Abstract
Gut microbiota play a significant role in host metabolic processes, and recent metagenomic surveys have revealed that they are involved in host immune modulation and influence host development and physiology (organ development). Initially, probiotics are identified as potential therapeutics to treat gastrointestinal disorders and to revitalize the disturbed gut ecosystem. Currently, studies are exploring the potential for expanded uses of probiotics for improving the health conditions in metabolic disorders that increase the risk of developing cardiovascular diseases such as hypertension. Further investigations are required to evaluate targeted and effective use of the wide variety of probiotic strains in various metabolic disorders to improve the overall health status of the host. This review addresses the causes of hypertension and the hypotensive effect of probiotics, with a focus on their mechanistic action.
Keywords: probiotics, hypertension, ACE, gut microbiota, metabolic disorders, metagenomics
Introduction
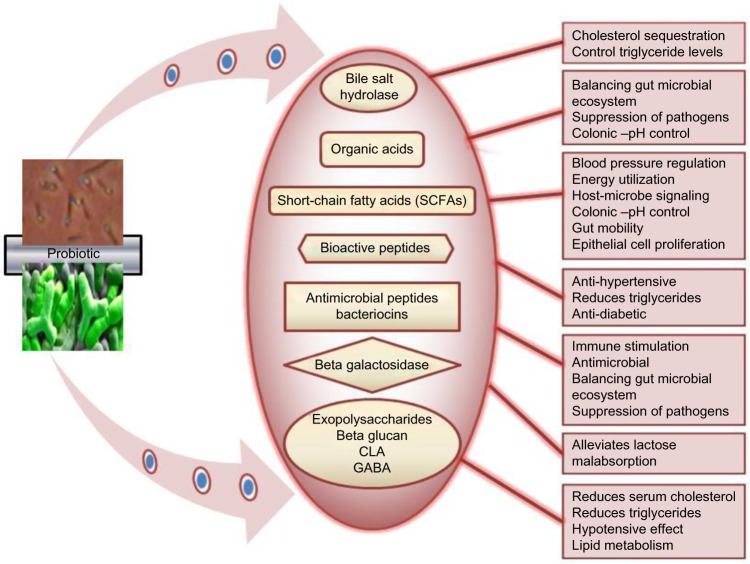
Probiotics have gained significant importance in the last few decades for their health promoting roles in the prevention and prophylaxis of various gut associated disorders, urogenital, and respiratory infections.1 They have also been shown to positively affect the host immune system through immunoglobulin production, and trigger cell-mediated immune responses as a frontline of defense.2,3 Probiotics are described as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”.4 The therapeutic potential and antimicrobial spectra of probiotics is a complex and a multifactorial process which involves the production of organic acids, hydrogen peroxide,5,6 bacteriocins, bacteriocin-like inhibitory substances,7,8 short-chain fatty acids (SCFAs), conjugated linoleic acid (CLA), and γ-amino butyric acid (GABA)9–12 (Figure 1).
Figure 1.
Probiotics and their metabolite-related health promoting functions.
Abbreviations: CLA, conjugated linoleic acid; GABA, γ-amino butyric acid.
Many studies have elucidated the health benefits and clinical effects of probiotics in gastrointestinal abnormalities including irritable bowel syndrome, irritable bowel disease, gastric ulcers, rotavirus, traveler’s and antibiotic-associated diarrhea, colorectal cancer, and in the alleviation of lactose malabsorption.13,14 Recently, probiotics have undergone scientific scrutiny for their potential in reducing the risk of cardiovascular diseases (CVDs) and they have been shown to be effective in improving the health conditions among the tested subjects with cardio-associated diseases.15–19 The estimated total number of adults with hypertension worldwide during the year 2000 was 1 billion and predicted to rise to 1.58 billion by the year 2025.20
Certain probiotic strains such as lactobacilli and bifidobacteria can effectively produce SCFAs, CLA, GABA,12,21–23 and ACE inhibitory peptides, which have shown potential hypotensive effects.24–26 Growing public awareness of diet-associated health issues has fueled the functional foods concept, foods that provide specific health benefits over and above their nutritional value,10 which can be produced under controlled fermentation conditions or enriched with added nutrients. Functional foods enhance the overall nutritional status with added vitamins and minerals including probiotics and their biogenic metabolites.27 Many studies have shown the health benefits associated with the consumption of functional foods incorporated with probiotics, such as cheese,28 milk, fermented milk products,21,29,30 and non-dairy beverages.31
In this review we emphasize the biochemistry of hypertension, diet associated disorders, and the role of probiotics in controlling elevated blood pressure (BP) levels, nutritional programming, and the application of probiotic cultures in reducing the onset and development of CVD.
Gut microbiology and human health
Metagenomics has revolutionized the field of microbiology by paving the way for culture-independent assessment and exploration of microbial communities residing in complex ecosystems. The mammalian gastrointestinal tract (GIT) is among the most densely populated with complex microbial communities and the colon merely harbors a load of ~1014 cells/host.32 The human intestinal microbiota plays a pivotal role in the host’s digestive process, gut maturation, epithelial cell development, and a regulatory switch to innate immunity, thereby contributing to host health33 and many more functions yet to be revealed which are associated with the gut microbiota. The metabolic capacity of the core gut microbiome is so prodigious that it has been considered as the “virtual organ” of the GIT,34,35 and it is known to influence human health and disease susceptibility in different ways.
