Abstract
Anatomical discoveries and a growing appreciation of the knee as a complex organ are driving innovations in patient care decision-making following anterior cruciate ligament (ACL) injury. Surgeons are increasing their efforts to restore combined mechanical-neurosensory ACL function and placing more consideration on when to reconstruct versus repair native anatomical structures. Surgical options now include primary repair with or without reinforcing the injured ACL with suture-based internal bracing, and growing evidence supports biological augmentation using platelet-rich plasma and mesenchymal stem cells to enhance tissue healing. Physical therapists and athletic trainers are increasing their efforts to facilitate greater athlete cognitive engagement during therapeutic exercise performance to better restore nonimpaired neuromuscular control activation amplitude and timing. Knee brace design and use needs to evolve to better match these innovations and their influence on the rehabilitation plan timetable. There is a growing appreciation for the multifaceted characteristics of the rehabilitation process and how they influence neuromuscular, educational, and psychobehavioral treatment goal achievement. Multiple sources may influence the athlete during the return to sports process and clinical outcome measures need to be refined to better evaluate these influences. This update summarizes contemporary ACL surgical, medical, and rehabilitation interventions and future trends.
Keywords: arthroscopy, knee, function, outcomes, decision-making
Introduction
This update provides an overview of knee surgical anatomy, neurological and biomechanical function, the influence of anterior cruciate ligament (ACL) injury on peripheral and central neurosensory and neuromotor function, upregulation of hip and downregulation of knee extensor muscle activation promoting a more hip biased knee extension strategy, contemporary ACL repair or reconstruction surgical approaches, the efficacy of biological augmentation methods such as platelet-rich plasma (PRP) and mesenchymal stem cells to facilitate ACL healing and remodeling, the evidence basis regarding postsurgical brace use, therapeutic exercise and psychobehavioral factor considerations, long-term neuromuscular function changes, the efficacy of returning to the same sport at the same intensity level, the influence of friends, family and significant others on athlete recovery, and improving clinical outcomes assessment efficacy.
Surgical anatomical discoveries and growing understanding
ACL surgery has become progressively less invasive and more anatomic. There is a growing appreciation for attempting to match native ACL anatomy and function during its reconstruction or repair.1 Associated with this is a better understanding that the ACL functions in close synchrony with other knee tissues.2 We still have much to learn regarding the subtleties of neuromuscular control activation responsiveness with knee joint loading.3 Interestingly, native ACL,1,4–10 meniscus,11 and associated knee structure surgical anatomy12–15 continues to be debated and elucidated. A growing body of research evidence suggests that an anatomical ACL reconstruction approach may be superior to a nonanatomical approach, particularly when remnant tissue is preserved.16,17 However, it remains to be seen how much of this new anatomical and functional information may translate into improved long-term clinical outcomes. Future ACL reconstruction or repair interventions will likely focus on improving the knee surgeon’s ability to more closely restore ACL insertional entheses,6,7 its ribbon-like bundle morphology,4 preserve the synovial sheath and its neurosensory-rich remnant tissue,16,17 and better manage associated medial collateral or anterolateral knee ligament injuries.2,18 As the evidence basis increases regarding the surgical anatomy, neurological function, and biomechanical function of these important structures, so will our ability to more precisely select and apply the appropriate surgical, medical, and rehabilitative intervention at precisely the right time during recovery.
ACL reconstruction simulates native mechanical function more closely than sensory function
Ahn et al19–21 have emphasized the importance of preserving the ACL remnant following injury to enhance future neurosensory function and improve clinical outcomes. Proximal and distal remnants of the torn ACL are mechanoreceptor-rich tissues. The surgical approach recommended by their group has incorporated a posterior arthroscopic portal to better identify the femoral ACL insertion while minimizing damage to vital remnant tissue.16,17 Currently, little is known about the precise mechanism by which synovial sheath and associated remnant tissue preservation may enhance the neurosensory function of the reconstructed ACL. Conceivably, it may assist with more effectively re-establishing neurosensory messaging from the center of the knee joint to the spinal cord (peripheral) and brain (central), thereby facilitating greater neuromuscular response precision.16,17 Whether through an anatomical single or double bundle surgical approach, the current state of ACL reconstruction is far more effective at replicating ACL mechanical than neurosensory function. Regarding the latter, we currently have very limited evidence supporting that this important component ever approaches premorbid conditions following ACL reconstruction, no matter how innovative the surgical, medical, or rehabilitative approach.
