSA. Reporter gene assays, specific inhibition by small interfering RNA (siRNA) transfections, and ChIP assays indicate that EGR-1/Sp1 transcription factors mediate TSA-induced NAG-1 expression. TSA also increases the stability of NAG-1 mRNA [112]. The mechanism of TSA-induced NAG-1 expression involves not only the interaction with Sp1 and EGR-1 but also multiple regulations at the transcriptional and posttranscriptional levels. However, the association between NAG-1 expression and the development or progression of glioma has not been well defined. Glioblastoma cell lines have a lower basal expression of NAG-1 than other gliomas and normal astrocytes. In fact, most primary human gliomas have very low levels of NAG-1 expression. NAG-1 basal expression appears to inversely correlate with tumor grade in glioma. Aberrant promoter hypermethylation is a common mechanism for silencing of tumor suppressor genes in cancer cells. In glioblastoma cell lines, basal NAG-1 expression was increased by the demethylating agent, 5-aza-2′-deoxycytidine. The NAG-1 promoter was densely methylated in several glioblastoma cell lines as well as in primary oligodendroglioma tumor samples, which have low basal expression of NAG-1 [112]. DNA methylation at two specific sites (−53 and +55 CpG sites) in the NAG-1 promoter was strongly associated with low NAG-1 expression. The methylation of the NAG-1 promoter at the −53 site blocks EGR-1 binding and thereby suppresses NAG-1 induction. Treatment of cells with low basal NAG-1 expression with NAG-1 inducer also did not increase NAG-1. Pre-incubation with 5-aza-2′-deoxycytidine increased NAG-1 basal expression, and subsequent incubation with a NAG-1 inducer increased NAG-1 expression. Thus, methylation of specific promoter sequences causes transcriptional silencing of the NAG-1 locus in gliomas and may ultimately contribute to tumor progression. Furthermore, methylation at the EGR-1 sites prevents the induction of NAG-1 by drugs and provides a rationale for explaining why some tumors are not suppressed by COX inhibitors. The methylation study was done on glioblastoma cells and glioblastoma tissue comparing tissue with low basal expression of NAG-1 to tissue/cells with high basal expression. However, many other tumors and cells are reported to highly express NAG-1 expression. The methylation status of other tumors has not been examined but one would suspect the CpG islands are not highly methylated in high-expressing tumors. Further studies are necessary to clarify the conflicting data on the expression of NAG-1 in tumors and the possible link to CpG island methylation. Clearly, the methylation of critical regions in the NAG-1 promoter will block an increase in expression after treatment with drugs or chemicals and provides a rationale for why some tumors may be resistant to drug treatment.
4.5 NAG expression and the inhibition of tumor growth by COX inhibitors
NSAIDs are the most widely used drugs for treatment of inflammatory diseases and long-term use of NSAIDs prevents development of several types of cancer. Both COX-dependent and COX-independent mechanisms have been proposed for the chemopreventive and antitumorigenic activities of NSAIDs. Laboratory studies suggest that NAG-1 exhibits pro-apoptotic and anti-tumorigenic activities in several types of cancer cells and inhibits intestinal tumor growth. NAG-1 expression is upregulated in cell culture models upon treatment with NSAIDs or other chemicals with cancer prevention activity. This finding raised a question of whether increases in NAG-1 expression by NSAID contribute to NSAID-induced inhibition of carcinogenesis.
NAG-1 expression is upregulated by several NSAIDs in a COX-independent manner in human cancer cells. NAG-1 was first identified by our laboratory from indomethacin-treated COX-deficient human colorectal cancer HCT116 cells [23]. Celecoxib has been shown to induce apoptosis in COX-2-deficient human gastric cancer cells via activation of NAG-1 expression and inhibition of AKt/GSK3beta [113]. Sulindac sulfide significantly induced NAG-1 expression in gastric cancer SNU601 cells that are devoid of COX-2 expression and increased apoptosis and decreased cell viability in this cell line [114]. To determine whether COX expression could affect NSAID-induced NAG-1 expression, HCT116 cells were transfected with COX-1 or COX-2. However, transient or stable transfection of the COX gene did not alter NSAID-induced NAG-1 expression in HCT116 cells [115]. Furthermore, NAG-1 expression was not affected by PGE2 and arachidonic acid levels [115]. The fact that NAG-1 expression can be induced by NSAIDs in a number of human cells lines and that NAG-1 induction by NSAIDs is not altered by COX expression or the presence of PGE2 or arachidonic acid, suggests a COX-2-independent mechanism of NSAIDs in the inhibition of carcinogenesis, which may be mediated by NAG-1.
NAG-1 induction has also been observed in animal models after feeding NSAIDs. Feeding C57/BL6 mice sulindac induced mNAG-1 expression in liver and colon tissues [97, 116]. Indomethacin treatment has been shown to induce the expression of NAG-1 mRNA level in human sinonasal cancer cell AMC-HN5 xenograft tumors at a dose-dependent manner. The volume of xenograft tumors of AMC-HN5 cells in indomethacin-treated nude mice was reduced compared to that in control mice [117]. In another study, celecoxib treatment increased NAG-1 expression in a dose-dependent manner in COX-2 knockout mice and wild-type littermates (COX-2+/+) [118]. NAG-1 induction upon NSAID feeding in animal models suggests a role of mNAG-1 in the suppression of tumor growth by NSAIDs in vivo. Based on these investigations, an increased expression of NAG-1 can be observed in mice treated with NSAIDs.