Microbial colonization of the host starts during birth, and the composition of the microbiota widely varies throughout host development.36,37 A variety of factors during and after birth, such as mode of birth (vaginal versus cesarean-section), feeding (breastfed or bottle fed), and antibiotics play a significant role in shaping the gut microbiota,38 which reflects their proximity in the health-disease state equilibrium during the growth and development of their host.39 Furthermore, the possible role of early colonizers during infancy and changes in their relative composition of the gut microbiota during childhood that can lead to the accumulation of body weight and obesity has been elucidated.40 A recent study revealed the presence of unique microbiome in the human placenta and its resemblance to their oral microbiome,41 suggesting that it is imperative to map the microbial networks residing at different niches to understand their role in health and disease.
Intestinal dysbiosis, diet, and metabolic disorders
Recent studies have revealed that many health maladies are as a result of significant perturbation in core gut microbial communities, and many parameters such as host-microbe crosstalk which are intrinsically linked to the microbial ecology and gut functionality. The influence of GIT microbiota composition and their possible link to obesity,42 diabetes,43,44 neural disorders,45,46 brain development,47 insulin resistance, and other metabolic disorders48 have been well documented. Furthermore, an aberration in the core-gut microbiota in TLR-5 deficient mice leads to the development of metabolic syndrome.49 Recently, more evidence has been accumulated by deciphering the role of gut microbiota in developing CVD. For example, gut microbiota metabolizes specific dietary nutrients which belong to the trimethylamine group (eg, choline, phosphatidylcholine, and L-carnitine) resulting in the formation of the pro-atherogenic compound called trimethylamine-N-oxide (TMAO),50,51 and the carnitine metabolic- pathway associated gene clusters are identified in the genomes of human microbiota.52 Altogether, compositional sequencing approaches coupled with transcriptomics studies extrapolated the microbe-host interaction and their interplay among various metabolic and biochemical pathways at the molecular level. However, information on the key role-players is still unclear and yet to be determined for modulation or to reprogram the microbial territories to overcome the health ailments.
There are many factors in developing hypertension, such as sedentary lifestyle, lipid and cholesterol metabolism (obesity), sodium sensitivity, personal habits (alcohol consumption, smoking), anxiety, stress, and vitamin D deficiency.22 There is direct evidence of the factors that control BP such as: a well programmed nutritional strategy and lifestyle, maintaining recommended body mass index (weight loss), reduced salt intake, “dietary approaches to stop hypertension”-type dietary pattern (vegetarian diet, more fruits, vegetables, and low-fat dairy products), increased potassium intake, and moderation of alcohol intake.53,54
It has been demonstrated that the risk of developing CVD was significantly lower in vegetarians when compared to omnivores.55–57 In this context, vegetarians had a significantly higher abundance of Bacteroides species and lower abundance of Prevotella species in their core gut microbiomes when compared to omnivores, and a reduced risk of developing CVDs.58 Similarly, a study in men (aged between 41–57 years), whose diet intake chiefly includes vegetables and fruits showed a reduced risk of developing high BP.59 These studies indicate the importance of dietary habits and their influence on overall health status and GIT microbiota.
One way of modulating the gut microbiota is by the consumption of probiotics, in particular, products containing lactic acid bacteria. In this context, Lactobacillus spp. and Bifidobacterium spp. have been extensively studied as probiotic microorganisms, while other groups such as Enterococcus, Oenococcus, Propionibacterium, Bacillus, Escherichia coli, Clostridium butyricum, and some yeast strains, such as Saccharomyces boulardii, have also been used. In recent years, functional foods containing probiotics have become popular within the food industry due to the heightened awareness of consumers toward these health-promoting foods.60 Nutritional programming to manipulate the composition of the intestinal microbiota through the administration of probiotics, prebiotics, and or synbiotics (a combination of probiotic and prebiotic) continues to receive much attention for preventing or attenuating the symptoms of metabolic-related diseases.
Biochemistry of BP
The maintenance of BP homeostasis is a complex process which is carefully regulated by a variety of inputs. Hypertension or BP, defined as systolic blood pressure (SBP) above 140 mmHg and diastolic blood pressure (DBP) above 90 mmHg, is one of the key risk factors for an individual prone to many diseases including coronary heart disease, cerebral hemorrhage, renal and cardiac failure.22,61,62 BP is controlled by a number of complex biochemical pathways. Typically, the renin-angiotensin system (RAS) is known to play a key role in BP regulation and sodium metabolism. In addition to RAS, the kinin-nitric oxide system, the neutral endopepti-dase system, and the endothelin-converting enzyme systems have been shown to produce additional vaso-regulatory peptides.63 However, RAS has been identified as one of the major controllers of BP among the others identified; which play a central role in controlling the level of other key vaso-active peptides.63 ACE is a carboxypeptidase responsible for the generation of the potent vasoconstrictor angiotensin II by releasing the C-terminal dipeptide His-Leu from angiotensin I, and is also responsible for the inactivation of the vasodilator bradykinin, which gives rise to a net hypertensive effect.63 Together these systems produce a wide array of peptides that collectively regulate BP, electrolyte balance, and fluid equilibrium via membrane bound receptors located in different tissues.64 RAS comprises of: i) AGT – a globular protein which serves as a substrate for ii) renin – an enzyme that catalyzes the proteolytic conversion of AGT to angiotensin I; iii) ACE (EC 3.4.15.1), a key enzyme of the RAS which controls the arterial BP and water-salt equilibrium in the body;65 and iv) angiotensin II receptor.66 The inhibition of ACE could lead to antihypertension. Recently, the influence of two sensory receptors for SCFAs (Olfr78 and GPR41) in BP regulation has been identified.67 BP is a multifactorial trait which is regulated by multiple biochemical pathways and all the networks are firmly interlinked.