Central representation changes and the sensorimotor cerebral cortex
Since reflexive neuromuscular pathways driven by neurosensory information from the native ACL are not fully re-established following surgery, premorbid ACL afferent information levels are not likely re-established within the sensory cortex.22–26 This change in central representation may facilitate compensatory movement plan programming in the motor cortex region of the brain.22,23 In the presence of deficient neurosensory signals from the ACL, this phenomenon may lead to the development of compensatory upregulation in hip and ankle extensor function and downregulation in knee extensor function.24–26 The use of a surgical approach that better preserves remnant neurosensory function would more likely avoid this tendency. With neuromuscular control activation training, the individual who has sustained an ACL injury may be able to become less dependent upon higher level brain function to maintain dynamic knee stability through more balanced lower extremity joint contributions. Through this type of training with a more intact neurosensory system, they may be able to activate protective neuromuscular responses with greater acuity and precision as they effectively perform movement tasks.3,25,26 Although neuromuscular control activation training can somewhat restore a less impaired balance between hip, knee, and ankle contributions to composite lower extremity extension, regular movement-specific training long after the rehabilitation and return to sports decision-making process may be needed to maintain this important function, particularly in the presence of a compromised sensorimotor system.3,25–27
Quadriceps inhibition, hip muscle facilitation?
It is well known that the quadriceps femoris or knee extensor muscle group is inhibited following ACL injury and surgery, particularly in the presence of knee joint effusion.24 When this occurs, hip extensor (including the hamstring muscle group) and ankle plantar flexor activation may be upregulated to compensate for impaired knee extensor function.24,28 Of potentially greater concern than a purely sagittal plane explanation of compensatory knee function are the frontal and transverse plane imbalances that may occur in the presence of upregulated hip abductor-external rotator neuromuscular activation and vastus medialis inhibition.24,28 Inhibition of the quadriceps femoris following ACL injury and surgery may be associated with progressive rectus femoris, hip abductor-external rotator, hip extensor, and ankle plantar flexor (particularly soleus) stiffness. Stiffness associated with heightened neuromuscular activation and/or overuse of these muscles may compromise lower quarter and low back region joint mobility and tissue extensibility in positions of function such as running, sprinting, sudden directional change movements/agility tasks, kicking, jumping, and jump landing.24 Hip region neuromuscular stiffness in the presence of impaired knee extensor function is related to associated frontal plane trunk and core region dysfunction which may further compromise knee function and safety.29 In association with this lack of neurosensory signals from the reconstructed ACL, the reflex-mediated hamstring muscle group protective function that existed prior to knee injury may not be replicated. Autogenous graft tissue harvest, whether bone-patellar tendon bone, semitendinosus-gracilis, or quadriceps tendon, further compromises neuromuscular knee function in addition to contributing to harvest site–related comorbidities.
Graft, preserve, replace with synthetics, or grow a new one
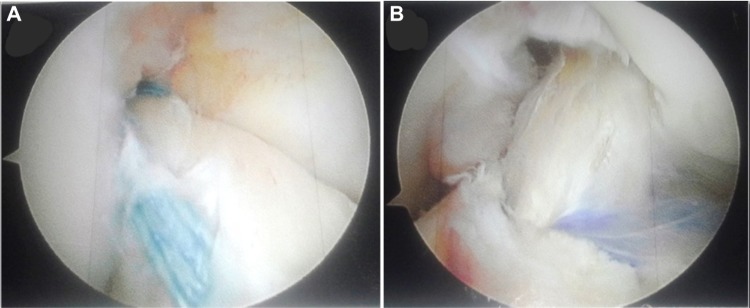
Traditional ACL graft options include autogenous bone-patellar tendon-bone or hamstring grafts as well as irradiated allograft alternatives (which have fallen into disfavor due to increased sudden failure rates among highly active younger individuals).30 In addition to these options, contemporary alternatives include soft tissue quadriceps tendon autografts or nonirradiated allografts,31,32 the “Ligament Augmentation and Reconstruction System”,33 xenografts,34 and in the not too distant future, laboratory developed grafts.35,36 Given the growing appreciation for restoring native ACL anatomy and both biomechanical and neurosensory function, current surgical innovations suggest strong potential for achieving a closer fusion between surgical and native ACL anatomy through primary repair with possible use of internal bracing (Figure 1A) rather than reconstruction (Figure 1B) for select cases.37–39 Internal bracing also provides an additional option for repairing adjacent knee tissues that function in synchrony with the ACL, such as the medial collateral (Figure 2) and anterolateral ligaments.2,38,40 With more ACL repair or reconstruction options, the previous “mechano-centric” surgical and rehabilitative focus will likely evolve to a greater decision-making balance between both mechanical and neurosensory function restoration, surgically preserving and enhancing the function of as much viable, proprioception-enhancing tissue as possible through insightful rehabilitation approaches.41
Figure 1.
