Abstract
In this study, we tested in vivo effectiveness of a previously developed poly-l-lactide/poly-ε-caprolactone armored vascular graft releasing heparin. This bioprosthesis was designed in order to overcome the main drawbacks of tissue-engineered vascular grafts, mainly concerning poor mechanical properties, thrombogenicity, and endothelialization. The bioprosthesis was successfully implanted in an aortic vascular reconstruction model in rabbits. All grafts implanted were patent at four weeks postoperatively and have been adequately populated by endogenous cells without signs of thrombosis or structural failure and with no need of antiplatelet therapy. The results of this preliminary study might warrant for further larger controlled in vivo studies to further confirm these findings.
Keywords: vascular graft, computer-aided tissue engineering, electrospinning, additive manufacturing, heparin, drug release
Introduction
Cardiovascular diseases are nowadays emerging as an overwhelming clinical reality leading to high morbidity and requiring several surgical procedures such as arterial bypass and vascular replacing. Currently used prosthetic vascular graft materials such as polyethylene terephthalate and polytetrafluoroethylene (PTFE) are often inadequate because of limitations regarding thrombogenicity, graft failure, and infection, especially when used for small-caliber vessel bypass grafting. On the other side, the use of autografts, such as internal thoracic artery or safenous vein, is harnessed by problems of poor quality, especially in the elderly, and inadequate size or length of the vascular conduit itself. Tissue engineering of vascular conduits is emerging as a cornerstone strategy to surmount the drawbacks experienced with surgical replacement with autologous vessels, allografts or xenografts, and prosthetic materials.1,2 Tissue-engineered vascular graft (TEVG) is emerging as a valid alternative to routinely used vascular prostheses. TEVG constructs are based on the use of biodegradable polymeric scaffolds that are used as frameworks for autologous vascular wall cells seeding and culturing.3 Animal studies and human trials demonstrated the effectiveness of TEVGs in vascular replacement for both large- (Ø > 6 mm)4 and small-diameter vessels (Ø < 4 mm).5 TEVGs seeded with autologous cells showed efficient endothelialization, stability over time, and freedom from infections. However, several issues concerning the clinical applications of TEVG still need to be answered. Reasonable and clinically suitable timing for scaffold preparation, mechanical properties, cell type, differentiation and growth inside the construct, donor scarcity, and thrombogenicity represent the major concerns.
The minimum time from harvest of autologous cells to the generation of a mature and durable conduit is approximately eight weeks.6,7
This limits the use of such conduits to elective procedures and requires local expertise and bioreactor facilities. Moreover issues concerning the patency rate of the graft should be considered as the use of engineered scaffold or even small intestine submucosa in several animal models has been shown an overall patency rate of 75 per cent (48 weeks) nothwithstanding aspirin and warfarin administration.8
In this context, bioengineering experimental efforts have been oriented toward the fabrication of scaffolds behaving as biological equivalents with histoarchitecture similar to the native vessels with the aim to stimulate and support cell engraftment and proliferation. Different approaches to the fabrication of TEVGs have been described.9 In this context, electrospinning has been claimed among the most promising manufacturing techniques for the production of polymeric fibrillar meshes resembling the extracellular matrix (ECM) organization with fiber diameters ranging in the nanometer or micrometer scale.10 With this system, it is possible to manufacture interconnected porous structures displaying desirable morphologic characteristics in biological environments as a high surface-to-volume ratio, as well as a high permeability.11 The manufacturing setup is amenable to produce tubular shapes10 and associated to all the techniques of cell seeding.12 Additionally, fibers are amenable to be functionalized with several compounds and growth factors without impairing their biological function and activity. We have previously developed poly-l-lactide (PLLA) electrospun tubular scaffold functionalized with heparin with the final aim to both assist cell differentiation and realize a drug delivery device to prevent graft thrombosis with encouraging results.13 Moreover, electrospun scaffolds, simulating the arrangement of ECM fibrillar proteins, showed a permissive effect on vascular remodeling of both the cellular and extracellular components of a graft, and might therefore constitute a suitable candidate for TEVGs fabrication.14 However, the ability to simulate biomechanical characteristics and behavior of the natural vessel wall represents an obstacle.15–18 Despite a number of efforts experimental efforts lavished in this direction, the mechanical properties of electrospun fibrillar matrices are still inappropriate to sustain the pressure loads soon after implantation in the vascular tree and to ensure structural and functional integrity in the later stages during the remodeling phases.19