Gut microbiota, probiotics, and BP homeostasis
Genomes of lactic acid bacteria (LAB) encode an array of proteolytic cassettes and peptide transporters.68 In general, LAB are cell factories for many proteolytic enzymes (present on cell envelope and intracellular peptidases) which are involved in the hydrolysis of peptide bonds generating short oligopeptides.69,70 Probiotics have been reported to exert ACE-inhibitory activity by producing antihypertensive bioactive peptides which are released during protein hydrolysis.10,71 Similar to ACE-inhibitory peptides, other peptides, casokinins and lactokinins, are also being released during enzymatic proteolysis of milk proteins and microbial fermentations.72 Hence, fermented milk products that are rich in bioactive peptides are considered as natural dietary sources to control hypertension. In addition to that, probiotic cultures with certain traits such as exopolysaccharides,73 CLA,74 and GABA production22,75 positively influence the host lipid metabolism and gut microbial compositions (Figure 1). The SCFAs produced by gut microbes, in particular propionate modulates BP levels via Gpr41 and Olfr78 receptors. Furthermore, Olfr78 knockout mice with reduced gut microbial biomass upon antibiotic treatment showed elevated BP levels.76 Similarly, reduced microbial richness and diversity has been observed in spontaneously hypertensive rats, with an increase in Firmicutes/Bacteroidetes ratio and decrease in acetate, butyrate-producing microbes,77 clearly indicating that our gut microbiota are master regulators of hypertension.
Vitamins, minerals, and BP
Deficiency in vitamin and mineral levels are also involved in developing BP. Vitamin D has been identified as one of the key role-players, among others (vitamin C and E). An insufficient vitamin D level has been observed in 50% of the world’s population and hypovitaminosis D leads to the development of hypertension. Furthermore, the antihypertensive effects of vitamin D are mediated by renoprotection, prevention of secondary hyperparathyroidism, vasodilation, suppression of the renin-angiotensin-aldosterone system, and anti-inflammatory effects have also been validated.78,79 Studies have shown the association between vitamin D deficiency and elevated BP levels among the individuals tested.78
Oral administration of probiotic Lactobacillus reuteri National Collections of Industrial, Marine and Food Bacteria (NCIMB) 30242 (Cardioviva) increased serum vitamin D levels by 14.9 nmol/L and the levels of other vitamins (A, E and β-carotene) were unaffected.80 Intensive research is required to understand the vitamin biosynthesis pathways of probiotic bacteria in vivo. Such studies aid us in developing live vitamin delivering cultures to combat vitamin deficiencies in the gut microenviroments. Furthermore, probiotic cultures are known to produce B vitamins such as folate (vitamin B9)81 and vitamin B12,82,83 which could be interesting in cases of vitamin deficiency. Until now only one study had shown the improvement of vitamin D levels upon probiotic administration in human subjects, therefore, there is a need for more clinical trials to support this hypothesis. The beneficial role of probiotics in improving cardiovascular health and in the reduction of BP cannot be ruled out; in order to confirm this role, more extensive studies are needed to understand the mechanisms underlying probiotic action. In a review by Ness et al, some studies have shown an inverse association between plasma vitamin C levels and BP, and a few reported an inverse association with vitamin C intake.84
In a recent meta-analysis, it has been found that a daily dose of vitamin C (500 mg) for a period of 8 weeks significantly reduced DBP by 1.67±0.72 mmHg.85 In contrast, administration of 500 mg of vitamin C for 5 years had no effect on BP.86 In conclusion, the association between vitamin C and controlling BP remains unclear due to the inconsistent results observed among research studies. Many minerals are involved in controlling BP levels; among them the major minerals positively involved in BP regulation are potassium, magnesium, and calcium.87 A study has shown that consumption of yogurt containing probiotic strains (Lactobacillus casei, L. reuteri, and Lactobacillus gasseri) containing yogurt increased apparent calcium absorption in growing rats.88 The ability of probiotics and prebiotics to increase micronutrient absorption has been examined in different studies. Although the obtained results were not uniform, an increased rate of mineral absorption was noticed in probiotic groups.89 In summary, due to the lack of a large number of studies on probiotics and their associative link with increased vitamin levels and mineral absorption, this area is still unclear and a solid conclusion cannot be drawn regarding the role of probiotics with respect to vitamins and minerals. Therefore, it is advisable to monitor the key vitamin and mineral levels in probiotic clinical trials to extrapolate the link between them.