(A) Primary ACL repair using suture-based internal bracing system. (B) Single-socket, double-bundle ACL reconstruction using soft tissue quadriceps tendon autograft with aperture and extra-cortical fixation.
Abbreviation: ACL, anterior cruciate ligament.
Figure 2.
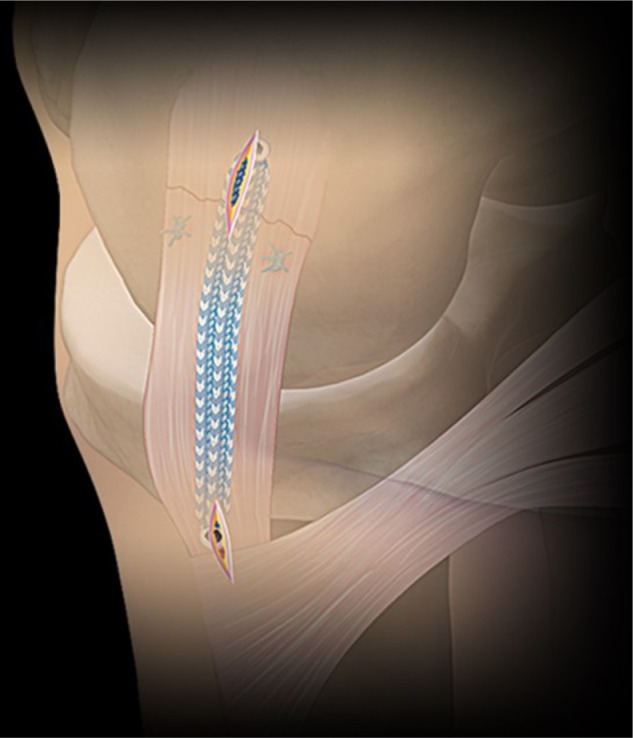
Primary medial collateral ligament repair using suture-based internal bracing system.
Note: This image provided courtesy of Arthrex, Inc.
The evidence basis of PRP and mesenchymal stem cells for ACL treatment
Increasing use of biologic tissue healing enhancement agents such as PRP and mesenchymal stem cells may improve the knee surgeons’ ability to accelerate the ACL repair or reconstruction healing timetable.42 Evidence supports PRP use to promote graft and graft harvest site healing; however, it does not support its use to facilitate bone tunnel integration or to prevent tunnel widening.42 Although these biologically mediated treatments may expand treatment options, much remains to be learned regarding the precise biochemical product that is needed to enhance a particular healing response, what delivery method to use, what dose to apply, how to achieve dosage consistency, when to apply the treatment, whether or not leukocytes should be included in the treatment,43,44 and what sources should be used.45 Much also remains unknown about what specific aspect of the healing response truly benefits from either PRP or mesenchymal stem cell enhancement. It is not known if the true effects are more anabolic, catabolic, or inhibitory and how this might influence the recovery timetable. Increasing PRP43,44 and mesenchymal stem cell45–47 use is beginning to exceed the level of scientific evidence that supports its efficacy. To date, much remains to be learned about the precise links between enhanced histological, biochemical, and biomechanical tissue properties and improved clinical outcomes. Because of these unknowns, the physical therapist, athletic trainer, and strength and conditioning specialists who treat patients following ACL surgery are confronted with difficult decisions regarding how to effectively apply, measure, and balance exercise dosages and joint tissue loads when these products are used. This is very fertile ground for interdisciplinary research between medical, surgical, and rehabilitation team members.
