Abstract
The proarrhythmic effects of new drugs have been assessed by measuring rapidly activating delayed-rectifier K+ current (IKr) antagonist potency. However, recent data suggest that even drugs thought to be highly specific IKr blockers can be arrhythmogenic via a separate, time-dependent pathway such as late Na+ current augmentation. Here, we report a mechanism for a quinolone antibiotic, sparfloxacin-induced action potential duration (APD) prolongation that involves increase in late L-type Ca2+ current (ICaL) caused by a decrease in Ca2+-dependent inactivation (CDI). Acute exposure to sparfloxacin, an IKr blocker with prolongation of QT interval and torsades de pointes (TdP) produced a significant APD prolongation in rat ventricular myocytes, which lack IKr due to E4031 pretreatment. Sparfloxacin reduced peak ICaL but increased late ICaL by slowing its inactivation. In contrast, ketoconazole, an IKr blocker without prolongation of QT interval and TdP produced reduction of both peak and late ICaL, suggesting the role of increased late ICaL in arrhythmogenic effect. Further analysis showed that sparfloxacin reduced CDI. Consistently, replacement of extracellular Ca2+ with Ba2+ abolished the sparfloxacin effects on ICaL. In addition, sparfloxacin modulated ICaL in a use-dependent manner. Cardiomyocytes from adult mouse, which is lack of native IKr, demonstrated similar increase in late ICaL and afterdepolarizations. The present findings show that sparfloxacin can prolong APD by augmenting late ICaL. Thus, drugs that cause delayed ICaL inactivation and IKr blockage may have more adverse effects than those that selectively block IKr. This mechanism may explain the reason for discrepancies between clinically reported proarrhythmic effects and IKr antagonist potencies.
Introduction
Drug-induced QT interval prolongation and the appearance of torsade de pointes (TdPs) are recognized as potential risks associated with the use of a wide range of noncardiovascular drugs including antibiotics [1–4]. Quinolone antibiotics have been suggested to have a class effect of blocking the human Ether-a-go-go-related gene (hERG) K+ channel expressing the rapid component of the delayed rectifier current (IKr) in the human heart, and thus prolong action potential duration (APD), which is associated with QT interval prolongation. The quinolone antibiotic sparfloxacin (SPX) has been withdrawn from U.S. drug market, because it was shown to induce QT interval prolongation and ventricular arrhythmia [5, 6]. Another quinolone, grepafloxacin, was withdrawn because it induced TdP, a polymorphic ventricular tachycardia (VT) linked to excessive QT interval prolongation [7]. Concern over the proarrhythmic effects of many other quinolone antibiotics continues to grow. In nonclinical studies, the proarrhythmic effects of clinically used quinolone antibiotics have been assessed by measuring their associated IKr antagonist potency. However, discrepancies between clinically reported proarrhythmic effects and in vitro observations exist. For example, the antibiotic moxifloxacin blocks IKr but has been associated with drug-induced long QT syndrome (LQT) only very rarely [8, 9].
Cardiac rhythm and contractility are regulated by the composite functions of cardiac myocyte ion channels. Specifically, the lengthening and flattening of action potential (AP) plateaus are determined by the sum of inward and outward currents. ICaL contribute to inward currents, maintaining the plateau phase of ventricular AP (phase 2). Inhibition of ICaL shortens the AP, whereas inhibition of outward IKr results in AP prolongation. Therefore, if both K+ and Ca2+ channels are inhibited, ICaL inhibition may counteract the IKr-blocking effects of quinolone antibiotics. Indeed, Xu et al.(2003) reported that drugs with dual blocking action against hERG K+ and Ca2+ channels are less likely to cause arrhythmias than drugs with selective blocking activity against hERG K+ current [10]. On the other hand, enhancing ICaL while blocking IKr may aggravate APD prolongation and/or generate early afterdepolarization (EAD) upstrokes.
During a normal AP, ICaL peaks early, triggering robust sarcoplasmic reticulum Ca2+ release before partially inactivating due to two processes: Ca2+-dependent inactivation (CDI), mediated by Ca2+ binding to calmodulin (CaM) tethered to the C-terminus of the channel, and voltage-dependent inactivation (VDI). The rate and degree of ICaL inactivation due to these processes during the late phase of the APD has a major effect on repolarization. This raises the possibility that instead of potentiating ICaL, modifying its shape by altering its inactivation kinetics might lead to APD prolongation and EAD. In a recent study, elimination of CDI in guinea pig ventricular myocytes via expression of Ca2+-insensitive CaM (CaM1234) was shown to produce “ultralong” Aps [11].
In the present study, we sought to determine the relevance of ICaL in APD prolongation effects of SPX. We analyzed the biophysical properties of L-type Ca2+ channels affecting APD. SPX reduced peak ICaL. However this quinolone antibiotic augmented late ICaL by attenuating CDI, which promoted APD prolongation in cardiac myocytes. In contrast, IKr blockers not associated with serious arrhythmias—ketoconazole, ciprofloxacin, enoxacin, ofloxacin, and levofloxacin produced no change in ICaL or decreased both peak and late ICaL, suggesting the role of increased late ICaL in arrhythmogenic effect. Our results suggest an importance of calcium channel inactivation in producing the arrhythmogenic effects of SPX and, as such, it is necessary to consider ICaL property changes when assessing drugs for QT prolonging and arrhythmogenic liability.
Materials and Methods
Cardiomyocyte isolation and culture
All animal care and experimental procedures complied with the National Institutes of Health guidelines, and the Institutional Animal Care and Use Committee of Konkuk University and Sungkyunkwan University approved this study. Neonatal ventricular myocytes were isolated from 1 to 2-day-old Sprague-Dawley (SD) rats (Nara Biotech, Seoul, Korea) by using a previously reported method [12]. Ventricular regions of neonatal rat hearts were excised (approximately, the lower third) and the tissues (approximately 1–2 mm) were minced on ice. The minced tissues were treated with a solution containing 0.1% collagenase (Wako, Japan), 0.1% trypsin, and 1% glucose in phosphate-buffered saline (Ca2+/Mg2+-free) at 37°C for 10 min. After the supernatant from the first digestion was removed, three 10-min digestions were performed using the same enzyme solution. The supernatants were stored in DMEM/F-12 culture medium containing 10% fetal bovine serum, 5% horse serum, penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively) in a 4°C ice chamber and the centrifuged for 7 min at 700 × g. The cell pellets were incubated at 37°C in a 95% O2 incubator for 1.5 h to attach non-cardiac myocytes to microscope cover glass. The cells were subsequently cultured on the cover glass for 3 days at 37°C and 95% O2. Cells cultured for 3–5 days were used for ICaL current recordings.