Probiotics as antihypertensive agents
A substantial body of evidence firmly supports the health benefits and clinical effects associated with probiotics and probiotic fermented foods based on in vitro and in vivo studies. In recent years probiotics and their potential role in maintaining cardiovascular and renal health has received much attention among the scientific communities. Numerous studies have shown either moderate or significant reduction in the ratios of SBP/DBP (Table 1). For example, administration of sour milk fermented with Lactobacillus helveticus LBK-16H containing bioactive tripeptides (commercialized as Evolus®; Valio Dairy, Helsinki, Finland) for 21 weeks reduced the mean SBP to 6.7 (±3.0) mmHg in 36 hypertensive subjects when compared to the control groups.26 Similarly, a mean reduction of SBP 5.2 (±8.1) mmHg and DBP 1.7 mmHg has been recorded in borderline hypertensive men (aged 23–59 years) given sour milk fermented with L. helveticus and Saccharomyces cerevisiae containing tripeptides (commercialized as Ameal S; Calpis Food Industry, Tokyo, Japan).90 It has been shown that administration of L. casei (LEx) cell lysate reduced BP, triglycerides, plasma cholesterol, and glucose levels when compared with the control group.91 In a study, oral administration of probiotic cultures Lactobacillus rhamnosus GG and Streptococcus thermophilus containing milk along with vegan food significantly improved lipid profiles and controlled the coliforms in the colon of rats.92 L. helveticus (LBK-16H strain) fermented sour milk containing ACE-inhibitory tripeptides attenuated the development of hypertension in spontaneously hypertensive rats.93 In a study, milk fermented with L. casei strain Shirota and Lactococcus lactis YIT 2027 and enriched with GABA (1 mg/mL) significantly reduced the mean SBP (17.4±4.3 mmHg) and DBP (7.5±5.7 mmHg) in mildly hypertensive patients.22 Furthermore, a meta-analysis based on 14 randomized placebo-controlled clinical trials has shown that probiotic fermented milk significantly reduced both SBP and DBP in pre-hypertensive and hypertensive subjects.94 Tanida et al showed that intraduodenal injection of Lactobacillus johnsonii La1 (1×108–9 CFU/day), or its metabolites, reduced hypertension and renal sympathetic nerve activity in urethane-anesthetized rats. This study suggests that La1 or its metabolites might lower BP by changing autonomic neurotransmission via the central histaminergic nerves and the suprachiasmatic nucleus in rats.95 In a double-blind, randomized placebo-controlled trial, consumption of a Lactobacillus plantarum 299v (2×1010/CFU/mL/day) fermented food product by 36 smokers for 6 weeks significantly reduced SBP (13±4 mmHg, P<0.001). Moreover, significant reductions were also observed in fibrinogen and low-density lipoprotein cholesterol, leptin, IL-6, and F2-isoprostane concentrations, which serve as biochemical markers for lipid peroxidation and oxidative stress.96 Lactic acid bacteria are able to metabolize the complex milk protein and aid in the release of short bioactive peptides which have ACE-inhibitory activity, thereby contributing to the modulation of hypertension.71,97,98 In another study, fermented soy milk probiotic cocktail (L. casei, Lactobacillus acidophilus, Lactobacillus bulgaricus, S. thermophilus, and Bifidobacterium longum) enriched with whey-separated bioactive peptides with high ACE-inhibitory activity positively reduced SBP in rats after 8 weeks of oral application.99 Earlier studies have shown the link between gut microbiota and TMAO levels in developing CVD. In a recent study, subjects who received probiotic L. casei Shirota (dose of 6.5×109 CFU thrice a day) for 12 weeks showed reduced levels of TMAO when compared to the control group.100 Even though the level of reduction is not significant, it is noteworthy to explore the beneficial role of probiotics in multiple aspects of improving the overall health status.
Table 1.
Anti-hypertensive effect of probiotics or probiotic fermented foods: in vivo studies
| Beneficial effect | Tested strains | Subjects | Dose (CFU) | Form | Result | Reference |
|---|---|---|---|---|---|---|
| Reduced systolic blood pressure (SBP) | Lactobacillus casei | 60 pre-diabetic patients (25–65 years old) | 7×109 | Capsule (500 mg) | SBP 3.10±2.2 mmHg | 89 |
| Lactobacillus acidophilus | 2×109 | Prebiotic | ||||
| Lactobacillus rhamnosus | 1.5×109 | Fructooligosaccharide | ||||
| Lactobacillus bulgaricus | 2×108 | |||||
| Bifidobacterium breve | 2×1010 | |||||
| Bifidobacterium longum | 7×109 | |||||
| Streptococcus thermophilus | 1.5×1010 | |||||
| Hypotensive effect | S. thermophilus | Meta-analysis | NA | NA | SBP 3.1±1.56 mmHg | 88 |
| Lactobacillus delbrueckii ssp. bulgaricus | 702 human subjects | DBP 1.09±0.06 mmHg | ||||
| L. acidophilus | ||||||