Bracing, not bracing, and weaning from the brace
When a patient limits weight bearing through prolonged crutch use, the lower extremity tissues get weaker and the patient becomes more dependent on the crutches.48 This same analogy likely exists with regard to long-term functional knee brace use. According to Wolff’s law, tissues that are not progressively loaded are unlikely to get stronger, potentially being “stress-shielded”.48,49 Frontal plane motion control to avoid sudden valgus-varus loading and sagittal plane motion control (to avoid hyperextension) either post-ACL injury, postsurgery, or prophylactically for primary prevention is supported by research evidence (Figure 3).50 Greater focus on collateral ligament and associated knee tissue repair, healing, and restoration of biomechanical integrity, including both surgical and/or biologically mediated treatment at the time of the index ACL surgery, may change the purpose, use timetable, and material properties of future knee braces to better match changing treatment goals across the rehabilitation plan. Current practice generally includes postsurgical brace use during the initial week or two of recovery to protect the quadriceps inhibited knee from experiencing sudden flexion under weight-bearing loads.50 After several months of rehabilitation, the patient is generally fit for a functional knee brace with greater transverse plane knee motion control as they progress to the more intense multiplanar joint loads associated with sport-specific training. Functional knee brace use is then often continued over the remainder of the first postsurgical year, particularly when soft tissue grafts or allografts are used and if the patient participates in high contact or collision sports such as football. The evidence base supporting post-surgical knee brace use tends to decrease across the healing continuum following ACL surgery.50–54 Even following knee brace prescription, safe training without brace use is essential to better simulate the loads needed for tissue healing, collagen deposition and remodeling, progressive restoration of biomechanical tissue integrity, and to develop a more responsive neuromuscular control activation system. With the increased focus on tissue repair and biologically augmented treatments that are intended to progressively restore tissue biomechanical properties, knee brace designs and use strategies both need to evolve beyond simply attempting to prevent premature graft or repair failure. The traditional singular focus on postsurgical knee joint protection must be balanced more with concerns related to the potential for knee brace–induced maladaptive lower extremity kinematic compensations, range of motion restrictions, and neuromuscular activation inhibition.50–54 Likewise, knee brace use needs to better match patient needs at each phase of the return to sports continuum. Over time the rehabilitation team needs to have a planned strategy to progressively wean the patient away from brace use once adequate biomechanical tissue integrity, near normal neuromuscular function, and high-level performance function have been re-established (Figure 4).
Figure 3.

Off-road motorcycle racer performing intensive primarily frontal plane agility challenge with functional knee brace support.
Figure 4.
American football athlete performing forward lunge-rotation (A); lateral slides with integrated shoulder elevation focusing on integrated upper-lower extremity and core neuromuscular activation and postural control using the Theraband CLX system (B); and full-speed pole cutting agility challenge with functional knee brace support (C).
The therapeutics of rehabilitation
Physical therapists and athletic trainers have a “wide palette” of therapeutic modality options to consider in treating patients following knee injury, repair, or reconstruction.27 The ultimate modality, however, in any well-designed rehabilitation program is the therapeutic exercises that drive the restoration of normal activity-specific functional movements, self-efficacy, fear-avoidance, and realistic self-appraisal of true functional capabilities and concerns.27 From the clinician’s perspective, it is important to focus on interventions designed to effectively address physical function, educational, and psychobehavioral goals in combination. Examples for this include attempting to improve self-efficacy while restoring lower extremity power (Figure 5), developing neuromuscular control activation while maintaining cognitive awareness (Figure 6), and learning how to effectively integrate trunk and core regions into sport movements in a manner that enhances both lower extremity injury prevention and performance (Figure 7).24 With appropriate selection and order, the therapeutic exercise environment is ideal for teaching proper body mechanics during the performance of high-quality, functionally relevant movements, addressing metabolic energy system demands, nutritional and recovery needs, and discussing appropriate work: rest intervals, in addition to targeted physical performance goals. The therapeutic exercise environment is also ideal for teaching, and achieving psychobehavioral treatment goals such as improving self-efficacy, decreasing fear avoidance and kinesiophobia. Well-learned, functionally valid therapeutic exercise lessons translate to increased athlete compliance, safer return to sports, a better understanding of the need to periodize training over the course of both the athletic season and offseason, and more successful treatment outcomes.27,55–58 Over the course of the rehabilitation plan, it is essential that the returning athlete be regularly and progressively exposed to comparable physical, environmental, and psychological stressors to which they will be exposed, in the sport that they will be returning to, as part of a comprehensive, progressive presport acclimatization process.
Figure 5.

Soccer athlete performing single-leg crossover hopping task with proper body mechanics instruction without knee brace support.
Figure 6.
Soccer, Taekwondo, and basketball athletes performing single-leg neuromuscular control tasks on Bosu ball without knee brace support.
Figure 7.
Soccer athlete performing single-leg lateral step-up with medicine ball held overhead (A) and progressive height two-legged hopping (B) to train self-efficacy and balanced lower extremity power without knee brace support.