In order to overcome this drawback, we recently developed a hybrid technique associating electrospinning and bioprinting to fabricate a bioresorbable scaffold for vascular tissue engineering. With the aim to improve mechanical and functional properties of the TEVG, a single-layer helical poly-ε-caprolactone (PCL) coil was bioprinted on the external surface as a reinforcement of an heparin-releasing PLLA tubular electrospun scaffold.20 This approach takes its inspiration from the so-called computer-aided tissue engineering (CATE), which has been reported to be an exciting resource to produce three-dimensional (3D) geometry constructs.21,22 Biofunctionalization with heparin permitted the creation of a drug delivery system that could overcome the thrombogenic issues of TEVG and at the same time provide a microenvironment suitable to stimulate endothelial differentiation. The PCL external reinforcement ameliorated the resistance to mechanical stress of the scaffold in comparison to nonarmored grafts, and more interestingly, when compared to autologous conduits, it showed better mechanical properties and stress–strain profile than a human saphenous vein while approximating to the ones of the internal thoracic artery. Additionally, it preserved the fibrillar ECM-like arrangement optimal for initial cell attachment and was able to stimulate the engraftment, proliferation, and endothelial differentiation of human bone marrow-derived mesenchymal stem cells. To our knowledge, no other approaches combining a heparin-releasing PLLA electrospun scaffold with a bioprinted PCL armor have been proposed in the literature.
On the basis of these experimental results, we decided to perform a preliminary proof-of-principle study to test the effectiveness of the previously developed TEVG in an in vivo rabbit model of aortoaortic vascular bypass.
Materials and Methods
Fabrication of the graft
Armored vascular grafts were prepared as previously described.20 Briefly, a 13% w/w PLLA (Sigma-Aldrich) solution in dichloromethane was combined with unfractioned heparin (sodium salt, 5000 UI/mL; Mspharma) using methanol as a cosolvent. A final concentration of heparin (with respect to PLLA) of 830 μg/g was obtained, corresponding to the dosage routinely used in literature and clinical settings.23 A tubular scaffold, 5 mm in diameter and 6 cm in length, was manufactured by means of electrospinning according to previously described methods.13,20
Subsequently, an outer PCL (MW 80 kDa; Sigma-Aldrich) armor was constructed by fusion deposition modeling techniques using a previously developed bioprinter.20
A detailed description of the morphological and mechanical characterization of the graft is reported elsewhere.20
In vivo experimental design and surgical procedure
A modified model of abdominal aorta vascular reconstruction was performed according to previously described protocols (Fig. 1).24 A total of 10 adult male New Zealand White rabbits weighting 1.8–2.1 kg were purchased (Charles River Laboratory) and housed under controlled conditions and normal diet for three weeks before experimentation. All animals were implanted with a PCL-armored heparin-releasing PLLA tubular scaffold according to the procedure further described. The study design did not include a control graft without heparin loading, as previous preliminary experiments using PCL-armored PLLA grafts showed an excessively low patency rate due to acute graft thrombotic occlusion. Additionally, nonarmored PLLA tubular grafts showed no adequate mechanical properties to support flow after implantation. Considering the preliminary nature of this study, designed as a proof-of-principle to test effectiveness of the newly developed armored heparin-releasing scaffold, we decided to not include control groups which would have been certainly inferior in performance to the study group. All procedures, care, and handling of the animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Regina Elena Institute.