Many studies have shown that sulindac fed to APC/Min mice inhibits polyp formation. However, the contribution of NAG-1 expression to the prevention of polyp formation by sulindac has not been determined. A number of sulindac derivatives (both sulfides and sulfoxides) were synthesized that lack of COX inhibitory activity to use as tools to study COX-2-independent mechanisms of NSAIDs. Two sulindac des-methyl (DM) analogues (DM-sulindac and DM-sulindac sulfide) devoid of COX activity induced NAG-1 expression in HCT-116 cells and the mouse rectal cancer cell line CMT93 [116]. Sulindac inhibited polyp development in the APC/Min mice, whereas DM-sulindac did not. Pharmacokinetic analysis showed sulindac was effectively converted to sulindac sulfide, the active metabolite, whereas DM-sulindac was rapidly excreted and not efficiently converted to the active DM-sulindac sulfide metabolite [116]. Further analysis of NAG-1 expression after sulindac treatment provided some clue as to the contribution of NAG-1 induction in sulindac-induced tumor inhibition in this study. mNAG-1 expression in the mouse intestinal tract is very low and not increased by sulindac feeding but we did observe significant induction of mNAG-1 expression in the liver upon feeding sulindac. Feeding the DM-sulindac did not alter NAG-1 expression. In contrast to humans, mouse liver is the major source of circulating mNAG-1 and thus sulindac feeding likely increases serum levels of mNAG-1 [97]. This conclusion is supported by another study to determine the role of NAG-1 in sulindac-induced inhibition of polyp formation in APC/Min mice. Zimmers et al. crossed APC/Min mice with NAG-1(−/−) mice, which did not alter polyp formation [91]. In this study, sulindac was effective in reducing the polyp formation in APC/Min mice that express wild-type NAG-1. However, sulindac did not reduce polyp formation in NAG-1(−/−) crossed with APC/Min mouse [91]. This finding suggests that NAG-1 is critical for anti-tumorigenic activity of sulindac in the APC/Min mouse model.
Knocking down NAG-1 in cell culture models was also used to elucidate the role of NAG-1 in NSAID-induced inhibition of cancer cell growth. In one study, sulindac sulfide induced NAG-1 expression in ovarian cancer SKOV3 cells and significantly suppressed cell growth [119]. Transfecting SKOV3 cells with the NAG-1 siRNA construct restored SKOV3 cell viability, suggesting sulindac sulfide-induced NAG-1 expression is responsible for the sulindac sulfide-induced cell growth arrest in SKVO3 cells [119]. By treating human prostate cancer PC-3 cells with NAG-1 siRNA, Wynne and Djakiew demonstrated that NAG-1 plays an important role in NSAID-induced inhibition of PC-3 cell migration [120]. Indomethacin induced apoptosis and NAG-1 expression in human sinonasal carcinoma AMC-HN5 cells, in which the indomethacin-induced apoptosis was suppressed by transfecting the cells with NAG-1 siRNA [121]. Collectively, significant reports suggest that NAG-1, in part, plays an important role in NSAID-induced inhibition of tumorigenesis both in in vivo and in in vitro models.
Inhibition of tumor growth by COX inhibitors is a complex process and involves COX inhibition and changes in gene expression. This conclusion is illustrated by a study of the growth of glioblastoma T98G cells on soft agar to estimate the antitumorigenic activity. Sulindac sulfide inhibited T98G cell colony growth on soft agar in a concentration-dependent manner [58]. Sulindac sulfide concentrations as high as 60–120 μM were required for the inhibition of cell growth and inhibition of prostaglandin formation. At these concentrations, sulidac sulfide not only induced NAG-1 expression but also inhibited EP4 receptor expression. Because the cells grown on soft agar are in a serum-containing media, concentrations required to effectively inhibit COX activity is higher than the ED50 value commonly reported that is based on assays in the absence of serum. Thus, the inhibition of tumor growth by sulindac sulfide is very complex both targeting COX and altering the expression of other critical proteins such as NAG-1 and EP4.
5 Summary and conclusion
The long-term use of NSAIDs reduces risk for colorectal cancer. Furthermore, the regular use of aspirin after diagnosis [122] of colorectal cancer can improve survival. However, long-term use of NSAIDs as prevention or therapeutic drugs is not acceptable because their prolonged use is linked to many adverse side effects. The nonselective COX inhibitors cause gastrointestinal adverse effects while the prolonged use of selective COX-2 inhibitors is linked to potentially serious cardiovascular side effects. Many of these side effects appear to be linked to total or global inhibition of prostaglandin formation. Developing safe and effective cancer prevention drugs is a difficult challenge because of the required long-term use.