| Lactobacillus kefiri | ||||||
| Anti-hypertensive effect | Lactobacillus helveticus | 46 hypertensive men (aged 23–59 years) | 160 g/day | Sour milk (Ameal S) | SBP 5.2±8.1 mmHg | 84 |
| Saccharomyces cerevisiae | DBP 1.7 mmHg | |||||
| Reduced blood pressure | L. casei | 28 hypertensive patients (14 males and 14 females) | NA | 400 mg cell lysate (LEx) | SBP 9±2 mmHg | 85 |
| DBP 6±2 mmHg | ||||||
| Reduced blood pressure levels | L. helveticus | 36 hypertensive subjects aged 40–80 years | 95 mL/day | Fermented milk (Ameal S) | 4 weeks | 90 |
| S. cerevisiae | SBP 9.4±3.6 mmHg | |||||
| 8 weeks | ||||||
| SBP 14.1±3.1 mmHg | ||||||
| DBP 6.9±2.2 mmHg | ||||||
| L. helveticus LBK-16H | 39 hypertensive subjects | 150 mL/day | Fermented milk (Evolus®) | SBP 6.7±3.0 mmHg | 91 | |
| DBP 3.6±1.9 mmHg | ||||||
| Reduction in high blood pressure levels | L. helveticus CM4 | Total 80 subjects | 12 g/day | Tablet | High–normal group | 92 |
| 40 – high–normal BP | SBP – no significant change | |||||
| 40 – mild hypertension (MH) | DBP 5.0±0.1 mmHg | |||||
| MH group | ||||||
| SBP 11.2±4.0 mmHg | ||||||
| DBP 6.5±0.1 mmHg | ||||||
| Reduced blood pressure levels | L. helveticus LBK-16H | 17 mild-hypertensive subjects | 150 mL/day | Fermented milk (Evolus®) containing Ile-Pro-Pro and Val-Pro-Pro tripeptides | 7.3% reduction | 26 |
| Lowering blood pressure | L. casei Strain Shirota | 39 MH patients 16 women and 23 men (aged between 28–81 years) | 100 mL/day | Fermented milk containing GABA | SBP 17.4±4.3 mmHg | 22 |
| DBP 7.2±5.7 mmHg | ||||||
| Lactococcus lactis YIT 2027 | ||||||
| Mean age 54.2 years | ||||||
| Lowers blood pressure | L. helveticus LBK-16H | 60 subjects (36 men, 24 women) | 150 mL/day | Fermented milk containing | 10 weeks (mean) | 93 |
| SBP 2.3 mmHg | ||||||
| 2.5–2.7 mg/150 mL | DBP ±0.5 mmHg | |||||
| Ile-Pro-Pro and Val-Pro-Pro tripeptides | ||||||
| Reduces blood pressure, triglyceride, and cholesterol levels | Group 1 | 70 healthy, | 450 mL/day | Fermented milk | 8 weeks mean | 94 |
| S. thermophilus | overweight, and | (yogurt) | Group 1 | |||
| (2 cultures) + | obese subjects | ΔSBP 4.4±1.8 mmHg | ||||
| L. acidophilus | 20 males | ΔDBP 3.4±1.5 mmHg | ||||
| (2 cultures) | 50 females | Group 2 | ||||
| Group 2 | 18–55 years old | ΔSBP 8.0±2.3 mmHg | ||||
| S. thermophilus | ΔDBP 4.0±2.3 mmHg | |||||
| (2 cultures) + | Group 3 | |||||
| Enterococcus faecium | ΔSBP 2.6±3.1 mmHg | |||||
| (Causido®) GAIO | ΔDBP 0.8±2.0 mmHg | |||||
| Group 3 | ||||||
| S. thermophiles | ||||||
| (2 cultures) + | ||||||
| L. rhamnosus | ||||||
| Significant reduction in SBP, cholesterol, and triglyceride levels | S. thermophilus | 20 healthy adults | 200 mL/day | Fermented milk containing whey protein concentrate | Significant reduction in SBP | 101 |
| TMC1543 | 6.8×108/mL and 2.6×107 respectively | (P<0.05) | ||||
| L. casei TMC0409 | ||||||
| Reduced blood pressure and body mass indexes | Lactobacillus plantarum | 40 subjects | 50 g/day | Probiotic cheese | Morning | 102 |
| TENSIA | ΔSBP 12.2±1.5 mmHg | |||||
| ΔDBP 4.0±0.9 | ||||||
| Evening | ||||||
| ΔSBP 8.8±0.9 mmHg | ||||||
| ΔDBP 1.6±1.2 mmHg |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; NA, not available.
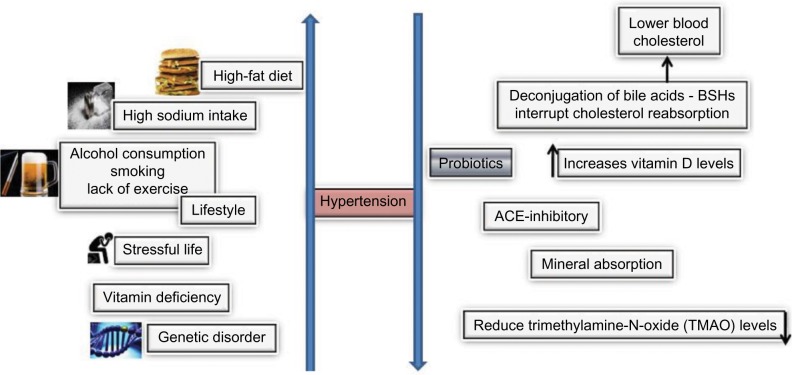
Altogether these studies support the antihypertensive activity of probiotics and consumption of probiotic fermented foods for improving overall health status and reducing the risk of developing CVDs. It is noteworthy that regulation of hypertension via administration of probiotics is cross-linked with several different mechanisms, such as improving lipid levels, triglyceride levels, bile acid deconjugation, and controlling body mass index (Figure 2). In addition, an increase in absorption of nutrients, phytoestrogens (act as vasodilatory factors), and reduction in plasma glucose levels may also influence the probiotic effect in BP regulation.17,65 Furthermore, this area of research needs to be examined thoroughly in more clinical studies to postulate the effect of probiotics in the regulation of hypertension.
Figure 2.
Causative agents of hypertension and potential modes of probiotic action on hypertension.
Abbreviation: BSHs, bile salt hydrolases.
Conclusion
An increasing number of clinical trials supporting the probiotic-dependent attenuation of hypertension and hypercholesterolemia could provide immense support for the application of such cultures to improve cardiovascular health. Hence, dietary intervention to correct gut microbiota could be an innovative nutritional therapeutic strategy for hypertension. The knowledge obtained on probiotic potential against CVDs is still at infancy stage and current findings suggest that hypotensive effects of probiotics are very promising and worth exploring to promote cardiovascular health.