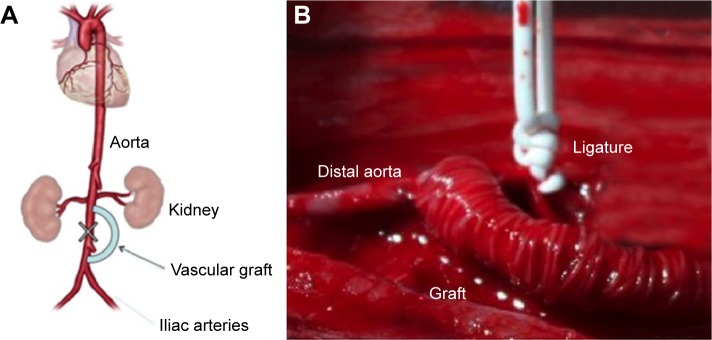
Figure 1.
(A) Schematic diagram of the experimental procedure. (B) Intraoperative photograph showing the PLLA armored scaffold implanted and the ligature of the segment of infrarenal aorta between the two anastomoses.
An optimized anesthesia protocol was selected in order to guarantee stabilization of cardiovascular function during the open chest procedure.25 Anesthesia was inducted by intramuscular (i.m.) administration of ketamine hydrochloride (25 mg/kg of body weight) and xylazine hydrochloride (15 mg/kg). After disappearance of the pedal reflex in the hindlimbs, rabbits were placed on a warming operative platform (37°C) in the supine position. A 23G vascular access was obtained through the marginal vein of the ear. The skin of the ventral abdomen was aseptically prepped with povidone–iodine solution. Briefly, a midline laparotomy incision was performed and the abdominal aorta exposed. Renal arteries were identified and following administration of 100 IU/kg of sodium heparin, proximal end-to-side anastomosis with the composite graft was constructed using a sidebiting microclamp ~1 cm below the origin or renal arteries using monofilament 8–0 polypropylene suture. Aorta was bypassed for a tract of ~4 cm and the distal end graft was then end-to-side anastomosed to aorta before the origin of iliac arteries using monofilament 8–0 polypropylene sutures. The infrarenal aorta between the two anastomoses was ligated so that all the blood flow to the inferior limbs was dependent on the graft (Fig. 1). Muscle layer and skin were closed with 3–0 polyglactin absorbable suture (Vycril; Ethicon). After closure of the abdomen, the animals were allowed to recover on a warming pad. The surgical procedure was completed within 30 minutes after the initiation of anesthesia. When responsive to stimuli and able to maintain an upright posture, rabbits were returned to the home cage and analgesia initiated with buprenorphine (0.5 mg/kg) and paracetamol (1 mg/kg). The first three days after surgery, buprenorphine (0.5 mg/kg b.i.d.) and cefuroxime (100 mg/kg b.i.d.) were administered subcutaneously. The animals received no antiaggregation therapy. Daily controls using handheld Doppler ultrasound system were performed to assess patency of the grafts and animals were examined for signs of inferior limbs weakness or paralysis. Four weeks after implantation, animals were humanely sacrificed and grafts were explanted for evaluation.
Contrast-enhanced vascular imaging
Before sacrifice, animals underwent CT scanning with intravenous contrast agent. Briefly, rabbits were anesthetized with midazolam at a dose of 2 mg/kg i.m. Iodinated contrast medium was injected in the marginal ear vein, and cardiac CT scans were obtained and reformatted in 3D using maximum intensity projection (MIP) and volume rendering algorithms.
Histology
Samples obtained were fixed in 4% paraformaldehyde and embedded in paraffin. Serial sections (6 μm thick) were cut and used for hematoxylin–eosin staining.
Results
At the moment of surgery, no evidence of transgraft leakage of blood was demonstrated. During clinical follow-up, no neurological events, infection, or other surgical complications have been noted. Use of sidebiting clamp and the end-to-side fashion of the anastomosis used prevented the known tendency to paraplegia resulting from cross-clamping of infrarenal aorta.