It is clear from the investigations summarized in this review that COX inhibitors can increase the expression or alter the activity of several proteins that can influence tumor development. These changes in expression or activity appear to be specific for the individual NSAIDs as shown in Fig. 5 and not related to the inhibition of COX activity. This observation offers hope for the development of a strategy to find drugs that can target specific proteins that influence cancer development, and thus avoid potential unacceptable side effects observed for generic inhibition of prostaglandins. For example, the development of specific antagonists for the EP receptors or drugs specifically reducing the expression of the EP2 or EP4 receptors would appear to be promising avenues of investigation.
Our laboratories have focused on investigating NAG-1 or GDF15 as a suppressor of tumor development. Many drugs and chemicals that prevent cancer development have in common the ability to increase the expression of this protein. The expression of NAG-1 is regulated by three tumor suppressor pathways: p53, EGR-1, and GSK-3β. The NAG-1 transgenic mouse expressing human NAG-1 ubiquitously has reduced tumor numbers and tumor size as estimated in mouse models for intestinal and pulmonary tumorigenesis. Other studies suggest NAG-1 may play a role in inhibiting prostate cancer but other results indicate a pro-tumorigenic role for NAG-1 in prostate cancer. Thus, contradictory evidence suggests both anti- and pro-tumorigenic activities for NAG-1 similar to what has been observed for other members of the TGF-β super family of proteins. The paradoxical biological activities and the signaling pathways of human NAG-1 are poorly characterized. Some results suggest some important differences between the NAG-1/GDF15 expression in mouse versus human organs and tissues causing mouse models of tumorigenesis to be of limited use. Identification and characterization of a NAG-1 receptor(s) would provide guidance to understanding the biological activity. One would expect the receptor to be similar to the type I and type II complexes observed for the TGF-β family members, but because a receptor(s) has not yet been identified, one could anticipate an unexpected receptor. Clearly studies in our laboratories and the published results from other investigators point to an important role for NAG-1/GDF15 in the inhibition of tumor growth by COX inhibitors. However, additional evidence, particularly related to human cancer is needed to support this hypothesis. Regardless of its role in cancer inhibition by COX inhibitors, NAG-1/GDF15 is a unique member of the TGF-β family with an array of diverse biological functions and could contribute to the understanding and treatment of many diseases including cancer, cardiovascular, and inflammatory diseases. A better understanding of the underlying mechanisms are necessary to elucidate how this protein inhibits growth at the early stages and then promotes proliferation, invasion, and metastases at the later stages of cancer.
Acknowledgments
We thank Misty Bailey (University of Tennessee), Dr. Shim, and Dr. Paul Wade (NIEHS) for their critical reading of this manuscript. We also thank Dr. Seong-Ho Lee (University of Tennessee) for his assistance on preparing the figures. This research was supported (in part) by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Contributor Information
Xingya Wang, Laboratory of Molecular Carcinogenesis, NIEHS, 111 TW Alexander Dr., Research Triangle Park, NC 27709, USA.
Seung Joon Baek, Biomedical and Diagnostic Sciences, College of Veterinary Medicine, University of Tennessee, Knoxville, TN 37996, USA.
Thomas Eling, Laboratory of Molecular Carcinogenesis, NIEHS, 111 TW Alexander Dr., Research Triangle Park, NC 27709, USA.
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backlund MG, Mann JR, Holla VR, Shi Q, Daikoku T, Dey SK, et al. Repression of 15-hydroxyprostaglandin dehydrogenase involves histone deacetylase 2 and snail in colorectal cancer. Cancer Research. 2008;68(22):9331–9337. doi: 10.1158/0008-5472.CAN-08-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, et al. 15-Hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Research. 2006;66(15):7818–7823. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 6.Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(50):17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacology and Therapeutics. 2004;103(2):147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nature Medicine. 2001;7(9):1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 9.Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, et al. Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Research. 2002;62(1):28–32. [PubMed] [Google Scholar]
- 10.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(2):591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiebich BL, Schleicher S, Spleiss O, Czygan M, Hull M. Mechanisms of prostaglandin E2-induced interleukin-6 release in astrocytes: possible involvement of EP4-like receptors, p38 mitogen-activated protein kinase and protein kinase C. Journal of Neurochemistry. 2001;79(5):950–958. doi: 10.1046/j.1471-4159.2001.00652.x. [DOI] [PubMed] [Google Scholar]