Ventricular myocytes from adult mice (> 5 months) or 2-week-old SD rats were isolated as previously described with minor modifications [13]. Briefly, ventricular myocytes were isolated by perfusing Ca2+-free NT solution containing collagenase (1 mg/ml, 4176, Worthington) and DL-dithiothreitol (1 mg/ml, D-0632, Sigma, St. Louis, MO, USA) through Langendorff columns at 37°C.
Electrophysiological recording
Cardiac myocytes were subjected to patch-clamp experiments. Whole-cell Ca2+ and Ba2+ currents were recorded using a conventional whole-cell patch-clamp configuration, outfitted with an EPC 8 patch-clamp amplifier (Heka, Germany). Voltage pulse generation was controlled using R-clamp software (R-clamp; provided by Dr. S.Y. Ryu). The data were digitized using the R-clamp software at a sampling rate of 5 kHz, after being low-pass filtered at 1 kHz. The patch pipettes were created from borosilicate glass capillaries (Clark Electromedical Instruments, Pangbourne, UK) by using a puller (PP-83; Narishige, Japan). Patch pipettes producing resistances of 1.5–2.5 MΩ in bathing solution were used. All experiments were performed at room temperature (20–25°C).
APs were recorded using a nystatin-perforated patch-clamp configuration, with an EPC10 patch-clamp amplifier (Heka, Germany). Data were digitized and current injection (125–175 pA, 9 ms) for AP generation were both controlled using Patch-Master software.
Solutions and Drugs
To record ICaL in cardiac myocytes, NMDG-Tyrode’s [143 mM N-methyl-d-glucamine (NMDG)-Cl, 5.4 mM CsCl, 0.33 mM NaH2PO4, 5 mM HEPES, 0.5 mM MgCl2, 1.8 mM CaCl2, 11 mM d-glucose, pH adjusted to 7.4 with HCl] was used as the bathing solution. The pipette solution contained 115 mM CsCl, 5 mM Mg-ATP, 10 mM HEPES, 5 mM ethylene glycol-bis (2-aminoethylether)-N,N,N,N,-tetraacetic acid (EGTA), and 5 mM creatine phosphate (disodium salt). The pH was adjusted to 7.3 by using CsOH. APs were recorded from single isolated myocytes in a perforated patch configuration by using nystatin (200 μg/ml). Normal Tyrode’s solution (143 mM NaCl, 5.4 mM KCl, 0.33 mM NaH2PO4, 5 mM HEPES, 0.5 mM MgCl2, 1.8 mM CaCl2, 11 mM d-glucose, pH adjusted to 7.4 with NaOH) was used as the bathing solution. The pipette solution for recoding APs contained 140 mM KCl, 10 mM HEPES, 5 mM EGTA, and 1 mM MgCl2, and the pH was adjusted to 7.2 with KOH.
To record INa in cardiac myocytes, 140 mM NaCl, 5 mM CsCl, 1.8 mM CaCl2, 1 mM MgCl2, 11 mM Glucose, 10 mM HEPES adjusted with NaOH (pH 7.4) was used as the bath solution. Nifedipine (1 μM), SN-6 (10 μM), and CdCl2 (100 μM) were used to block L-type Ca2+ currents, NCX currents, and T-type Ca2+ currents, respectively. The pipette solution contained 20 mM CsCl, 100 mM Cs-Asp, 10 mM EGTA, 10 mM HEPES, 20 mM TEA-Cl, 5 mM Mg-ATP adjusted to 7.25 with CsOH.
Unless otherwise stated, all chemicals and drugs were purchased from Sigma-Aldrich; SPX (Fluca, 56968) and E4031 (Sigma, M5060) were prepared as stock solutions in dimethyl sulfoxide. The drugs were diluted in the bathing solution on the day of the experiment.
Statistical analysis
The results are shown as mean ± standard error of the mean. Student’s t-tests or Fisher's exact test were performed to test for significance as appropriate using SigmaPlot. P values <0.05 were deemed to be statistically significant.
Results
SPX induces APD prolongation in the presence of IKr blocker
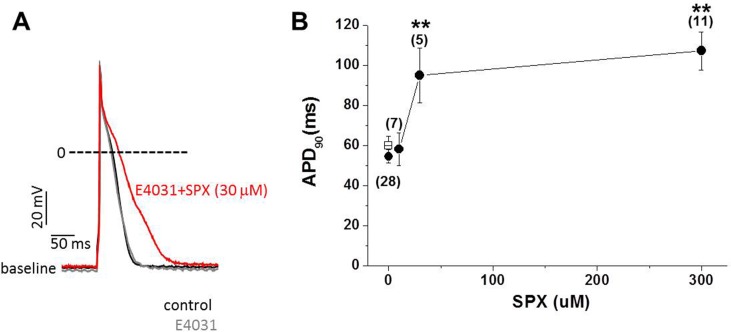
To test the hypothesis that factors other than IKr modulation play important roles in SPX- induced APD prolongation, the effects of SPX applied at concentrations between 10 and 300 μM were investigated in 2-week-old rat ventricular myocytes in the presence of E4031, a selective IKr blocker. APs were elicited by electrical stimulations delivered using a patch pipette in current clamp mode, at a stimulation frequency of 2 Hz. As shown in Fig 1A, 30 μM SPX significantly prolonged APD in the presence of 1 μM E4031. The time required for 90% repolarization (APD90), increased from 54.5 ± 3.3 to 95.0 ± 13.6 ms after 10 min of 30-μM SPX treatment (paired t-test; n = 7, P < 0.05).
Fig 1. The effect of SPX on APs recorded in the presence of E4031.
Action potentials were recorded by pacing myocytes at 2 Hz. In the presence of 10 μM E4031, SPX (10–300 μM) was applied. Data were obtained at 37°C by using the perforated patch technique. A, Examples of action potentials recorded before (gray) and after (red) SPX exposure at a concentration of 30 μM. The control action potential (black) before E-4031 treatment was also overlaid for comparison. B, APD90 was plotted as a function of SPX concentration. Asterisks indicate statistical significance (paired t-test; *P < 0.05, **P < 0.01). Error bars indicate standard error. The numbers in parentheses indicate the number of cells tested.
No changes in resting membrane potential (RMP) or in AP overshoot potentials were observed during SPX treatments. The mean RMP and overshoot potential values after SPX treatment were −68.5 mV (n = 7; P > 0.05 vs. −70.8 mV in the absence of SPX) and 53.5 mV (n = 7; P > 0.05 vs. 52.7 mV in the absence of SPX), respectively. The steady-state APD90 values obtained at various SPX concentrations are summarized in Fig 1B. These data indicate that SPX-induced APD prolongation is not only attributable to blocking IKr, but is also influenced by additional channel modulation.