However, more studies are required for a better understanding of gut microbiota-host crosstalk and biochemical networks underlying control of hypertension. As BP is interlinked with other metabolic disorders, it is necessary to examine the outcomes in a meticulous manner to get a clear picture of probiotic action against CVDs.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787–796. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73(2 Suppl):444S–450S. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 3.Klaenhammer TR, Kleerebezem M, Kopp MV, Rescigno M. The impact of probiotics and prebiotics on the immune system. Nat Rev Immunol. 2012;12(10):728–734. doi: 10.1038/nri3312. [DOI] [PubMed] [Google Scholar]
- 4.Food and Agriculture Organization of the United Nations/World Health Organization . Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. FAO/WHO; 2001. [Accessed December 19, 2015]. Available from: ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf. [Google Scholar]
- 5.Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000;66(5):2001–2005. doi: 10.1128/aem.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atassi F, Servin AL. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii ncc933 and vaginal strain Lactobacillus gasseri ks120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol Lett. 2010;304(1):29–38. doi: 10.1111/j.1574-6968.2009.01887.x. [DOI] [PubMed] [Google Scholar]
- 7.Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG. Bac-teriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A. 2007;104(18):7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: A probiotic trait? Appl Environ Microbiol. 2012;78(1):1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (lab) Crit Rev Food Sci Nutr. 1999;39(1):13–126. doi: 10.1080/10408699991279187. [DOI] [PubMed] [Google Scholar]
- 10.Stanton C, Ross RP, Fitzgerald GF, Sinderen DV. Fermented functional foods based on probiotics and their biogenic metabolites. Curr Opin Biotechnol. 2005;16(12):198–203. doi: 10.1016/j.copbio.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Vandenbergh PA. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiology Reviews. 1993;12(1–3):221–237. [Google Scholar]
- 12.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113(2):411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 13.Upadrasta A, Stanton C, Hill C, Fitzgerald G, Ross RP. Tsakalidou E, Papadimitriou K. Stress responses of lactic acid bacteria. Springer US; 2011. Improving the stress tolerance of probiotic cultures: Recent trends and future directions; pp. 395–438. [Google Scholar]
- 14.Sudha RM, Bhonagiri S. Efficacy of Bacillus coagulans strain unique is-2 in the treatment of patients with acute diarrhea. International Journal of Probiotics and Prebiotics. 2012;7(1):33–37. [Google Scholar]
- 15.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5(6):719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fariborz A, Aziz H. Dairy probiotic foods and coronary heart disease: A review on mechanism of action. Intech; 2012. [Google Scholar]
- 17.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64(4):897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 18.Liong MT. Probiotics: a critical review of their potential role as antihypertensives, immune modulators, hypocholesterolemics, and perimenopausal treatments. Nutr Rev. 2007;65(7):316–328. doi: 10.1111/j.1753-4887.2007.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 19.Lye HS, Kuan CY, Ewe JA, Fung WY, Liong MT. The improvement of hypertension by probiotics: Effects on cholesterol, diabetes, renin, and phytoestrogens. Int J Mol Sci. 2009;10(9):3755–3775. doi: 10.3390/ijms10093755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y. Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive wistar-kyoto rats. Br J Nutr. 2004;92(3):411–417. doi: 10.1079/bjn20041221. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Shirai T, Ochiai H, et al. Blood-pressure-lowering effect of a novel fermented milk containing [gamma]-aminobutyric acid (gaba) in mild hypertensives. Eur J Clin Nutr. 2003;57(3):490–495. doi: 10.1038/sj.ejcn.1601555. [DOI] [PubMed] [Google Scholar]
- 23.Hennessy AA, Ross RP, Devery R, Stanton C. The health promoting properties of the conjugated isomers of alpha linolenic acid. Lipids. 2011;46(2):105–119. doi: 10.1007/s11745-010-3501-5. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Ledesma B, Amigo L, Ramos M, Recio I. Angiotensin converting enzyme inhibitory activity in commercial fermented products. Formation of peptides under simulated gastrointestinal digestion. J Agric Food Chem. 2004;52(6):1504–1510. doi: 10.1021/jf034997b. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto A, Hanagata H, Matsumoto E, Kawamura Y, Koizumi Y, Yanagida F. Angiotensin I converting enzyme inhibitory activities of various fermented foods. Biosci Biotechnol Biochem. 1995;59(6):1147–1149. doi: 10.1271/bbb.59.1147. [DOI] [PubMed] [Google Scholar]
- 26.Seppo L, Kerojoki O, Suomalainen T, Korpela R. The effect of a Lactobacillus helveticus lbk-16 h fermented milk on hypertension: A pilot study on humans. Milchwissenschaft. 2002;57(3):124–127. [Google Scholar]
- 27.Stanton C, Gardiner G, Meehan H, et al. Market potential for probiotics. Am J Clin Nutr. 2001;73(2 Suppl):476S–483S. doi: 10.1093/ajcn/73.2.476s. [DOI] [PubMed] [Google Scholar]
- 28.Ross RP, Fitzgerald G, Collins K, Stanton C. Cheese delivering biocultures–probiotic cheese. Australian Journal of Dairy Technology. 2002;57:71–78. [Google Scholar]
- 29.Xiao JZ, Kondo S, Takahashi N, et al. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J Dairy Sci. 2003;86(7):2452–2461. doi: 10.3168/jds.S0022-0302(03)73839-9. [DOI] [PubMed] [Google Scholar]