- 12.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sciences. 2003;74(2–3):143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. Journal of Biological Chemistry. 2003;278(37):35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 14.Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Research. 2003;63(17):5218–5223. [PubMed] [Google Scholar]
- 15.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. Journal of Biological Chemistry. 2003;278(14):12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 16.Han S, Roman J. Suppression of prostaglandin E2 receptor subtype EP2 by PPARgamma ligands inhibits human lung carcinoma cell growth. Biochemical and Biophysical Research Communications. 2004;314(4):1093–1099. doi: 10.1016/j.bbrc.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Baron JA. Aspirin and NSAIDs for the prevention of colorectal cancer. Recent Results in Cancer Research. 2009;181:223–229. doi: 10.1007/978-3-540-69297-3_21. [DOI] [PubMed] [Google Scholar]
- 18.Iwama T. NSAIDs and colorectal cancer prevention. Journal of Gastroenterology. 2009;44(Suppl 19):72–76. doi: 10.1007/s00535-008-2265-7. [DOI] [PubMed] [Google Scholar]
- 19.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annual Review of Medicine. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 20.Olsen JH, Friis S, Poulsen AH, Fryzek J, Harving H, Tjonneland A, et al. Use of NSAIDs, smoking and lung cancer risk. British Journal of Cancer. 2008;98(1):232–237. doi: 10.1038/sj.bjc.6604151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li DD, et al. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Research and Treatment. 2009;117(1):141–150. doi: 10.1007/s10549-008-0228-6. [DOI] [PubMed] [Google Scholar]
- 22.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(6):572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Molecular Pharmacology. 2001;59(4):901–908. [PubMed] [Google Scholar]
- 24.Zhang X, Morham SG, Langenbach R, Young DA. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. The Journal of Experimental Medicine. 1999;190(4):451–459. doi: 10.1084/jem.190.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu CH, McEntee MF, Whelan J. Sulindac causes rapid regression of preexisting tumors in Min/+ mice independent of prostaglandin biosynthesis. Cancer Research. 1997;57(19):4267–4273. [PubMed] [Google Scholar]
- 26.Baek SJ, Eling TE. Changes in gene expression contribute to cancer prevention by COX inhibitors. Progress in Lipid Research. 2006;45(1):1–16. doi: 10.1016/j.plipres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Ikawa H, Kamitani H, Calvo BF, Foley JF, Eling TE. Expression of 15-lipoxygenase-1 in human colorectal cancer. Cancer Research. 1999;59(2):360–366. [PubMed] [Google Scholar]
- 28.Nixon JB, Kim KS, Lamb PW, Bottone FG, Eling TE. 15-Lipoxygenase-1 has anti-tumorigenic effects in colorectal cancer. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2004;70(1):7–15. doi: 10.1016/j.plefa.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Shureiqi I, Chen D, Lee JJ, Yang P, Newman RA, Brenner DE, et al. 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. Journal of the National Cancer Institute. 2000;92(14):1136–1142. doi: 10.1093/jnci/92.14.1136. [DOI] [PubMed] [Google Scholar]
- 30.Shureiqi I, Chen D, Day RS, Zuo X, Hochman FL, Ross WA, et al. Profiling lipoxygenase metabolism in specific steps of colorectal tumorigenesis. Cancer Prev Res (Phila) 2010;3(7):829–838. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo X, Wu Y, Morris JS, Stimmel JB, Leesnitzer LM, Fischer SM, et al. Oxidative metabolism of linoleic acid modulates PPAR-beta/delta suppression of PPAR-gamma activity. Oncogene. 2006;25(8):1225–1241. doi: 10.1038/sj.onc.1209160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JS, Baek SJ, Bottone FG, Jr., Sali T, Eling TE. Overexpression of 15-lipoxygenase-1 induces growth arrest through phosphorylation of p53 in human colorectal cancer cells. Molecular Cancer Research. 2005;3(9):511–517. doi: 10.1158/1541-7786.MCR-05-0011. [DOI] [PubMed] [Google Scholar]
- 33.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91(3):325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Glasgow W, George MD, Chrysovergis K, Olden K, Roberts JD, et al. 15-Lipoxygenase-1 activates tumor suppressor p53 independent of enzymatic activity. International Journal of Cancer. 2008;123(12):2741–2749. doi: 10.1002/ijc.23855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverman ES, Collins T. Pathways of Egr-1-mediated gene transcription in vascular biology. American Journal of Pathology. 1999;154(3):665–670. doi: 10.1016/S0002-9440(10)65312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krones-Herzig A, Mittal S, Yule K, Liang H, English C, Urcis R, et al. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Research. 2005;65(12):5133–5143. doi: 10.1158/0008-5472.CAN-04-3742. doi:10.1158/0008-5472.can-04-3742. [DOI] [PubMed] [Google Scholar]
- 37.Nair P, Muthukkumar S, Sells SF, Han SS, Sukhatme VP, Rangnekar VM. Early growth response-1-dependent apoptosis is mediated by p53. Journal of Biological Chemistry. 1997;272(32):20131–20138. doi: 10.1074/jbc.272.32.20131. [DOI] [PubMed] [Google Scholar]