Effects of SPX on ICaL
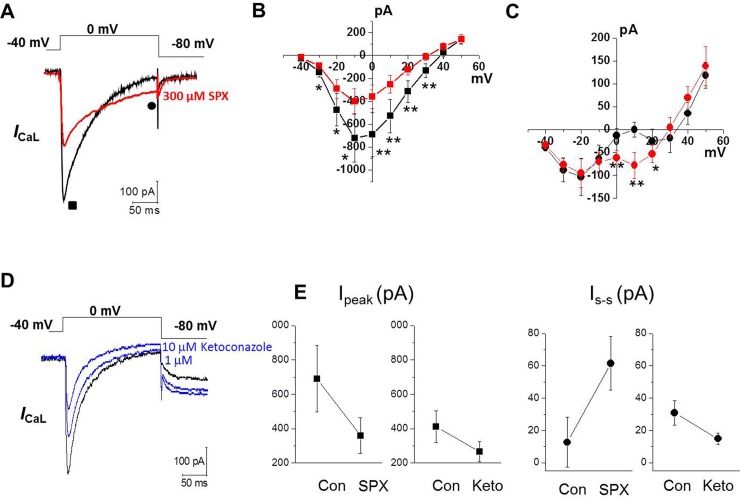
We next examined whether SPX enhances ICaL in neonatal cardiomyocytes. By holding Em at −50 mV, we could successfully isolate ICaL from ICaT measurements (S1 Fig). Therefore, we used holding potential of −50 mV for all voltage-clamp experiments, excluding those reported in Fig 2, in which we applied a double pulse at −40 mV followed by 0 mV from a holding potential of −80 mV [14]. To eliminate voltage-gated K+ currents and Na+ currents, a Cs+-rich pipette solution and Na+-free (substituted with NMDG+) bath solution was used. Fig 2A shows representative ICaL measurements under control conditions and in the presence of 300 μM SPX, recorded from the same ventricular myocytes. The amplitude of the peak ICaL was reduced after SPX treatment. The I–V curves indicated that the peak ICaL was decreased after 300 μM SPX treatment at all potentials ranging from −40 to +50 mV, without altering the I–V relationship (n = 10; Fig 2B). Inactivation, however, was slowed by SPX treatment. Slower ICaL decay values resulted in larger current amplitudes in the presence of SPX at the end of the 200 ms pulse (Fig 2C). The amplitude of this current was larger over the voltage range 0 to +20 mV—i.e. the range in which the AP plateau typically occurs.
Fig 2. Effect of SPX on ICaL.
A, Representative traces of ICaL during a 200-ms voltage-clamp pulse from −40 to 0 mV before (black) and after (red) exposure to 300 μM SPX. B–C, current–voltage (I-V) relationships of Ipeak (■, B) and Iend of pulse (●, C) under control conditions (black) and after application of 300 μM SPX (red) (n = 10). D, Representative traces of ICaL to 1 μM and 10 μM of ketoconazole (blue) (n = 4). E, The comparison of Ipeak (■, left) and Is-s (●, right) from ICaL before and after 300 μM SPX (n = 10) or 10 μM ketoconazole (n = 4). *P < 0.05 **P < 0.01.
We also examined the effects on ICaL of multiple IKr blockers that have not been associated with severe arrhythmias. Ciprofloxacin, enoxacin, ofloxacin, and levofloxacin are quinolone antibiotics with variable IKr potencies that are rarely associated with LQTS risk [15, 16]. They had no effect on ICaL (S2 Fig). We also examined the effect on ICaL of ketoconazole an antifungal agent which is known to block IKr but is not associated with TdP risk [17, 18]. Ketoconazole did decrease ICaL (Fig 2D). In contrast to SPX, however, it reduced late ICaL as well as peak ICaL (Fig 2E). Taken together, these data suggest that SPX increased late ICaL that might be related with its ability to induce arrhythmias.
Effect of SPX on the Inactivation Kinetics of ICaL
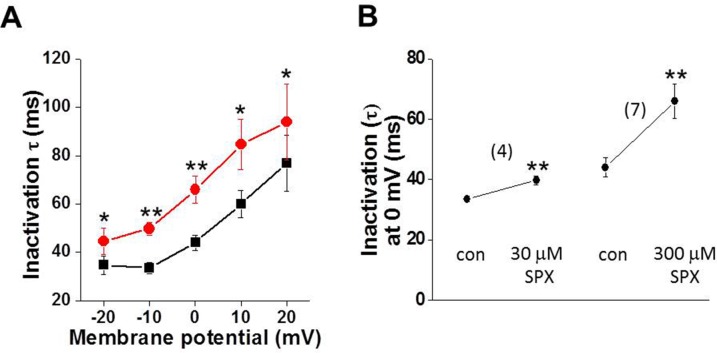
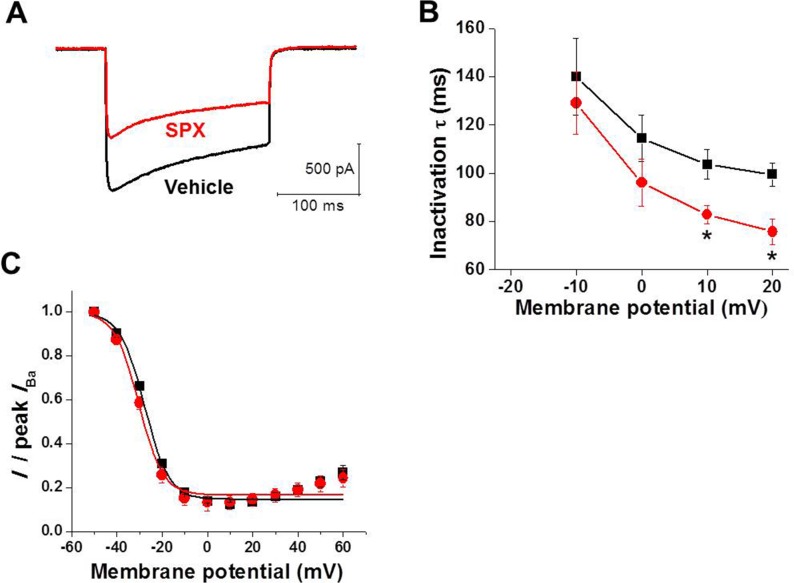
To further investigate the effect of SPX on inactivation kinetics, the decay phase of ICaL was fitted using an exponential function. When the decay phase was fitted using a monoexponential function, the time constant (τ) was significantly increased by SPX treatment (Fig 3A). The voltage dependence of τ remained unchanged in the presence of SPX. As shown in Fig 3B, the effects of SPX on inactivation kinetics were dose-dependent. Taken together, these results suggest that SPX induces APD prolongation by slowing ICaL inactivation.