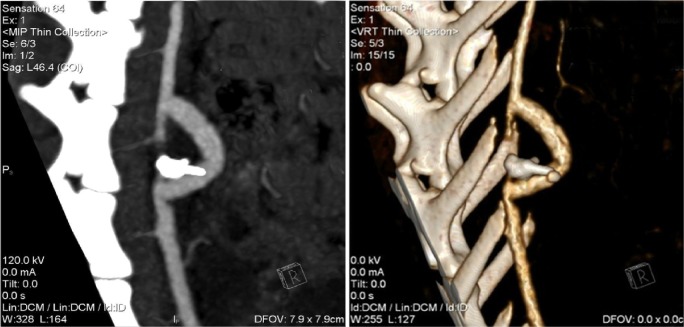
All the grafts and iliac axes were patent at four weeks postimplant and perfusion preserved in the inferior limbs. Kidneys were adequately perfused (Fig. 2).
Figure 2.
Computed tomographic angiography study. Left: contrast-enhanced imaging. Note patency of the TEGV. Right: 3D reconstruction with MIP and volume rendering algorithms.
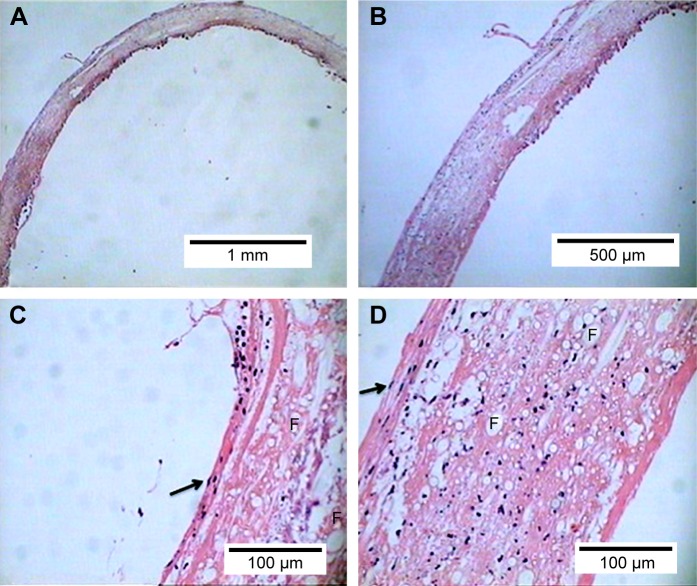
At explant the graft preserved their integrity with no signs of significant intraluminal thrombosis or exuberant foreign-body inflammatory reaction. At histology scaffolds appeared uniformly cellularized with a variety elements colonizing the scaffold fibrillar framework. Polymer fibers could be seen with cells engrafted within scaffold meshes with deposition of ECM. Interestingly, the inner surface was populated by elongated cells with rare cytoplasm, a high nucleus/cytoplasm ratio, and nuclei protruding in the lumen. This endothelial-like morphology was not encountered within the wall of the scaffold, in which cellular elements looked more irregular and immersed in an eosinophil connectival atmosphere. The outer side of the tubular graft was populated by spindle-shaped cells with characters resembling proliferating fibroblasts (Fig. 3). Taken together, these findings might reliably suggest the idea of self-colonization of the scaffold by endogenous cells that progressively acquired different phenotypes within the scaffold.
Figure 3.
Histological analysis. Hematoxylin–eosin staining. The scaffold appeared to be densely colonized by different cellular elements that progressively acquired different phenotypic characteristics according to the region of the TEVG in which they engrafted. (A) 5× magnification. (B) 10× magnification. (C) 40× magnification of the inner side of the TEVG. Note flat elongated cells with nucleus protruding in the lumen (arrow) organized in an endothelial-like fashion. (D) 40× magnification of the outer side of the TEVG. Note spindle-shaped cells reliably representing fibroblasts (arrow). F indicates fibers of polymer in both cross and long axis section.