- 38.Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Molecular Pharmacology. 2005;67(2):356–364. doi: 10.1124/mol.104.005108. [DOI] [PubMed] [Google Scholar]
- 39.Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nature Cell Biology. 2001;3(12):1124–1128. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- 40.Cho KN, Sukhthankar M, Lee SH, Yoon JH, Baek SJ. Green tea catechin (−)-epicatechin gallate induces tumour suppressor protein ATF3 via EGR-1 activation. European Journal of Cancer. 2007;43(16):2404–2412. doi: 10.1016/j.ejca.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitlock NC, Bahn JH, Lee SH, Eling TE, Baek SJ. Resveratrol-induced apoptosis is mediated by early growth response-1, Kruppel-like factor 4, and activating transcription factor 3. Cancer Prevention Research (Philadelphia, Pa.) 2011;4(1):116–127. doi: 10.1158/1940-6207.CAPR-10-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi K, Lee SH, Kim JS, Wimalasena J, Kitajima S, Baek SJ. Activating transcription factor 3 and early growth response 1 are the novel targets of LY294002 in a phosphatidylinositol 3-kinase-independent pathway. Cancer Research. 2006;66(4):2376–2384. doi: 10.1158/0008-5472.CAN-05-1987. [DOI] [PubMed] [Google Scholar]
- 43.Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. European Journal of Cancer. 2005;41(16):2438–2448. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Abdelrahim M, Baker CH, Abbruzzese JL, Sheikh-Hamad D, Liu S, Cho SD, et al. Regulation of vascular endothelial growth factor receptor-1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer Research. 2007;67(7):3286–3294. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 45.Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Molecular Pharmacology. 2005;68(2) doi: 10.1124/mol.105.011825. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q, et al. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. Journal of Biological Chemistry. 1998;273(50):33750–33758. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- 47.Tymms MJ, Ng AY, Thomas RS, Schutte BC, Zhou J, Eyre HJ, et al. A novel epithelial-expressed ETS gene, ELF3: human and murine cDNA sequences, murine genomic organization, human mapping to 1q32.2 and expression in tissues and cancer. Oncogene. 1997;15(20):2449–2462. doi: 10.1038/sj.onc.1201427. [DOI] [PubMed] [Google Scholar]
- 48.Chang CH, Scott GK, Kuo WL, Xiong X, Suzdaltseva Y, Park JW, et al. ESX: a structurally unique Ets overex-pressed early during human breast tumorigenesis. Oncogene. 1997;14(13):1617–1622. doi: 10.1038/sj.onc.1200978. [DOI] [PubMed] [Google Scholar]
- 49.Oettgen P, Alani RM, Barcinski MA, Brown L, Akbarali Y, Boltax J, et al. Isolation and characterization of a novel epithelium-specific transcription factor, ESE-1, a member of the ets family. Molecular and Cellular Biology. 1997;17(8):4419–4433. doi: 10.1128/mcb.17.8.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng AY, Waring P, Ristevski S, Wang C, Wilson T, Pritchard M, et al. Inactivation of the transcription factor Elf3 in mice results in dysmorphogenesis and altered differentiation of intestinal epithelium. Gastroenterology. 2002;122(5):1455–1466. doi: 10.1053/gast.2002.32990. [DOI] [PubMed] [Google Scholar]
- 51.Brembeck FH, Opitz OG, Libermann TA, Rustgi AK. Dual function of the epithelial specific ets transcription factor, ELF3, in modulating differentiation. Oncogene. 2000;19(15):1941–1949. doi: 10.1038/sj.onc.1203441. [DOI] [PubMed] [Google Scholar]
- 52.Lee S-H, Bahn JH, Choi CK, Whitlock NC, English AE, Safe S, et al. ESE-1/EGR-1 pathway plays a role in tolfenamic acid-induced apoptosis in colorectal cancer cells. Molecular Cancer Therapeutics. 2008;7(12):3739–3750. doi: 10.1158/1535-7163.MCT-08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23(3):425–434. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 54.Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, et al. Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. Journal of Biological Chemistry. 2000;275(26):20127–20135. doi: 10.1074/jbc.M909580199. [DOI] [PubMed] [Google Scholar]
- 55.Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE. Expression of NAG-1, a transforming growth factor-beta superfamily member, by troglitazone requires the early growth response gene EGR-1. Journal of Biological Chemistry. 2004;279(8):6883–6892. doi: 10.1074/jbc.M305295200. [DOI] [PubMed] [Google Scholar]
- 56.Kambe A, Iguchi G, Moon Y, Kamitani H, Watanabe T, Eling TE. Regulation of EP4 expression via the Sp-1 transcription factor: inhibition of expression by anti-cancer agents. Biochimica et Biophysica Acta. 2008;1783(6):1211–1219. doi: 10.1016/j.bbamcr.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foord SM, Marks B, Stolz M, Bufflier E, Fraser NJ, Lee MG. The structure of the prostaglandin EP4 receptor gene and related pseudogenes. Genomics. 1996;35(1):182–188. doi: 10.1006/geno.1996.0337. [DOI] [PubMed] [Google Scholar]