Fig 3. Effects of SPX on ICaL inactivation time course.
A, Summary of the effects of SPX (300 μM) on the time course (time constants, τ) of inactivation at various membrane potentials (n = 4–5). B, Summary of the concentration-dependent slowing of ICaL inactivation by SPX at 0 mV (n = 4–5). *P < 0.05 **P < 0.01.
SPX reduces Ca2+-dependent ICaL inactivation
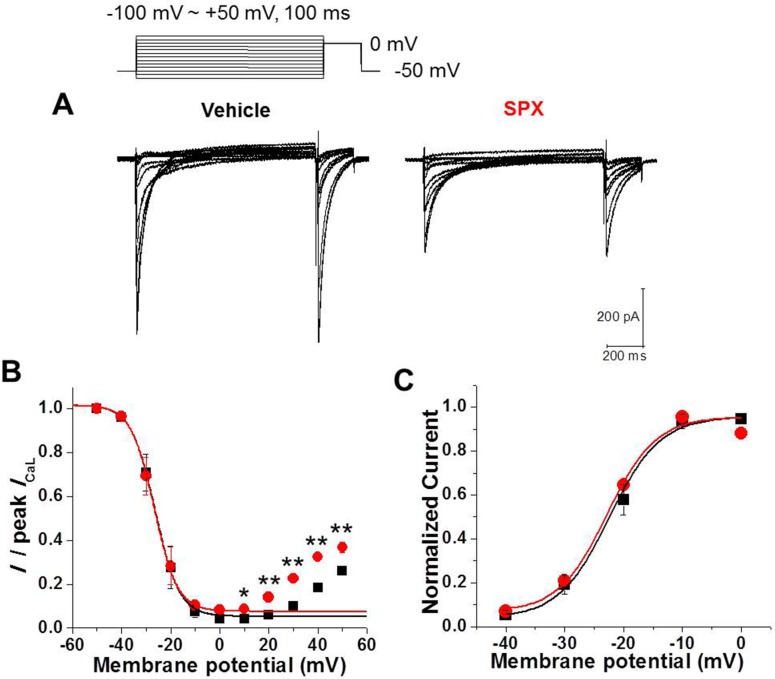
The effects of SPX on late ICaL inactivation were investigated using conventional double-pulse protocol. Prepulses 1000 ms in duration and at various potentials ranging from −50 to +50 mV in 10 mV steps preceded a 100-ms test pulse at 0 mV. The superimposed current responses to the test pulse (0 mV) are shown in Fig 4A (left, control; right, 300 μM SPX). The slow inactivating ICaL in the presence of SPX indicates that SPX exerts a pharmacological effect (Fig 4A, right).
Fig 4. Effect of SPX on the steady-state inactivation of ICaL.
A, The steady-state inactivation levels were measured by using double-pulse protocol. B, Steady-state inactivation curves for the ICaL in the absence and presence of SPX (300 μM). C, Voltage dependence of ICaL activation in the absence and presence of SPX (300 μM). The half-activation voltage was not significantly changed by SPX (n = 10, paired t-test, P > 0.05).
Under control conditions, inactivation increased sharply as the prepotential increased from −40 to −20 mV, reaching a maximum of ~95.8% at +10 mV (Fig 4B). At prepulse potentials greater than +20 mV, the extent of inactivation decreased, resulting in a U-shaped ICa,L inactivation curve. The data from −100 to +10 mV were fitted using the following Boltzmann equation:
where V is the membrane potential, V1/2 is the membrane potential of half-maximum inactivation, and k is the slope of the inactivation curve. A1 represents the maximal amplitude and A2 is the amplitude of the non-inactivating component of ICa,L. V1/2 was −26.2 ± 1.4 mV and k was +4.9 ± 0.7 mV under control condition.
The current availability curves produced in the presence of SPX (Fig 4B) indicate that SPX significantly reduced steady-state inactivation. When the data from −100 to +10 mV were fitted using the Boltzmann equation, the A2 value, the amplitude of the non-inactivating component of ICa,L, was increased by SPX (0.087 ± 0.008 vs. 0.042 ± 0.012 in control, n = 5, P < 0.01). Neither V1/2 nor k were affected (-26.6 ± 1.5 mV and +4.8 ± 0.8 mV, respectively; P > 0.05). In addition, at prepulse potentials greater than 0 mV, ICaL amplitudes in the presence of SPX were greater than those under control conditions (P < 0.01; paired t-test; n = 5).
We confirmed that no differences in the steady-state activation curves (Fig 4C) were present before and after SPX treatment. The voltages for half-activation were −22.2 ± 0.1 mV (n = 10) in the control and −23.2 ± 1.2 mV (n = 10) in the presence of SPX (paired t-test, P > 0.05). These data suggest that SPX attenuates inactivation, leading to slower ICaL decay.
L-type Ca2+ channels can be inactivated by two different mechanisms: CDI and VDI. The Ca2+-dependent aspect of L-type Ca2+ channel inactivation is dependent on Ca2+ entry. Therefore, essentially all inactivation of Ba2+ current through the L-type Ca2+ channels is voltage dependent. The substitution of Ba2+ ions for Ca2+ has been used widely to separate the contribution VDI from CDI to the macroscopic ICaL. We confirmed the results reported previously, showing the Ca2+ dependence of CDI in our cells and that the effects of SPX are changed by replacing Ca2+ with Ba2+ (Fig 5). As shown in Fig 5A, SPX did not slow Ba2+ current inactivation. SPX did not increase the inactivation time constant, but instead reduced it, indicating that the attenuation of ICaL inactivation by SPX was abolished (Fig 5B). In addition, SPX had little effect on the steady-state inactivation of Ba2+ currents (Fig 5C). These results confirm that SPX specifically modulates CDI.
Fig 5. Effects of SPX on Ba2+ currents.
A, Representative traces of ICaL with external Ba2+ replacing Ca2+ as charge carrier during a 200 ms voltage-clamp pulse from −50 to 0 mV before (black) and after (red) exposure to 300 μM SPX. B, Voltage-dependence of Ba2+ current inactivation time constants (τ) under control conditions (black) and in the presence of 300 μM SPX (red). Data points (mean ± standard error) are from 5 cells. C, The steady-state inactivation curves of Ba2+ currents under control conditions (black) and in the presence of 300 μM SPX (red) (n = 3). *P < 0.05.