Returning to the same sport at the same or higher level
Much has been recently discussed regarding the decision-making process needed to determine when a patient is ready to safely return to unrestricted sports participation following ACL surgery.24 This decision-making process requires information related to actual and perceived physical function, psychobehavioral status, general conditioning level, and many other factors.3 Multiple inputs from multiple sources generally leads to the most accurate assessment. On the basis of the pre- and postsurgery Tegner Activity Level Scale scores of 99 subjects post-ACL reconstruction, Rodriguez-Roiz et al59 reported that 91.9% of patients returned to regular sports activity, and 75% returned within the first postoperative year. However, only 51 patients (51.5%) perceived that they had returned to the same level within their sport of choice. Twenty-four percent of patients changed their sport because of reinjury fear (66%), less time available to participate (11%), knee pain (17%), or knee instability (6%). In a systematic review and meta-analysis of papers that discussed return to sports following ACL reconstruction and rehabilitation, Ardern et al60 reported that 81% of subjects returned to any sport. However, only 65% returned to their preinjury level within their preferred sport, and only 55% returned to a competitive level sport following surgery. Key factors that increased the likelihood for a successful return to preinjury level sport participation included symmetrical hop test performance, younger age, male sex, playing elite sport, and having a positive psychological response. The authors suggested that some athletes may change sports postsurgery, change priorities regarding sport participation, or return to participation at a lower level than the preinjury level and still be satisfied with their outcome. Return to sports success determinants should be individualized and based on the patient’s goals.60
In studying 31 patients between 18 and 40 years of age at ≥2 years postprimary ACL reconstruction for factors that influenced sports cessation, Tjong et al61 identified fear, priority, and personality as key factors. Fear was the most encountered factor. Fear, however, was not limited solely to the fear of reinjury. This fear also included nonphysical factors such as potential income loss, fear of having to repeat the rehabilitation process, and fear of skill deficiency at their preferred sport. Changes in life priorities affected nearly half of the subjects who did not return to sport. Many patients were in transitional life stages moving from high school to university life, from being single to being married with a family, and changing from being a full-time student to working full time. These changes led to widely ranging shifts in life priorities. Subjects who returned successfully to their sport placed a higher priority on exercise for stress relief. The third factor was personality. Self-motivated, highly competitive, and “Type A” personality type individuals (high ambition, energy, and competitiveness) were more likely to return to sport. Cautious individuals, those who possessed a more relaxed life outlook, procrastinators, or individuals who lacked self-confidence were less likely to return to sports. Only one subject in this cohort reported knee joint laxity and decreased knee range of motion impairments as the reasons for not returning to sport. In a systematic review, Czuppon et al62 reported that higher postsurgical quadriceps femoris strength, less knee effusion, lower pain levels, less instability, lower kinesiophobia, greater athletic confidence, higher presurgery knee self-efficacy, and higher presurgery self-motivation were all factors associated with a successful return to sport following ACL reconstruction.
Numerous diverse factors can influence how an athlete feels about returning to their sport following ACL surgery.59–62 Motivation, fear, cognitive appraisal, or numerous other perceptions may necessitate the need to modify a given patient’s rehabilitation plan in a highly individualized manner. Time for reflection is needed. The knee joint may be structurally and functionally sound. However, it could just be that the athlete has changed their mind regarding the role of a particular sport in their life and may be ready to move on to other activities. This may be particularly true in revision cases where attempting to return to sports that possess a combination of high reinjury risk and frequent pivoting requirements combine to increase the likelihood of the knee condition progressing to osteoarthritis.
The injury is permanent and the function changes
According to Erickson et al,63 most college and professional American football team physicians recommend return to sport by 6 (55.8%) or 9 (12.3%) months post-ACL reconstruction. No surgeon recommended waiting ≥12 months before returning to sport.63 Additionally, most surgeons (64%) did not recommend knee brace use by football running backs once they were returned to play following ACL reconstruction using a bone patellar tendon bone autograft (86%).
Just because the ACL has been reconstructed and physical function has been largely restored through rehabilitation and focused sport-specific training does not mean that premorbid conditions have been re-established. Despite advances in surgical, medical, and rehabilitative strategies, the current state of ACL reconstruction does not remotely replicate the native ligament in terms of neurosensory function, regional bone mineral density, insertional entheses, anatomy, or physiology.