Discussion
The two major concerns in TEVGs rely on the prevention of thrombosis and mechanical resistance of the construct when implanted in vivo. Acute graft failure for thrombotic occlusion or structural deterioration with aneurysmal degeneration or rupture are considered the main drawbacks of TEVG harnessing their actual clinical application.26–28 In this proof-of-principle study, a PCL-armored heparin-releasing PLLA graft showed good patency rate and structural integrity in a previously described model of aortoaortic bypass.24 We avoided an aortic interposition graft model for a number of reasons. First, to avoid the well-known tendency to paraplegia after infrarenal aortic cross-clamp in rabbits.29,30 Second, to simulate the clinical scenario normally characterized by end-to-side anastomoses rather than end-to-end sutured grafts. Third, to test the antithrombogenic properties of the TEVG in a condition characterized by a higher risk of turbulence and hemodynamic irregularity, as for a 90° anastomosis, rather than a full-channel straight anastomosis between conduits of similar diameters. The TEVG remained patent throughout the duration of the study and no signs of embolic or neurological events were noted. The latter acquires a significant value considering that, for the characteristics of the model used, the entire limbs perfusion was dependent on the graft and no antiplatelet agents were given as thrombosis prophylaxis. The scaffold appeared to be densely colonized by different cellular elements that progressively acquired different phenotypic characteristics according to the region of the TEVG in which they engrafted. The inner side was populated by elongated flat cells organized in an endothelial-like fashion. Cells within the scaffold wall and in the outer side showed characters of both quiescent and active fibroblast, indicating the contemporaneous presence of different degrees of cell activity in terms of neo-ECM deposition. The outer layers of the scaffolds were colonized by spindle-shaped cells, which might either represent fibroblasts or smooth muscle cells. As shown in our previous work, heparin functionalization exerted a significant influence on cell differentiation toward vascular endothelium.13 Besides the well-known action on thrombosis prevention,31,32 heparin is essential for endothelial cell adhesion and homeostasis, ameliorates cell engraftment into the scaffold, and provides signals for cells survival and differentiation.33,34 Additionally, heparin binds a number of angiogenic growth factors, such as Vascular endothelial growth factor (VEGF) and Basic Fibroblast Growth Factor (bFGF).33 Therefore, scaffold functionalization with heparin would permit to attract and concentrate in the scaffold the soluble angiogenic growth factors released in the blood stream during a vascular injury. This might reliably explain the flourishing cell colonization within the scaffold after four weeks and their differentiation toward an endothelial-like phenotype. However, specific immunophenotype analysis of both resident and blood stream cells should be performed to support this hypothesis. Conversely, the fibroblastic/smooth muscle cell phenotype observed in the outer side of the scaffold might represent either a further evolution of the same population of cells colonizing the scaffold from the blood stream or the results of a foreign body reaction from the tissue surrounding the graft. In the first case, we might reliably speculate that both biological and mechanical factors (ie, shear stress at luminal side) have influenced the differentiation of the cells. However, in the in vivo hemodynamic conditions, several other factors might be involved in these phenomena and exert specific effects on the system. In the second case, despite PLLA is known to be highly biocompatible and to not elicit strong inflammatory reactions, the connective tissue surrounding the TEVG might have constituted a fibroblastic layer around the prosthesis. Clearly, further studies are required to elucidate the mechanism underlying these findings.
Different from other approaches and in light of a translational inspiration, in this study, we did not preseed scaffolds with autologous cells. Indeed, in order to overcome many of the biological, economical, logistic, and ethical concerns, which are currently known to hurdle the clinical application of cellular-based therapy, we decided to explore a strategy that avoids the use of cells but mostly relies on the biomimetic design of the scaffold and its paracrine effect as a drug-eluting device and might, therefore, be more rapidly translatable to the clinical practice. The rationale underlying this study concerned the possibility to exploit the endogenous reparative capabilities of the body and to guide these regenerative resources toward tissue restoration by means of a tailored absorbable material. Circulating endothelial progenitor cells or bone marrow-derived cells recruited from the blood stream might therefore colonize an artificial matrix that, for its internal structure, closely simulates the native ECM atmosphere and actively emanates biological signals to support their survival and differentiation. Recruited cells would in fact differentiate within a 3D environment that closely mimics the organization of the vascular ECM and guarantees progressive cell growth and neotissue formation.