Use-dependency
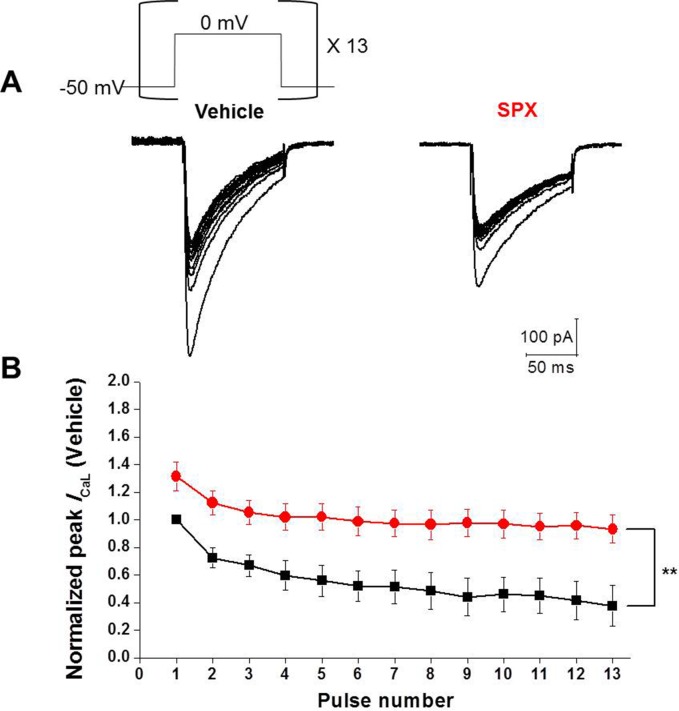
Since SPX slowed the inactivation of ICaL by inhibiting CDI, it is expected that repetitive application of depolarizing voltage steps may cause less accumulation of ICaL inactivation in the presence of SPX. In order to prove this hypothesis, a series of depolarizing step pulses at frequencies of 2Hz were applied (Fig 6A, inset). Fig 6A and 6B shows superimposed current traces of cells in the absence and presence of SPX, respectively. When the depolarizing step pulses were applied repetitively under control conditions, the ICaL peak amplitudes were gradually decreased (50.6 ± 7.3% of the initial level at pulse 13; Fig 6C). In the presence of SPX (300 μM), however, this gradual decrease was significantly attenuated, with the ICaL peak amplitude being 63.3 ± 5.7% of the initial level by pulse 13 (Fig 6C). Fig 6C illustrates the gradual decrease of ICaL peak amplitudes during repetitive pulses in the absence and presence of SPX.
Fig 6. Use-dependency of SPX on ICaL.
A, Repetitive application of depolarizing pulses (shape is shown in the figure inset) to 0 mV from a holding potential of −50 mV gradually decreased the ICaL amplitude. The frequency of depolarizing pulses was 2 Hz. In the presence of SPX, the gradual decrease of ICaL was significantly attenuated. B, Summary of the use-dependent effect of SPX. Normalized current levels are plotted against pulse number (A and B, n = 6; black, control; red, SPX). The amplitudes of steady-state currents of both control and SPX group were normalized by the first current of the control. *P < 0.05 **P < 0.01 (n = 6).
Recovery from inactivation
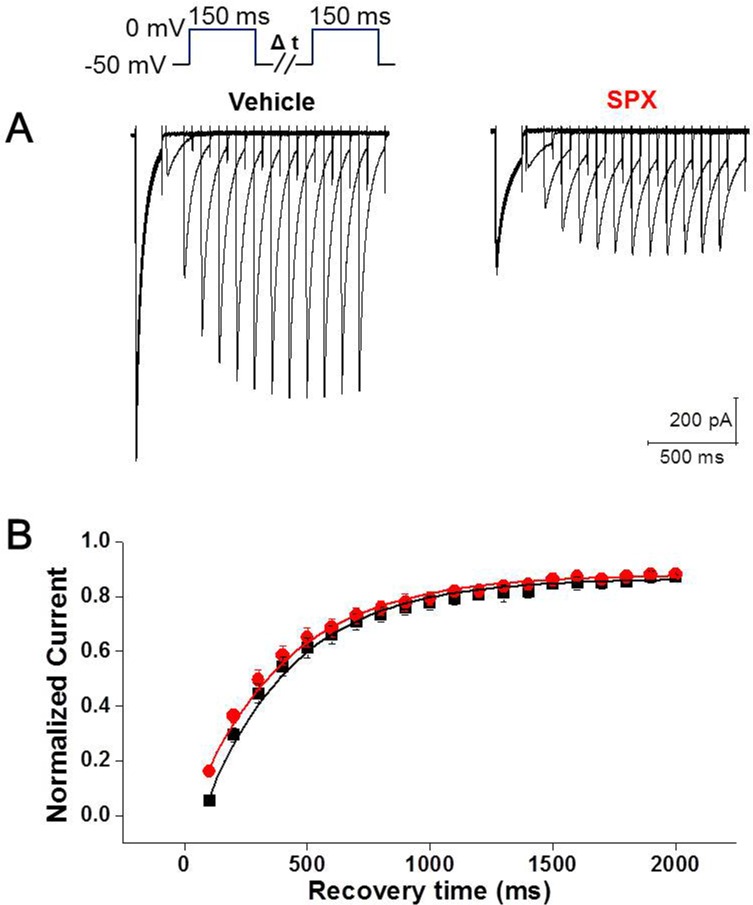
Inactivation and recovery from inactivation are closely related processes and are critical factors that determine channel function. Recovery from inactivation was investigated by eliciting sustained depolarization (200 ms), followed by recovery intervals of increasing durations, and then applying a subsequent test pulse (Fig 7A, inset). In comparison to the control, recovery from inactivation was not changed by SPX treatment (Fig 7A and 7B). Data were fitted to a single exponential function. The time constants for recovery from inactivation were 365.4 ± 20.0 ms (n = 7) in the control and 382.6 ± 22.9 ms (n = 7) in the presence of SPX (paired t-test, P > 0.05).
Fig 7. Effect of SPX on time course of the recovery from inactivation.
A, Raw current tracings in the absence and presence of SPX. The voltage pulse protocol is shown as a figure inset. B, Summary of time courses of the recovery from inactivation in the absence and presence of SPX (n = 6; black, vehicle; red, SPX). *P < 0.05 **P < 0.01.