- 58.Kambe A, Yoshioka H, Kamitani H, Watanabe T, Baek SJ, Eling TE. The cyclooxygenase inhibitor sulindac sulfide inhibits EP4 expression and suppresses the growth of glioblastoma cells. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(12):1088–1099. doi: 10.1158/1940-6207.CAPR-09-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu D, Wolfgang CD, Hai T. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. Journal of Biological Chemistry. 2006;281(15):10473–10481. doi: 10.1074/jbc.M509278200. [DOI] [PubMed] [Google Scholar]
- 60.Yin HW, Lei F, Wang AL, Cheng J, Zhou Y. Bioelectrical impedance assay to monitor changes in aspirin-treated human colon cancer HT-29 cell shape during apoptosis. Analytical Letters. 2007;40:85–94. [Google Scholar]
- 61.Yan C, Boyd DD. ATF3 regulates the stability of p53: a link to cancer. Cell Cycle. 2006;5(9):926–929. doi: 10.4161/cc.5.9.2714. [DOI] [PubMed] [Google Scholar]
- 62.Fan F, Jin S, Amundson SA, Tong T, Fan W, Zhao H, et al. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene. 2002;21(49):7488–7496. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- 63.Huang X, Li X, Guo B. KLF6 induces apoptosis in prostate cancer cells through up-regulation of ATF3. Journal of Biological Chemistry. 2008;283(44):29795–29801. doi: 10.1074/jbc.M802515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan C, Wang H, Boyd DD. ATF3 represses 72-kDa type IV collagenase (MMP-2) expression by antagonizing p53-dependent trans-activation of the collagenase promoter. Journal of Biological Chemistry. 2002;277(13):10804–10812. doi: 10.1074/jbc.M112069200. [DOI] [PubMed] [Google Scholar]
- 65.Stearns ME, Kim G, Garcia F, Wang M. Interleukin-10 induced activating transcription factor 3 transcriptional suppression of matrix metalloproteinase-2 gene expression in human prostate CPTX-1532 Cells. Molecular Cancer Research. 2004;2(7):403–416. [PubMed] [Google Scholar]
- 66.Bottone FG, Jr., Moon Y, Kim JS, Alston-Mills B, Ishibashi M, Eling TE. The anti-invasive activity of cyclooxygenase inhibitors is regulated by the transcription factor ATF3 (activating transcription factor 3). Molecular Cancer Therapeutics. 2005;4(5):693–703. doi: 10.1158/1535-7163.MCT-04-0337. [DOI] [PubMed] [Google Scholar]
- 67.Lee SH, Bahn JH, Whitlock NC, Baek SJ. Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene. 2010;29(37):5182–5192. doi: 10.1038/onc.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McEntee MF, Chiu CH, Whelan J. Relationship of beta-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis. 1999;20(4):635–640. doi: 10.1093/carcin/20.4.635. [DOI] [PubMed] [Google Scholar]
- 69.Dihlmann S, Siermann A, von Knebel Doeberitz M. The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene. 2001;20(5):645–653. doi: 10.1038/sj.onc.1204123. [DOI] [PubMed] [Google Scholar]
- 70.Dihlmann S, Klein S, Doeberitz Mv MK. Reduction of beta-catenin/T-cell transcription factor signaling by aspirin and indomethacin is caused by an increased stabilization of phosphorylated beta-catenin. Molecular Cancer Therapeutics. 2003;2(6):509–516. [PubMed] [Google Scholar]
- 71.Greenspan EJ, Madigan JP, Boardman LA, Rosenberg DW. Ibuprofen inhibits activation of nuclear β-catenin in human colon adenomas and induces the phosphorylation of GSK-3β. Cancer Prevention Research. 2011;4(1):161–171. doi: 10.1158/1940-6207.CAPR-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(21):11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, et al. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. Journal of Biological Chemistry. 1998;273(22):13760–13767. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- 74.Bottner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1). Gene. 1999;237(1):105–111. doi: 10.1016/s0378-1119(99)00309-1. [DOI] [PubMed] [Google Scholar]
- 75.Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochimica et Biophysica Acta. 1997;1354:40–44. doi: 10.1016/s0167-4781(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 76.Albertoni M, Shaw PH, Nozaki M, Godard S, Tenan M, Hamou MF, et al. Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in glioblastoma cells independently of p53 and HIF-1. Oncogene. 2002;21(27):4212–4219. doi: 10.1038/sj.onc.1205610. [DOI] [PubMed] [Google Scholar]
- 77.Lawton LN, Bonaldo MF, Jelenc PC, Qiu L, Baumes SA, Marcelino RA, et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene. 1997;203:17–26. doi: 10.1016/s0378-1119(97)00485-x. [DOI] [PubMed] [Google Scholar]
- 78.Bauskin AR, Zhang H-P, Fairlie WD, He XY, Russell PK, Moore AG, et al. The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-β superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO Journal. 2000;19(10):2212–2220. doi: 10.1093/emboj/19.10.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bauskin AR, Brown DA, Junankar S, Rasiah KK, Eggleton S, Hunter M, et al. The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Research. 2005;65(6):2330–2336. doi: 10.1158/0008-5472.CAN-04-3827. [DOI] [PubMed] [Google Scholar]