The concept of fabricating a scaffold recapitulating native ECM and delivering molecules like heparin, which are able to stimulate stem cells differentiation and also to produce crucial systemic effects, has been previously explored and validated by our group also in nonvascular fields.35–38 In this study, we aimed at developing a device able to boost the endogenous process of reendothelialization and, contemporaneously, to simplify and improve the pharmacological handling and the outcomes of the graft once in the clinical scenario. The idea of combining biomaterials with different resorption timings to progressively accompany vascular reconstitution over time is also novel. Additionally, the proposed manufacturing technique is amenable to be used in the context of GMP facilities in view of a potential translational application.
In conclusion, this preliminary study assessed the feasibility and the in vivo effectiveness of a heparin-releasing armored tubular scaffold. Scaffold has been adequately populated by endogenous cells and did not show signs of thrombosis or structural failure with no need of antiplatelet therapy. If confirmed by larger studies, we might reliably speculate that this construct could constitute an attractive alternative in the panorama of tissue engineering of vascular grafts overcoming the majority of the limits currently known for TEVG and allowing for a potentially easier clinical management.
Limitations
Among the limitations of this study, the authors acknowledge the lack of an immunohistological analysis of the cells colonizing the scaffolds to demonstrate the phenomena of endothelialization and smooth muscular differentiation speculated in the text. The scarcity of a specific antibody to detect rabbit markers limited this study. Second, the lack of a quantitative and qualitative analysis of the neo-matrix deposited within the biopolymer in order to support the findings on the vascular remodeling of the TEVG. Third, the lack of a control group implanted with a nonfunctionalized PLLA graft or with current state-of-the-art graft routinely used in clinical practice. As described earlier, our preliminary in vivo study demonstrated an excessive acute failure of the graft for thrombosis in nonheparin-functionalized scaffolds, which led us to abandon this approach. Conversely, a number of reasons prevented to add a polyethylene terephthalate or PTFE graft control group in the study. First, the technical difficulty in performing the vascular reconstruction model used considering the discrepancy in caliber of the native aorta and the smallest clinically available prosthetic graft, as no other experiences with these type of grafts are reported in the literature for a rabbit model. Second, the discrepancy in caliber would have added an additional bias considering the possibility of thrombosis because of hemodynamic irregularity or turbulence. Third, this group would have implied to administer antiplatelet agents, as normally required in clinical settings to avoid thrombosis, weakening the main aims of this study, ie, to test the actual ability of a TEVG to constitute a valid alternative to autologous conduits guaranteeing patency and mechanical resistance. In this context, a longer term follow-up would have been useful to evaluate vascular reconstitution and potential side effects. Finally, this study was only preliminary and speculative in its nature and was performed as a proof-of-principle to test the in vivo effectiveness of our previously developed armored prosthesis in terms of patency and resistance. The results of this preliminary study might warrant for further larger controlled in vivo studies to further confirm these findings.