Effect of SPX on ICaL and AP in adult mouse cardiac myocytes
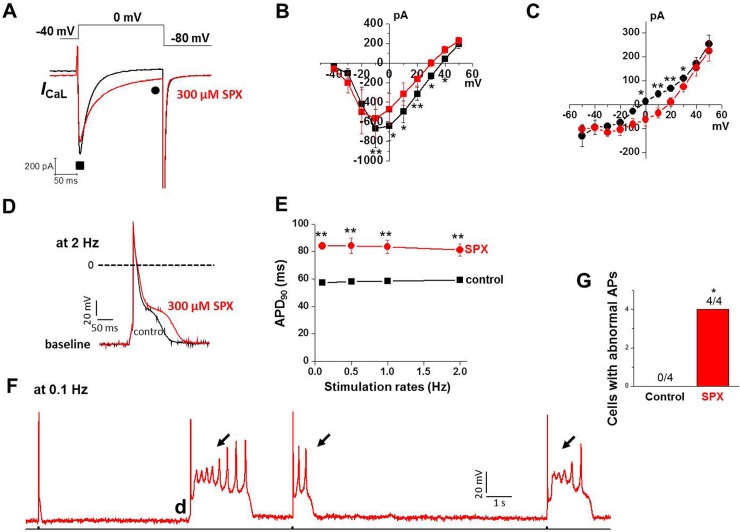
We then examined whether SPX still increases late ICaL and APDs in cardiac myocytes from adult mouse (6 month old), which is lack of native IKr. We performed experiments similar to that shown in Fig 2. Fig 8A and 8C show that late ICaL in adult mouse ventricular myocytes was significantly increased by exposure to 300 μM SPX for 10 min. Interestingly, SPX-induced reduction of peak ICaL in adult mouse ventricular myocytes was not so pronounced as that observed in neonatal cardiac myocytes (Fig 8B). Consistent with increase in late ICaL, AP prolongation was observed when adult mouse cardiac myocytes were treated with SPX (300 μM) (Fig 8D & 8E). Fig 8E summarizes these results and shows that SPX prolonged APD90 over a range of stimulation rate in adult mouse cardiomyocytes. In addition, with exposure to SPX, triggered beats arising from early and delayed afterdepolarizations were observed in all cardiac myocytes examined at slow stimulation rate (n = 4); an example is shown in Fig 8F. In the absence of drug exposure, no afterdepolarizations were observed in cells (n = 4, Fig 8G). These findings exclude the potential E4031 effect and support the idea that SPX can increase late ICaL and APDs in cardiac myocytes. Recently it was demonstrated that chronic exposure to some IKr blockers also increases cardiac late Na+ current, which is probably regarded as another mechanism for the drug-induced Q-T prolongation and TdP in patients chronically exposed to (non)-cardiac drugs in clinics [19]. We examined whether chronic exposure (5 hrs) to SPX enhances late Na+ currents in adult mouse ventricular myocytes. However no differences in late Na+ currents were observed between control and SPX-treated cells (S3 Fig), suggesting that the SPX effect on APs could not be attributed to a change in late Na+ currents.
Fig 8. Effect of SPX on ICaL and AP in adult mouse cardiac myocytes.
A, Representative traces of ICaL during a 200-ms voltage-clamp pulse from −40 to 0 mV before (black) and after (red) exposure to 300 μM SPX. B–C, current–voltage (I-V) relationships of Ipeak (■, B) and Iend of pulse (●, C) under control conditions (black) and after application of 300 μM SPX (red) (n = 4). D. SPX prolonged APs of adult mouse cardiomyocyte at a stimulation rate of 2 Hz. E, Plot of APD90 over a range of stimulation rate in adult mouse cardiomyocytes in the absence and presence of SPX. F. Example of AP recorded after 30 min exposure to SPX at a slow stimulation rate (0.1 Hz). EADs (arrows) and delayed afterdepolarization (DAD; d) were observed, G. Summary data showing frequency of afterdepolarizations in control and SPX-treated cells. Incidence of EAD or DAD was analyzed using Fisher's exact test. *P < 0.05, **P < 0.01.
Discussion
It has been argued that the extent of IKr block is imperfect at best as a predictor of effects of a drug in a human subject [20, 21]. Proposed reasons for this discrepancy include a time-dependent effect on biosynthesis of hERG channel or on cell surface trafficking [22, 23] or failure of in vitro testing to consider other ion channel actions such as ICaL or late Na+ currents [19, 24]. Our data showed that SPX markedly prolonged APD in a concentration-dependent manner in cardiac myocytes that lack IKr, suggesting that SPX can be arrhthmogenic via an IKr-independent pathway. SPX reduced peak ICaL but augmented late ICaL recorded several hundred milliseconds after a step depolarization and thus associated with APD prolongation. This effect is not seen with IKr blockers not associated with severe arrhythmias (ciprofloxacin, enoxacin, ofloxacin, and levofloxacin). We further showed that an antifungal agent ketoconazole, a potent IKr blocker without severe arrhythmias reduced both peak and late ICaL, suggesting a close relationship between late ICaL and arrhythmogenesity. Detailed analysis showed that SPX treatment reduced the Ca2+-dependent component of steady-state inactivation, indicating that SPX attenuated CDI. Consequently, the steady-state levels of ICaL were increased in the presence of SPX compared to that of the control. Consistent with the observed SPX-induced CDI attenuation, SPX had little effect on the inactivation time constant and steady-state inactivation once extracellular Ca2+ was replaced with Ba2+, a scenario in which essentially all inactivation is voltage dependent. The progressive use-dependent decrease of ICaL, which was assessed by applying repetitive voltage pulses at 2Hz, was less pronounced in the presence of SPX, indicating the positive effect of SPX on ICaL occurred in a use-dependent manner. The recovery from inactivation of ICaL was not altered by SPX. Taken together, our data suggest that SPX attenuates CDI, and the resulting slower ICaL decay might contribute to SPX‐associated EAD and TdP.
The positive effects of SPX on ICaL were concentration dependent, and SPX started to slow Ca2+ channel inactivation at a treatment concentration of 10 μM (Fig 3). Moreover, SPX-induced APD prolongation in the presence of E4031 was evident at 30 μM (Fig 1). Because the steady-state plasma concentration of SPX in healthy volunteers and patients was 1.8 μM and the hERG IC50 value is 18 μM [16], these results suggest that the slowing of ICaL inactivation may attributable to SPX-induced LQT or arrhythmia under clinical conditions.
We demonstrated that SPX attenuated late ICaL inactivation, especially at depolarized potentials (≥0 mV) without voltage shift of steady-state curve. Therefore, inactivation curve was more U-shaped in the presence of SPX (Fig 4). These results suggest that SPX specifically interrupts the Ca2+-dependent component of ICaL inactivation, having little effect on the voltage-dependent component. In support of this hypothesis, when Ba2+ was used as the ICaL charge carrier (Fig 5), SPX-induced inactivation slowing and the consequent increase in the late ICaL were abolished (Fig 5).
Our data showed that SPX treatment reduced peak ICaL amplitude as well as slowed its inactivation. These two changes have opposing effects on APD. However, the results of previous studies suggest that the kinetics of ICaL inactivation, rather than the amplitude modulates its effects on the APD restitution slope and reentry [25]. Consistent with this concept, SPX induced APD prolongation, because of the dominance of suppressed ICaL inactivation in controlling APD compared to the ongoing reduction of ICaL amplitude. In a context in which there is a concomitant reduction of repolarizing current, which should shorten APs, the slowed ICaL decay is an important factor in tipping the balance towards EAD formation.