- 30.Itsaranuwat P, Al-Haddad KS, Robinson RK. The potential therapeutic benefits of consuming ‘health-promoting’ fermented dairy products: a brief update. International Journal of Dairy Technology. 2003;56(4):203–210. [Google Scholar]
- 31.Gawkowski D, Chikindas ML. Non-dairy probiotic beverages: the next step into human health. Benef Microbes. 2013;4(2):127–142. doi: 10.3920/BM2012.0030. [DOI] [PubMed] [Google Scholar]
- 32.Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bocci V. The neglected organ: bacterial flora has a crucial immunostimulatory role. Perspect Biol Med. 1992;35(2):251–260. doi: 10.1353/pbm.1992.0004. [DOI] [PubMed] [Google Scholar]
- 35.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336(6086):1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter J, Ley R. The human gut microbiome: Ecology and recent evolutionary changes. Annu Rev Microbiol. 2011;65:411–429. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 38.Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Dogra S, Sakwinska O, Soh SE, et al. Dynamics of infant gut micro-biota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. MBio. 2015;6(1):e02419–e02414. doi: 10.1128/mBio.02419-14. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalliomäki M, Carmen Collado M, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 41.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 43.Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 45.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 46.Foster JA, Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Curr Psychiatry Rep. 2013;15(2):337. doi: 10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121(6):2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-n-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Y, Jameson E, Crosatti M, Schäfer H, Rajakumar K, Bugg TDH, Chen Y. Carnitine metabolism to trimethylamine by an unusual riesketype oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension a scientific statement from the American heart association. Hypertension. 2006;47(2):296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 54.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkins DJ, Kendall CW, Marchie A, et al. The Garden of Eden–plant based diets, the genetic drive to conserve cholesterol and its implications for heart disease in the 21st century. Comp Biochem Physiol A Mol Integr Physiol. 2003;136(1):141–151. doi: 10.1016/s1095-6433(02)00345-8. [DOI] [PubMed] [Google Scholar]
- 57.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus a systematic review and meta-analysis. Circulation. 2010;121(21):2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miura K, Greenland P, Stamler J, Liu K, Daviglus ML, Nakagawa H. Relation of vegetable, fruit, and meat intake to 7-year blood pressure change in middle-aged men the Chicago western electric study. Am J Epidemiol. 2004;159(6):572–580. doi: 10.1093/aje/kwh085. [DOI] [PubMed] [Google Scholar]
- 60.Shanahan F, McCarthy J. Functional foods and probiotics: time for gastroenterologists to embrace the concept. Curr Gastroenterol Rep. 2000;2(5):345–346. doi: 10.1007/s11894-000-0030-z. [DOI] [PubMed] [Google Scholar]
- 61.Peters J. Molecular basis of human hypertension: the role of angiotensin. Baillières Clin Endocrinol Metab. 1995;9(3):657–678. doi: 10.1016/s0950-351x(95)80672-5. [DOI] [PubMed] [Google Scholar]
- 62.Marc Y, Llorens-Cortes C. The role of the brain renin-angiotensin system in hypertension: implications for new treatment. Prog Neurobiol. 2011;95(2):89–103. doi: 10.1016/j.pneurobio.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 63.FitzGerald RJ, Murray BA, Walsh DJ. Hypotensive peptides from milk proteins. J Nutr. 2004;134(4):980S–988S. doi: 10.1093/jn/134.4.980S. [DOI] [PubMed] [Google Scholar]
- 64.Morgan L, Pipkin FB, Kalsheker N. Angiotensinogen: molecular biology, biochemistry and physiology. Int J Biochem Cell Biol. 1996;28(11):1211–1222. doi: 10.1016/s1357-2725(96)00086-6. [DOI] [PubMed] [Google Scholar]
- 65.Ebel B, Lemetais G, Beney L, et al. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr. 2014;54(2):175–189. doi: 10.1080/10408398.2011.579361. [DOI] [PubMed] [Google Scholar]
- 66.Griendling KK, Murphy TJ, Alexander RW. Molecular biology of the renin-angiotensin system. Circulation. 1993;87(6):1816–1828. doi: 10.1161/01.cir.87.6.1816. [DOI] [PubMed] [Google Scholar]
- 67.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5(2):202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu M, Bayjanov JR, Renckens B, Nauta A, Siezen RJ. The proteolytic system of lactic acid bacteria revisited: a genomic comparison. BMC Genomics. 2010;11:36. doi: 10.1186/1471-2164-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordon JI. Honor thy gut symbionts redux. Science. 2012;336(6086):1251–1253. doi: 10.1126/science.1224686. [DOI] [PubMed] [Google Scholar]
- 71.Korhonen H. Milk-derived bioactive peptides: From science to applications. Journal of Functional Foods. 2009;1(2):177–187. [Google Scholar]
- 72.FitzGerald RJ, Meisel H. Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br J Nutr. 2000;84(Suppl 1):S33–37. doi: 10.1017/s0007114500002221. [DOI] [PubMed] [Google Scholar]
- 73.London LEE, Kumar AHS, Wall R, et al. Exopolysaccharide-producing probiotic lactobacilli reduce serum cholesterol and modify enteric microbiota in ApoE-deficient mice. J Nutr. 2015;144(12):1956–1962. doi: 10.3945/jn.114.191627. [DOI] [PubMed] [Google Scholar]
- 74.Marques TM, Wall R, O’Sullivan O, et al. Dietary trans-10, cis-12-conjugated linoleic acid alters fatty acid metabolism and microbiota composition in mice. Br J Nutr. 2015;113(5):728–738. doi: 10.1017/S0007114514004206. [DOI] [PubMed] [Google Scholar]