Footnotes
ACADEMIC EDITOR: Anuj Chauhan, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 507 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: CS. Analyzed the data: FN, FdM, AR. Wrote the first draft of the manuscript: CS, PS, FS. Contributed to the writing of the manuscript: FN, AR, MC, MT. Agree with manuscript results and conclusions: AR, FS, MC, MT. Jointly developed the structure and arguments for the paper: CS, AR, FN, FS, PS. Made critical revisions and approved final version: FS, MC, MT, AR, FN. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Xu ZC, Zhang WJ, Li H, et al. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials. 2008;29(10):1464–1472. doi: 10.1016/j.biomaterials.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Yang F, Murugan R, Wang S, et al. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 3.Shinoka T, Shum-Tim D, Ma PX, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115(3):536–545. doi: 10.1016/S0022-5223(98)70315-0. discussion 545–536. [DOI] [PubMed] [Google Scholar]
- 4.Shin’oka T, Matsumura G, Hibino N, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129(6):1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 5.He W, Ma Z, Teo WE, et al. Tubular nanofiber scaffolds for tissue engineered small-diameter vascular grafts. J Biomed Mater Res A. 2009;90(1):205–216. doi: 10.1002/jbm.a.32081. [DOI] [PubMed] [Google Scholar]
- 6.Carr HM, Vohra R, Sharma H, et al. Endothelial cell seeding kinetics under chronic flow in prosthetic grafts. Ann Vasc Surg. 1996;10(5):469–475. doi: 10.1007/BF02000595. [DOI] [PubMed] [Google Scholar]
- 7.Rosenman JE, Kempczinski RF, Pearce WH, et al. Kinetics of endothelial cell seeding. J Vasc Surg. 1985;2(6):778–784. [PubMed] [Google Scholar]
- 8.Greisler HP, Cziperle DJ, Kim DU, et al. Enhanced endothelialization of expanded polytetrafluoroethylene grafts by fibroblast growth factor type 1 pretreatment. Surgery. 1992;112(2):244–254. discussion 254–245. [PubMed] [Google Scholar]
- 9.Spadaccio C, Chello M, Trombetta M, et al. Drug releasing systems in cardiovascular tissue engineering. J Cell Mol Med. 2009;13(3):422–439. doi: 10.1111/j.1582-4934.2008.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mo XM, Xu CY, Kotaki M, et al. Electrospun P. (LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25(10):1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Badami AS, Kreke MR, Thompson MS, et al. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27(4):596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Inai R, Kotaki M, et al. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10(7–8):1160–1168. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- 13.Spadaccio C, Rainer A, Centola M, et al. Heparin-releasing scaffold for stem cells: a differentiating device for vascular aims. Regen Med. 2010;5(4):645–657. doi: 10.2217/rme.10.25. [DOI] [PubMed] [Google Scholar]
- 14.Hashi CK, Zhu Y, Yang GY, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104(29):11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes CP, Sell SA, Boland ED, et al. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59(14):1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Murugan R, Ramakrishna S. Nano-featured scaffolds for tissue engineering: a review of spinning methodologies. Tissue Eng. 2006;12(3):435–447. doi: 10.1089/ten.2006.12.435. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Yoo JJ, Lim GJ, et al. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J Biomed Mater Res A. 2007;83(4):999–1008. doi: 10.1002/jbm.a.31287. [DOI] [PubMed] [Google Scholar]
- 18.Mironov V, Kasyanov V, Markwald RR. Nanotechnology in vascular tissue engineering: from nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008;26(6):338–344. doi: 10.1016/j.tibtech.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Nederberg F, Zhang L, et al. Hierarchical assembly of nanostructured organosilicate networks via stereocomplexation of block copolymers. Nano Lett. 2008;8(1):294–301. doi: 10.1021/nl0726813. [DOI] [PubMed] [Google Scholar]
- 20.Centola M, Rainer A, Spadaccio C, et al. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication. 2010;2(1):014102. doi: 10.1088/1758-5082/2/1/014102. [DOI] [PubMed] [Google Scholar]