Although the precise molecular sites that are responsible for the CDI is not entirely clear yet, it has been demonstrated that two kinds of Ca2+-binding sites model (i.e., high-affinity slow and low-affinity fast kinetic binding sites) successfully simulated the CDI obtained by experiments [26, 27]. The high affinity binding site was expected to be present very near at inner channel mouth and not to be accessible by intracellular Ca2+ buffers such as EGTA or BPATA. Therefore, this CDI is attributable by the influx of Ca2+ itself and can’t be excluded by pipette EGTA or BAPTA. It can only be excluded by the substitution of Ca2+ with other ions such as Ba2+ for the charge carrier. Classically, the ‘domain’ model of CDI can explain well this high affinity Ca2+ binding site model [26–28]. The low-affinity Ca2+ binding site can explain well the ‘shell’ model of CDI, in which global increase in cytosolic [Ca2+]i mediates the CDI [27, 28]. The Ca2+ that are released from intracellular store such as sarcoplasmic reticulum (SR) took the biggest part in the ‘shell’ or ‘low-affinity Ca2+ binding site’ models [27]. Therefore, the release-dependent inactivation (RDI) was primarily responsible for CDI in the ‘low-affinity Ca2+ binding site’ model [27]. High concentrations of pipette Ca2+ buffer such as BAPTA can effectively exclude this CDI that is mediated by the low-affinity Ca2+ binding site. Since we used pipette solution with 10 mM EGTA in the present study, it is expected that SR Ca2+ is depleted and the CICR is largely prevented. Therefore, the CDI of this study is thought to be primarily mediated by the high affinity Ca2+ binding site probably very near at the channel mouth. Taken together, the slowing of inactivation time course of ICaL by SPX was not secondary phenomenon due to the decreases in peak ICaL and intracellular [Ca2+], but due to SPX-induced specific inhibition of CDI that is mediated by high affinity Ca2+-binding site (that is, an EGTA-insensitive site). Moreover, lack of effects on the inactivation time courses by ketoconazole (Fig 2), at concentrations that inhibit the peak ICaL similarly to those of SPX, also indicates that the CDI of the present study is not mediated by the global intracellular [Ca2+] increase.
In conclusion, the present findings demonstrate the role of ICaL in SPX-induced APD prolongation. Our results suggest that modification of ICaL properties, in addition to IKr antagonistic activities, should be considered when assessing the proarrhythmic potential of drugs. Especially new drug evaluation will need to look beyond effect on peak ICaL and examine drug effects on late ICaL of which perturbation induces abnormal repolarization.
Supporting Information
L-type and T-type Ca2+ channels in neonatal rat cardiomyocytes A, Ca2+ currents were elicited by depolarizing voltage steps from a holding potential of −80 mV in the absence and presence of nifedipine (1 μM) or nifedipine (1 μM) plus NiCl2 (100 μM). B, Current–voltage (I–V) relationships of the peak Ca2+ current (holding potential −80 mV) in the absence and presence of Ca2+ channel inhibitors (black, control; red, Nifedipine; green, Nifedipine + NiCl2. C, Ca2+ currents were elicited by depolarizing voltage steps from a holding potential of −50 mV in the absence and presence of nifedipine (1 μM). D, Current–voltage (I–V) relationships of the peak Ca2+ currents in the absence and presence of nifedipine (holding potential −50 mV; black, control; red, nifedipine).
(TIF)
Effects of quinolones on ICaL Representative traces showing the effect of 1 mM ciprofloxacin (A), 1 mM enoxacin (B), 1 mM ofloxacin (C), and 1 mM levofloxacin (D) on ICaL. Representative traces of ICaL during a 200-ms voltage-clamp pulse from −40 to 0 mV before (black) and after (red) exposure to 300 μM SPX. Lower panels (E−H) summarize the concentration-response of the quinolones.
(TIF)
Chronic exposure to SPX does not increase late Na+ current A, Examples of Na+ current recorded 5 hours after isolation in the absence (vehicle; left), or in the presence of SPX (right). The selective late current blocker ranolazine did not affect Na+ current in SPX-treated cells as well as cells under control condition. B, Summary data show that there was no effect on late Na+ current of 5-hour exposure to SPX in adult mouse ventricular myocytes.
(TIF)
Acknowledgments
This work was supported by Grant [08172KFDA508] from the Korea Food and Drug Administration in 2008, Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [NRF-2009-0071242], and Basic Science Research Program through the NRF funded by the Ministry of Science, ICT & Future Planning [NRF-2012R1A2A2A01046878].
Abbreviations
- AP
action potential
- APD
action potential duration
- CDI
Ca2+-dependent inactivation
- EAD
early afterdepolarization
- hERG
human Ether-à-go-go-related gene
- ICaL
L-type Ca2+ current
- ICaT
T-type Ca2+ current
- IKr
rapidly activating delayed-rectifier K+ current
- LQT
long QT
- SPX
sparfloxacin
- TdP
Torsade de Pointes
- VT
ventricular tachycardia
- VDI
voltage-dependent inactivation
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Grant [08172KFDA508] from the Korea Food and Drug Administration in 2008, Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [NRF-2009-0071242], and the Basic Science Research Program through the NRF funded by the Ministry of Science, ICT & Future Planning [NRF-2012R1A2A2A01046878].