- 75.Lyte M, Cryan JF, Wall R, et al. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- 76.Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110(11):4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. 2009;6(10):621–630. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 79.Kienreich K, Tomaschitz A, Verheyen N, et al. Vitamin D and cardiovascular disease. Nutrients. 2013;5(8):3005–3021. doi: 10.3390/nu5083005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(7):2944–2951. doi: 10.1210/jc.2012-4262. [DOI] [PubMed] [Google Scholar]
- 81.Santos F, Wegkamp A, de Vos WM, Smid EJ, Hugenholtz J. High-level folate production in fermented foods by the B12 producer Lacto-bacillus reuteri JCM1112. Appl Environ Microbiol. 2008;74(10):3291–3294. doi: 10.1128/AEM.02719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taranto MP, Vera JL, Hugenholtz J, De Valdez GF, Sesma F. Lactobacillus reuteri CRL1098 produces cobalamin. J Bacteriol. 2003;185(18):5643–5647. doi: 10.1128/JB.185.18.5643-5647.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santos F, Vera JL, van der Heijden R, et al. The complete coenzyme b12 biosynthesis gene cluster of Lactobacillus reuteri CRL1098. Microbiology. 2008;154(Pt 1):81–93. doi: 10.1099/mic.0.2007/011569-0. [DOI] [PubMed] [Google Scholar]
- 84.Ness AR, Chee D, Elliott P. Vitamin C and blood pressure–an overview. J Hum Hypertens. 1997;11(6):343–350. doi: 10.1038/sj.jhh.1000423. [DOI] [PubMed] [Google Scholar]
- 85.Juraschek SP, Guallar E, Appel LJ, Miller ER., 3rd Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95(5):1079–1088. doi: 10.3945/ajcn.111.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim MK, Sasaki S, Sasazuki S, Okubo S, Hayashi M, Tsugane S. Lack of long-term effect of vitamin c supplementation on blood pressure. Hypertension. 2002;40(6):797–803. doi: 10.1161/01.hyp.0000038339.67450.60. [DOI] [PubMed] [Google Scholar]
- 87.Houston MC. Treatment of hypertension with nutraceuticals, vitamins, antioxidants and minerals. Expert Rev Cardiovasc Ther. 2007;5(4):681–691. doi: 10.1586/14779072.5.4.681. [DOI] [PubMed] [Google Scholar]
- 88.Ghanem KZ, Badawy IH, Abdel-Salam AM. Influence of yoghurt and probiotic yoghurt on the absorption of calcium, magnesium, iron and bone mineralization in rats. Milchwissenschaft. 2004;59(9):472–475. [Google Scholar]
- 89.Sheridan PO, Bindels LB, Saulnier DM, et al. Can prebiotics and pro-biotics improve therapeutic outcomes for undernourished individuals? Gut Microbes. 2014;5(1):74–82. 90. doi: 10.4161/gmic.27252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mizushima S, Ohshige K, Watanabe J, et al. Randomized controlled trial of sour milk on blood pressure in borderline hypertensive men. Am J Hypertens. 2004;17(8):701–706. doi: 10.1016/j.amjhyper.2004.03.674. [DOI] [PubMed] [Google Scholar]
- 91.Nakajima K, Hata Y, Osono Y, Hamura M, Kobayashi S, Watanuki M. Antihypertensive effect of extracts of Lactobacillus casei in patients with hypertension. Journal of Clinical Biochemistry and Nutrition. 1995;18(3):181–187. [Google Scholar]
- 92.Al-Okbi SY, Mohamad D, Hamed T, Afifi AA, Mohamad SH. Reduction of the risk of cardiovascular diseases through dietary mixtures and probiotic. The Medical Journal of Cairo University. 2010;78(2) [Google Scholar]
- 93.Sipola M, Finckenberg P, Santisteban J, Korpela R, Vapaatalo H, Nurminen M-L. Long-term intake of milk peptides attenuates development of hypertension in spontaneously hypertensive rats. J Physiol Pharmacol. 2001;52:745–754. [PubMed] [Google Scholar]
- 94.Dong JY, Szeto IM, Makinen K, et al. Effect of probiotic fermented milk on blood pressure: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110(7):1188–1194. doi: 10.1017/S0007114513001712. [DOI] [PubMed] [Google Scholar]
- 95.Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. Effects of intraduodenal injection of Lactobacillus johnsonii la1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett. 2005;389(2):109–114. doi: 10.1016/j.neulet.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 96.Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002;76(6):1249–1255. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
- 97.Donkor ON, Henriksson A, Singh TK, Vasiljevic T, Shah NP. Ace-inhibitory activity of probiotic yoghurt. International Dairy Journal. 2007;17(11):1321–1331. [Google Scholar]
- 98.Hayes M, Stanton C, Slattery H, et al. Casein fermentate of Lac-tobacillus animalis DPC6134 contains a range of novel propeptide angiotensin-converting enzyme inhibitors. Appl Environ Microbiol. 2007;73(14):4658–4667. doi: 10.1128/AEM.00096-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsai JS, Lin YS, Pan BS, Chen TJ. Antihypertensive peptides and γ-aminobutyric acid from prozyme 6 facilitated lactic acid bacteria fermentation of soymilk. Process Biochemistry. 2006;41(6):1282–1288. [Google Scholar]
- 100.Tripolt NJ, Leber B, Triebl A, Köfeler H, Stadlbauer V, Sourij H. Effect of Lactobacillus casei Shirota supplementation on trimethylamine-n-oxide levels in patients with metabolic syndrome: An open-label, randomized study. Atherosclerosis. 2015;242(1):141–144. doi: 10.1016/j.atherosclerosis.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 101.Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci. 2000;83(2):255–263. doi: 10.3168/jds.S0022-0302(00)74872-7. [DOI] [PubMed] [Google Scholar]
- 102.Sharafedtinov KK, Plotnikova OA, Alexeeva RI, et al. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients–a randomized double-blind placebo-controlled pilot study. Nutr J. 2013;12:138. doi: 10.1186/1475-2891-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]