- 21.Giannitelli SM, Accoto D, Trombetta M, et al. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014;10(2):580–594. doi: 10.1016/j.actbio.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Giannitelli SM, Mozetic P, Trombetta M, et al. Combined additive manufacturing approaches in tissue engineering. Acta Biomater. 2015;24:1–11. doi: 10.1016/j.actbio.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Lobo BL, Greene WL. ACCP Consensus Conference on Antithrombotic Therapy. Indicate specific low molecular weight heparin product and dosage. Chest. 1996;110(3):866. doi: 10.1378/chest.110.3.866-a. [DOI] [PubMed] [Google Scholar]
- 24.Tillman BW, Yazdani SK, Lee SJ, et al. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials. 2009;30(4):583–588. doi: 10.1016/j.biomaterials.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 25.De Mulder PA, Van Kerckhoven RJ, Adriaensen HF, et al. Continuous total intravenous anesthesia, using propofol and fentanyl in an open-thorax rabbit model: evaluation of cardiac contractile function and biochemical assessment. Lab Anim Sci. 1997;47(4):367–375. [PubMed] [Google Scholar]
- 26.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98(1):25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 27.L’Heureux N, Dusserre N, Marini A, et al. Technology insight: the evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4(7):389–395. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 28.Stegemann JP, Kaszuba SN, Rowe SL. Review: advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng. 2007;13(11):2601–2613. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tetik O, Islamoglu F, Goncu T, et al. Reduction of spinal cord injury with pentobarbital and hypothermia in a rabbit model. Eur J Vasc Endovasc Surg. 2002;24(6):540–544. doi: 10.1053/ejvs.2002.1753. [DOI] [PubMed] [Google Scholar]
- 30.Wisselink W, Money SR, Crockett DE, et al. Ischemia-reperfusion injury of the spinal cord: protective effect of the hydroxyl radical scavenger dimethylthiourea. J Vasc Surg. 1994;20(3):444–491. doi: 10.1016/0741-5214(94)90144-9. discussion 449–450. [DOI] [PubMed] [Google Scholar]
- 31.Chandy T, Rao GH, Wilson RF, et al. Delivery of LMW heparin via surface coated chitosan/peg-alginate microspheres prevents thrombosis. Drug Deliv. 2002;9(2):87–96. doi: 10.1080/10426500290095584. [DOI] [PubMed] [Google Scholar]
- 32.Philbrick JT. Review: extended-duration prophylaxis with heparin prevents deep venous thrombosis in hip or knee replacement. ACP J Club. 2002;136(1):9. [PubMed] [Google Scholar]
- 33.Chabut D, Fischer AM, Colliec-Jouault S, et al. Low molecular weight fucoidan and heparin enhance the basic fibroblast growth factor-induced tube formation of endothelial cells through heparan sulfate-dependent alpha6 overexpression. Mol Pharmacol. 2003;64(3):696–702. doi: 10.1124/mol.64.3.696. [DOI] [PubMed] [Google Scholar]
- 34.Serizawa N, Takei Y, Okubo H, et al. Effect of low-molecular-weight heparin on the commitment of bone marrow cells to liver sinusoidal endothelial cells in CCl(4)-induced liver injury. Hepatol Res. 2006;34(4):207–213. doi: 10.1016/j.hepres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Spadaccio C, Rainer A, De Porcellinis S, et al. A G-CSF functionalized PLLA scaffold for wound repair: an in vitro preliminary study. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:843–846. doi: 10.1109/IEMBS.2010.5626796. [DOI] [PubMed] [Google Scholar]
- 36.Spadaccio C, Rainer A, Trombetta M, et al. A G-CSF functionalized scaffold for stem cells seeding: a differentiating device for cardiac purposes. J Cell Mol Med. 2011;15(5):1096–1108. doi: 10.1111/j.1582-4934.2010.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spadaccio C, Rainer A, Trombetta M, et al. Poly-L-lactic acid/hydroxyapatite electrospun nanocomposites induce chondrogenic differentiation of human MSC. Ann Biomed Eng. 2009;37(7):1376–1389. doi: 10.1007/s10439-009-9704-3. [DOI] [PubMed] [Google Scholar]
- 38.Rainer A, Spadaccio C, Sedati P, et al. Electrospun hydroxyapatite-functionalized PLLA scaffold: potential applications in sternal bone healing. Ann Biomed Eng. 2011;39(7):1882–1890. doi: 10.1007/s10439-011-0289-2. [DOI] [PubMed] [Google Scholar]