- 80.Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131(5):1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 81.Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prevention Research (Phila Pa) 2009;2(5):450–458. doi: 10.1158/1940-6207.CAPR-09-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakamura T, Scorilas A, Stephan C, Yousef GM, Kristiansen G, Jung K, et al. Quantitative analysis of macrophage inhibitory cytokine-1 (MIC-1) gene expression in human prostatic tissues. British Journal of Cancer. 2003;88(7):1101–1104. doi: 10.1038/sj.bjc.6600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, Batra SK. Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene. 2010;29(9):1293–1302. doi: 10.1038/onc.2009.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and nonsteroidal anti-inflammatory drug-activated gene-1. Molecular Pharmacology. 2005;68(6):1782–1792. doi: 10.1124/mol.105.017046. [DOI] [PubMed] [Google Scholar]
- 85.Jutooru I, Chadalapaka G, Chintharlapalli S, Papineni S, Safe S. Induction of apoptosis and nonsteroidal anti-inflammatory drug-activated gene 1 in pancreatic cancer cells by a glycyrrhetinic acid derivative. Molecular Carcinogenesis. 2009;48(8):692–702. doi: 10.1002/mc.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelly JA, Lucia MS, Lambert JR. p53 controls prostate-derived factor/macrophage inhibitory cytokine/NSAID-activated gene expression in response to cell density, DNA damage and hypoxia through diverse mechanisms. Cancer Letters. 2009;277(1):38–47. doi: 10.1016/j.canlet.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 87.Yang H, Filipovic Z, Brown D, Breit SN, Vassilev LT. Macrophage inhibitory cytokine-1: a novel biomarker for p53 pathway activation. Molecular Cancer Therapeutics. 2003;2(10):1023–1029. [PubMed] [Google Scholar]
- 88.Kim KK, Lee JJ, Yang Y, You KH, Lee JH. Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells. Carcinogenesis. 2008;29(4):704–712. doi: 10.1093/carcin/bgn031. [DOI] [PubMed] [Google Scholar]
- 89.Chen SJ, Karan D, Johansson SL, Lin FF, Zeckser J, Singh AP, et al. Prostate-derived factor as a paracrine and autocrine factor for the proliferation of androgen receptor-positive human prostate cancer cells. Prostate. 2007;67(5):557–571. doi: 10.1002/pros.20551. [DOI] [PubMed] [Google Scholar]
- 90.Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(5):450–458. doi: 10.1158/1940-6207.CAPR-09-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zimmers TA, Gutierrez JC, Koniaris LG. Loss of GDF-15 abolishes sulindac chemoprevention in the ApcMin/+ mouse model of intestinal cancer. Journal of Cancer Research and Clinical Oncology. 2010;136(4):571–576. doi: 10.1007/s00432-009-0691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamaguchi K, Lee SH, Eling TE, Baek SJ. Identification of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) as a novel downstream target of phosphatidylinositol 3-kinase/AKT/GSK-3beta pathway. Journal of Biological Chemistry. 2004;279(48):49617–49623. doi: 10.1074/jbc.M408796200. [DOI] [PubMed] [Google Scholar]
- 93.Gitenay D, Baron VT. Is EGR1 a potential target for prostate cancer therapy? Future Oncology. 2009;5(7):993–1003. doi: 10.2217/fon.09.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Therapy. 2006;13(2):115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shappell SB, Manning S, Boeglin WE, Guan YF, Roberts RL, Davis L, et al. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia. 2001;3(4):287–303. doi: 10.1038/sj.neo.7900166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fairlie WD, Zhang HP, Wu WM, Pankhurst SL, Bauskin AR, Russell PK, et al. The propeptide of the transforming growth factor-beta superfamily member, macrophage inhibitory cytokine-1 (MIC-1), is a multifunctional domain that can facilitate protein folding and secretion. Journal of Biological Chemistry. 2001;276(20):16911–16918. doi: 10.1074/jbc.M010000200. [DOI] [PubMed] [Google Scholar]
- 97.Kim KS, Baek SJ, Flake GP, Loftin CD, Calvo BF, Eling TE. Expression and regulation of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) in human and mouse tissue. Gastroenterology. 2002;122(5):1388–1398. doi: 10.1053/gast.2002.32972. [DOI] [PubMed] [Google Scholar]
- 98.Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, et al. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009;150(4):1688–1696. doi: 10.1210/en.2008-0952. [DOI] [PubMed] [Google Scholar]
- 99.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nature Medicine. 2007;13(9):1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 100.Koopmann J, Buckhaults P, Brown DA, Zahurak ML, Sato N, Fukushima N, et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clinical Cancer Research. 2004;10(7):2386–2392. doi: 10.1158/1078-0432.ccr-03-0165. [DOI] [PubMed] [Google Scholar]
- 101.Brown DA, Lindmark F, Stattin P, Balter K, Adami HO, Zheng SL, et al. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clinical Cancer Research. 2009;15(21):6658–6664. doi: 10.1158/1078-0432.CCR-08-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eitel I, Blase P, Adams V, Hildebrand L, Desch S, Schuler G, et al. Growth-differentiation factor 15 as predictor of mortality in acute reperfused ST-elevation myocardial infarction: insights from cardiovascular magnetic resonance. Heart. 2011;97(8):632–640. doi: 10.1136/hrt.2010.219543. [DOI] [PubMed] [Google Scholar]