Physical therapists and athletic trainers have long professed about the need to re-establish strength, power, and functional performance test bilateral equivalence to approximately 80% to >90% as a criteria to achieve return to sports readiness status. Others have designed more elaborate algorithms combining multiple inventories including functional performance tests, clinical examination, perceived function and psychobehavioral inventories, and biomechanical testing.64,65 Regardless of the specific criteria and the threshold score that signals safe return to play readiness, current outcomes research suggests that normalcy in terms of restoring the premorbid condition seldom if ever occurs. Additionally, the possible negative influence of the index ACL injury and surgery on contralateral lower extremity function suggests that comparisons based solely on “noninjured” lower extremity function may be of limited validity.66 Although some athletes return to the same competitive level in the same sport in which they sustained their index ACL injury, most do not. In addition to aforementioned physical and psychobehavioral factors, variables such as subject age at the time of the index ACL injury, socioeconomic considerations regarding continued sports participation, and changing motivations over time may influence the return to sport decision-making process. Given the knee joint neurosensory compromise that occurs when reconstructing a sensitive neurosensory structure with a comparatively “sensory inert” graft, it is not surprising that compensatory neuromuscular control activation, increased hip region muscle stiffness, fast twitch muscle fiber atrophy at the quadriceps femoris, and gastrocnemius of the involved lower extremity and imbalanced lower extremity loading patterns occur to enable continued function.25,26,56 Following ACL injury and surgery, patients display a greater tendency to employ a hip strategy when performing progressive step-up and jumping tasks. This enables the gluteus maximus and hamstring muscles to substitute for quadriceps femoris function through the knee for composite lower extremity extension during closed kinetic chain exercises and movements.25,26,56 An essential part of knee joint recovery is the re-establishment of balanced hip, knee and ankle contributions to composite lower extremity extension.24
With well-designed rehabilitation, this increased hip postural control strategy bias can be somewhat mediated and athletes can function at very close to preinjury levels. This may be more likely when neurosensory ACL function is either preserved or restored. However, long-term maintenance training is needed to prevent recurrent quadriceps femoris inhibition.24 Factors to monitor also include regular thigh and calf girth assessments to evaluate fast twitch muscle fiber health, high knee flexion-hip extension range of motion (Figures 8A and B), gluteal muscle extensibility (Figure 8C) verification, lower extremity neuromuscular control activation responsiveness to perturbation and the capacity to effectively regulate intrinsic neuromuscular stiffness, bone mineral density in the ACL surgery region of interest,57,66 central representation differences,22,23 and long-term psychobehavioral considerations.24,58 Lastly, instrumented anterior translational laxity and pivot shift glide measurements should be monitored to ensure that mechanical graft or repair integrity is not being compromised as the athlete progresses through more demanding sport-specific tasks.
Figure 8.
Quadriceps femoris and hip flexor stretching (including rectus femoris). Start position (A) and end position (B). Crossed knee to chest gluteal region stretch (C).
Notes: Both stretches are maintained for 30 seconds for two repetitions at each lower extremity.
The others
The surgical, medical, and rehabilitation team play an important role in helping the athlete manage the cognitive appraisal of their condition and of return to play readiness. Others, however, may have equal if not greater influences. Whether participating in a team or in an individual sport, the athlete who is recovering from an ACL injury and surgery may be influenced by unrealistic and often bewildering information offered by many people and other information sources such as internet websites. Individuals who often contribute their opinion include family members, coaches, teammates, friends, and teachers. Some may advise that the athlete is trying to return too quickly, while others insist that the athlete should have been back on the field several months earlier. Some of these individuals may state that they or someone they know experienced a similar injury and recovered more quickly. Despite the fact that they possess no details of case similarities or differences, that does not stop them from pontificating about what the athlete can expect to experience during the recovery process, sometimes even over the remaining years of their life! Statements such as these may increase the athlete’s anxiety level building upon their desire to demonstrate how hard the work they put in during rehabilitation has paid off. This may lead them to attempt tasks prior to permission from the rehabilitation team. The same athlete may also fear that they might disappoint their supporters given the disparity between their own cognitive appraisal of their condition and that of others.
Coaches have a strong passion to win.67 Their contributions to an athlete’s recovery no matter how well intended may be inherently biased due to the importance of winning to either keep or to enhance their coaching position and prestige. Even the coach who encourages an athlete to delay returning to a sport for safety reasons likely expects total recovery upon release. They often do not consider how extended playing time, back-to-back games, playing both sides of the scrimmage line in American football, or repetitious lower extremity impact loading may increase the athlete’s knee reinjury risk. Teammates may also influence the athlete to return too early through their desire to win, or to return later, possibly to increase playing time for themselves.