References
- 1.Cubeddu LX. QT prolongation and fatal arrhythmias: a review of clinical implications and effects of drugs. Am J Ther. 2003;10(6):452–7. . [DOI] [PubMed] [Google Scholar]
- 2.Raehl CL, Patel AK, LeRoy M. Drug-induced torsade de pointes. Clin Pharm. 1985;4(6):675–90. . [PubMed] [Google Scholar]
- 3.Shah RR. Pharmacogenetic aspects of drug-induced torsade de pointes: potential tool for improving clinical drug development and prescribing. Drug Saf. 2004;27(3):145–72. . [DOI] [PubMed] [Google Scholar]
- 4.Viskin S, Justo D, Halkin A, Zeltser D. Long QT syndrome caused by noncardiac drugs. Prog Cardiovasc Dis. 2003;45(5):415–27. 10.1053/pcad.2003.00101 . [DOI] [PubMed] [Google Scholar]
- 5.Olsen KM. Pharmacologic agents associated with QT interval prolongation. J Fam Pract. 2005;Suppl:S8–S14. . [PubMed] [Google Scholar]
- 6.Psaty BM. Clinical trial design and selected drug safety issues for antibiotics used to treat community-acquired pneumonia. Clin Infect Dis. 2008;47 Suppl 3:S176–9. 10.1086/591400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson ME, Mazur A, Yang T, Roden DM. Potassium current antagonist properties and proarrhythmic consequences of quinolone antibiotics. J Pharmacol Exp Ther. 2001;296(3):806–10. . [PubMed] [Google Scholar]
- 8.Bloomfield DM, Kost JT, Ghosh K, Hreniuk D, Hickey LA, Guitierrez MJ, et al. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther. 2008;84(4):475–80. . [DOI] [PubMed] [Google Scholar]
- 9.Dale KM, Lertsburapa K, Kluger J, White CM. Moxifloxacin and torsade de pointes. Ann Pharmacother. 2007;41(2):336–40. 10.1345/aph.1H474 . [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Yan GX, Wu Y, Liu T, Kowey PR. Electrophysiologic effects of SB-237376: a new antiarrhythmic compound with dual potassium and calcium channel blocking action. J Cardiovasc Pharmacol. 2003;41(3):414–21. . [DOI] [PubMed] [Google Scholar]
- 11.Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99(26):17185–90. 10.1073/pnas.262372999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung DJ, Kim JG, Won KJ, Kim B, Shin HC, Park JY, et al. Blockade of K+ and Ca2+ channels by azole antifungal agents in neonatal rat ventricular myocytes. Biol Pharm Bull. 2012;35(9):1469–75. . [DOI] [PubMed] [Google Scholar]
- 13.Yoon JY, Ahn SH, Oh H, Kim YS, Ryu SY, Ho WK, et al. A novel Na+ channel agonist, dimethyl lithospermate B, slows Na+ current inactivation and increases action potential duration in isolated rat ventricular myocytes. Br J Pharmacol. 2004;143(6):765–73. 10.1038/sj.bjp.0705969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hille B. Ion channels of excitable membranes 3rd ed. Sunderland, Mass.: Sinauer; 2001. xviii, 814 p. p. [Google Scholar]
- 15.Frothingham R. Rates of torsades de pointes associated with ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Pharmacotherapy. 2001;21(12):1468–72. . [DOI] [PubMed] [Google Scholar]
- 16.Kang J, Wang L, Chen XL, Triggle DJ, Rampe D. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol Pharmacol. 2001;59(1):122–6. . [DOI] [PubMed] [Google Scholar]
- 17.Tsai WC, Tsai LM, Chen JH. Combined use of astemizole and ketoconazole resulting in torsade de pointes. J Formos Med Assoc. 1997;96(2):144–6. . [PubMed] [Google Scholar]
- 18.Zimmermann M, Duruz H, Guinand O, Broccard O, Levy P, Lacatis D, et al. Torsades de Pointes after treatment with terfenadine and ketoconazole. Eur Heart J. 1992;13(7):1002–3. . [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C, et al. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation. 2014;130(3):224–34. 10.1161/CIRCULATIONAHA.113.007765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann P, Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. J Pharmacol Toxicol Methods. 2006;53(2):87–105. 10.1016/j.vascn.2005.07.003 . [DOI] [PubMed] [Google Scholar]
- 21.Lu HR, Vlaminckx E, Hermans AN, Rohrbacher J, Van Ammel K, Towart R, et al. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines. Br J Pharmacol. 2008;154(7):1427–38. 10.1038/bjp.2008.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, et al. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66(1):33–44. 10.1124/mol.66.1.33 . [DOI] [PubMed] [Google Scholar]
- 23.Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, et al. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 2005;312(1):316–23. 10.1124/jpet.104.073692 . [DOI] [PubMed] [Google Scholar]
- 24.Kuryshev YA, Wang L, Wible BA, Wan X, Ficker E. Antimony-based antileishmanial compounds prolong the cardiac action potential by an increase in cardiac calcium currents. Mol Pharmacol. 2006;69(4):1216–25. 10.1124/mol.105.019281 . [DOI] [PubMed] [Google Scholar]
- 25.Mahajan A, Sato D, Shiferaw Y, Baher A, Xie LH, Peralta R, et al. Modifying L-type calcium current kinetics: consequences for cardiac excitation and arrhythmia dynamics. Biophys J. 2008;94(2):411–23. 10.1529/biophysj.106.98590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markevich NI, Pimenov OY, Kokoz YM. Analysis of the modal hypothesis of Ca2+-dependent inactivation of L-type Ca2+ channels. Biophys Chem. 2005;117(2):173–90. 10.1016/j.bpc.2005.04.017 . [DOI] [PubMed] [Google Scholar]
- 27.Ryu JS, Kim WT, Lee JH, Kwon JH, Kim HA, Shim EB, et al. Analysis of factors affecting Ca(2+)-dependent inactivation dynamics of L-type Ca(2+) current of cardiac myocytes in pulmonary vein of rabbit. J Physiol. 2012;590(Pt 18):4447–63. 10.1113/jphysiol.2012.229203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budde T, Meuth S, Pape HC. Calcium-dependent inactivation of neuronal calcium channels. Nat Rev Neurosci. 2002;3(11):873–83. 10.1038/nrn959 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
L-type and T-type Ca2+ channels in neonatal rat cardiomyocytes A, Ca2+ currents were elicited by depolarizing voltage steps from a holding potential of −80 mV in the absence and presence of nifedipine (1 μM) or nifedipine (1 μM) plus NiCl2 (100 μM). B, Current–voltage (I–V) relationships of the peak Ca2+ current (holding potential −80 mV) in the absence and presence of Ca2+ channel inhibitors (black, control; red, Nifedipine; green, Nifedipine + NiCl2. C, Ca2+ currents were elicited by depolarizing voltage steps from a holding potential of −50 mV in the absence and presence of nifedipine (1 μM). D, Current–voltage (I–V) relationships of the peak Ca2+ currents in the absence and presence of nifedipine (holding potential −50 mV; black, control; red, nifedipine).
(TIF)
Effects of quinolones on ICaL Representative traces showing the effect of 1 mM ciprofloxacin (A), 1 mM enoxacin (B), 1 mM ofloxacin (C), and 1 mM levofloxacin (D) on ICaL. Representative traces of ICaL during a 200-ms voltage-clamp pulse from −40 to 0 mV before (black) and after (red) exposure to 300 μM SPX. Lower panels (E−H) summarize the concentration-response of the quinolones.
(TIF)
Chronic exposure to SPX does not increase late Na+ current A, Examples of Na+ current recorded 5 hours after isolation in the absence (vehicle; left), or in the presence of SPX (right). The selective late current blocker ranolazine did not affect Na+ current in SPX-treated cells as well as cells under control condition. B, Summary data show that there was no effect on late Na+ current of 5-hour exposure to SPX in adult mouse ventricular myocytes.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.