- 104.Maisel A. Biomarkers in heart failure. Does prognostic utility translate to clinical futility? Journal of the American College of Cardiology. 2007;50(11):1061–1063. doi: 10.1016/j.jacc.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 105.Wiklund FE, Bennet AM, Magnusson PK, Eriksson UK, Lindmark F, Wu L, et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging Cell. 2010;9(6):1057–1064. doi: 10.1111/j.1474-9726.2010.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moore AG, Brown DA, Fairlie WD, Bauskin AR, Brown PK, Munier ML, et al. The transforming growth factor-ss superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. Journal of Clinical Endocrinology and Metabolism. 2000;85(12):4781–4788. doi: 10.1210/jcem.85.12.7007. [DOI] [PubMed] [Google Scholar]
- 107.Fairlie WD, Russell PK, Wu WM, Moore AG, Zhang HP, Brown PK, et al. Epitope mapping of the transforming growth factor-beta superfamily protein, macrophage inhibitory cytokine-1 (MIC-1): identification of at least five distinct epitope specificities. Biochemistry. 2001;40(1):65–73. doi: 10.1021/bi001064p. [DOI] [PubMed] [Google Scholar]
- 108.Lindmark F, Zheng SL, Wiklund F, Bensen J, Balter KA, Chang B, et al. H6D polymorphism in macrophage-inhibitory cytokine-1 gene associated with prostate cancer. Journal of the National Cancer Institute. 2004;96(16):1248–1254. doi: 10.1093/jnci/djh227. [DOI] [PubMed] [Google Scholar]
- 109.Hayes VM, Severi G, Southey MC, Padilla EJ, English DR, Hopper JL, et al. Macrophage inhibitory cytokine-1 H6D polymorphism, prostate cancer risk, and survival. Cancer Epidemiology, Biomarkers & Prevention. 2006;15(6):1223–1225. doi: 10.1158/1055-9965.EPI-06-0063. [DOI] [PubMed] [Google Scholar]
- 110.Cheng I, Krumroy LM, Plummer SJ, Casey G, Witte JS. MIC1 and IL1RN genetic variation and advanced prostate cancer risk. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(6):1309–1311. doi: 10.1158/1055-9965.EPI-07-0165. [DOI] [PubMed] [Google Scholar]
- 111.Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, et al. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clinical Cancer Research. 2003;9(7):2642–2650. [PubMed] [Google Scholar]
- 112.Kadowaki M, Yoshioka H, Kamitani H, Watanabe T, Wade PA, Eling TE. DNA methylation-mediated silencing of nonsteroidal anti-inflammatory drug-activated gene (NAG-1/GDF15) in glioma cell lines. Int J Cancer. 2011 doi: 10.1002/ijc.26082. doi:10.1002/ijc.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pang RP, Zhou JG, Zeng ZR, Li XY, Chen W, Chen MH, et al. Celecoxib induces apoptosis in COX-2 deficient human gastric cancer cells through Akt/GSK3beta/NAG-1 pathway. Cancer Letters. 2007;251(2):268–277. doi: 10.1016/j.canlet.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 114.Jang TJ, Kang HJ, Kim JR, Yang CH. Nonsteroidal anti-inflammatory drug activated gene (NAG-1) expression is closely related to death receptor-4 and -5 induction, which may explain sulindac sulfide induced gastric cancer cell apoptosis. Carcinogenesis. 2004;25(10):1853–1858. doi: 10.1093/carcin/bgh199. [DOI] [PubMed] [Google Scholar]
- 115.Baek SJ, Wilson LC, Lee CH, Eling TE. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): inhibition of cyclooxygenase and induction of NSAID-activated gene. Journal of Pharmacology and Experimental Therapeutics. 2002;301(3):1126–1131. doi: 10.1124/jpet.301.3.1126. [DOI] [PubMed] [Google Scholar]
- 116.Wang X, Kingsley PJ, Marnett LJ, Eling TE. The role of NAG-1/GDF15 in the inhibition of intestinal polyps in APC/Min mice by sulindac. Cancer Prevention Research (Philadelphia) 2011;4(1):150–160. doi: 10.1158/1940-6207.CAPR-10-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim JH, Chang JH, Rhee KH, Yoon JH, Kwon SH, Song K, et al. Cyclooxygenase inhibitors induce apoptosis in sinonasal cancer cells by increased expression of nonsteroidal anti-inflammatory drug-activated gene. International Journal of Cancer. 2008;122(8):1765–1773. doi: 10.1002/ijc.23302. [DOI] [PubMed] [Google Scholar]
- 118.Iguchi G, Chrysovergis K, Lee SH, Baek SJ, Langenbach R, Eling TE. A reciprocal relationship exists between non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) and cyclooxygenase-2. Cancer Letters. 2009;282(2):152–158. doi: 10.1016/j.canlet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim JS, Baek SJ, Sali T, Eling TE. The conventional nonsteroidal anti-inflammatory drug sulindac sulfide arrests ovarian cancer cell growth via the expression of NAG-1/MIC-1/GDF-15. Molecular Cancer Therapeutics. 2005;4(3):487–493. doi: 10.1158/1535-7163.MCT-04-0201. [DOI] [PubMed] [Google Scholar]
- 120.Wynne S, Djakiew D. NSAID inhibition of prostate cancer cell migration is mediated by Nag-1 induction via the p38 MAPK-p75(NTR) pathway. Molecular Cancer Research. 2010;8(12):1656–1664. doi: 10.1158/1541-7786.MCR-10-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diener HC. Secondary stroke prevention with antiplatelet drugs: have we reached the ceiling? International Journal of Stroke. 2006;1(1):4–8. doi: 10.1111/j.1747-4949.2005.00016.x. [DOI] [PubMed] [Google Scholar]
- 122.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. Journal of the American Medical Association. 2009;302(6):649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]