Following ACL injury, surgery and rehabilitation athletes are confronted with widely ranging and often diametrically opposed opinions from a wide variety of significant, and unsolicited, “not so significant” others. True advocacy focuses on defusing confrontational situations, and helping the athlete navigate through a plethora of puzzling information in the presence of often daily changes in physical function and cognitive appraisal perceptions. The injured athlete needs time to discuss, and reflect about their injury. They also need to know that moving on to another sport or recreational interest is not equivalent to failure on their part. There is a growing appreciation for monitoring an athlete’s psychobehavioral status throughout the rehabilitation process, how these characteristics translate in conjunction with improved physical function, the athlete’s desire to return to sports participation, and how these decisions may change across the life span.
Improving clinical outcome measurement methods
The tried and true mantra following orthopedic surgery intervention is that nothing spoils good results like long-term outcomes. Another mantra that may be equally if not more appropriate is that nothing spoils good results like measuring all essential factors in a valid manner and with high precision. These factors include much more than just objective and subjective physical function levels, knee joint laxity, and impairment level neuromuscular strength and range of motion measurements. Factors such as self-efficacy, kinesiophobia, cognitive reinjury risk perceptions, and health locus of control should be evaluated as well.3,24,58 Release back to unrestricted sports participation timing based solely on time postsurgery does not assure that adequate neuromuscular control activation has been re-established to protect the surgical knee joint.68
For the return to unrestricted sports participation plan to be most effective, it should represent a layered or tiered approach that bridges key transition periods within the recovery continuum (ie, effective quadriceps femoris activation, weight bearing, unrestricted range of motion, strength and power restoration, straight ahead running and sprinting, cutting and agility maneuvers, sport-specific training, return to practice, and return to competition) obtaining input from multiple sources. Release to unrestricted sports participation based solely on restored physical function does not assure that cognitive appraisal and psychobehavioral readiness has been restored. Even with careful restoration of each essential factor, another challenge is maintaining these proficiencies in the presence of progressively increasing physical, biomechanical, and psychobehavioral stressors such as can only be reproduced in the sports practice or game environment. A large part of the return to sports and secondary injury prevention plan should include not just steps to progressively acclimatize the athlete to these stressors, but also to ensure that they have learned how to both maintain their current functional status and continue to progress following release from care. Merely achieving rehabilitation and conditioning goals, no matter how advanced, without having established a plan by which these goals can be maintained and improved upon sets the stage for ipsilateral knee reinjury or contralateral knee injury. Clinical outcome measures need to be developed, which more precisely and responsively measure each of these factors across the recovery continuum while concurrently identifying essential links between physical, psychobehavioral, and cognitive components.3 This is a very fertile area for interdisciplinary research.
Conclusion
As surgical, medical, and rehabilitation ACL injury care innovations evolve, there is a growing need to use a team approach with return to play decision-making and to refine clinical outcome measurement tools to better ensure that athletes are not only restoring physical function, but are also restoring neurosensory and neuromuscular control activation acuity, psychobehavioral goals, sport performance capability, as well as learning how to maintain high level functional status and continue to progress following release from care. Primary ACL repair with or without the use of internal bracing augmentation may better preserve both the mechanical and neurosensory balance that is vital to helping prevent knee reinjury. Maintaining or re-establishing ACL neurosensory properties should better enable the patient to benefit from innovative neuromuscular control activation and sensorimotor training rehabilitation strategies. More well-designed clinical studies are needed in this area to determine the true efficacy of less invasive, remnant preserving, surgical approaches.
Acknowledgments
All subjects provided permission for use of their image in this publication.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nyland J, Caborn DN, Jakob R. Knee arthroscopy: the science of art. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2457–2458. doi: 10.1007/s00167-015-3701-x. [DOI] [PubMed] [Google Scholar]
- 2.Nyland J, Doral MN, Lee YHD, et al. Medial collateral ligament and anterior cruciate ligament synergy: functional interdependence. In: Doral MN, Karlsson J, editors. Sports Injuries Prevention, Diagnosis, Treatment and Rehabilitation. 2nd ed. Berlin, Germany: Springer Verlag; 2015. pp. 1131–1143. Chapter 92. [Google Scholar]
- 3.Nyland J, MacKinlay KGW, Wera J, Krupp RJ. Return to play decision-making following anterior cruciate ligament reconstruction: multi-factor considerations. In: Doral MN, Karlsson J, editors. Sports Injuries Prevention, Diagnosis, Treatment and Rehabilitation. 2nd ed. Berlin, Germany: Springer Verlag; 2015. pp. 1491–1502. Chapter 120. [Google Scholar]
- 4.Smigielski R, Zdanowicz U, Drwięga M, Ciszek B, Ciszkowska-Łysoń B, Siebold R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: a cadaveric study including 111 knees. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3143–3150. doi: 10.1007/s00167-014-3146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siebold R, Schuhmacher P, Fernandez F, et al. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3136–3142. doi: 10.1007/s00167-014-3058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaulieu ML, Carey GE, Schlecht SH, Wojtys EM, Ashton-Miller JA. A quantitative comparison of the microscopic anatomy of the human ACL femoral and tibial entheses. J Orthop Res. 2015;33:1811–1817. doi: 10.1002/jor.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai C, Guo L, Yang L, Wu Y, Gou J, Li B. Regional fibrocartilage variations in human anterior cruciate ligament tibial insertion: a histological three-dimensional reconstruction. Connect Tissue Res. 2015;56:18–24. doi: 10.3109/03008207.2014.970183. [DOI] [PubMed] [Google Scholar]
- 8.Steckel H, Starman JS, Baums MH, Klinger HM, Schultz W, Fu FH. Anatomy of the anterior cruciate ligament double bundle structure: a macroscopic evaluation. Scand J Med Sci Sports. 2007;17(4):387–392. doi: 10.1111/j.1600-0838.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 9.Steckel H, Vadala G, Davis D, Fu FH. 2D and 3D 3-tesla magnetic resonance imaging of the double bundle structure in anterior cruciate ligament anatomy. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1151–1158. doi: 10.1007/s00167-006-0185-8. [DOI] [PubMed] [Google Scholar]
- 10.Starman JS, Vanbeek C, Armfield DR, et al. Assessment of normal ACL double bundle anatomy in standard viewing planes by magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):493–499. doi: 10.1007/s00167-006-0266-8. [DOI] [PubMed] [Google Scholar]
- 11.Smigielski R, Becker R, Zdanowicz U, Ciszek B. Medial meniscus anatomy-from basic science to treatment. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):8–14. doi: 10.1007/s00167-014-3476-5. [DOI] [PubMed] [Google Scholar]
- 12.Spencer L, Burkhart TA, Tran MN, et al. Biomechanical analysis of simulated clinical testing and reconstruction of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(9):2189–2197. doi: 10.1177/0363546515589166. [DOI] [PubMed] [Google Scholar]
- 13.Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–328. doi: 10.1111/joa.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewison CE, Tran MN, Kaniki N, Remtulla A, Bryant D, Getgood AM. Lateral extra-articular tenodesis reduces rotational laxity when combined with anterior cruciate ligament reconstruction: a systematic review of the literature. Arthroscopy. 2015;31:2022–2034. doi: 10.1016/j.arthro.2015.04.089. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606–1615. doi: 10.1177/0363546515578253. [DOI] [PubMed] [Google Scholar]
- 16.Koga H, Muneta T, Yagishita K, et al. Evaluation of a behind-remnant approach for femoral tunnel creation in remnant-preserving double-bundle anterior cruciate ligament reconstruction – comparison with a standard approach. Knee. 2015;22(3):249–255. doi: 10.1016/j.knee.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21:906–913. doi: 10.1007/s00167-012-2023-5. [DOI] [PubMed] [Google Scholar]
- 18.Thambyah A, Lei Z, Broom N. Microanatomy of the medial collateral ligament enthesis in the bovine knee. Anat Rec (Hoboken) 2014;297:2254–2261. doi: 10.1002/ar.23001. [DOI] [PubMed] [Google Scholar]
- 19.Ahn JH, Lee SH. Risk factors for knee instability after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Mar 19; doi: 10.1007/s00167-015-3568-x. Epub. [DOI] [PubMed] [Google Scholar]
- 20.Ahn JH, Lee SH, Choi SH, Lim TK. Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. Am J Sports Med. 2010;38(9):1768–1777. doi: 10.1177/0363546510368132. [DOI] [PubMed] [Google Scholar]
- 21.Ahn JH, Lee YS, Lee SH. Creation of an anatomic femoral tunnel with minimal damage to the remnant bundle in remnant-preserving anterior cruciate ligament reconstruction using an outside-in technique. Arthrosc Tech. 2014;3(1):e175–e179. doi: 10.1016/j.eats.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtney CA, Rine RM. Central somatosensory changes associated with improved dynamic balance in subjects with anterior cruciate ligament deficiency. Gait Posture. 2006;24(2):190–195. doi: 10.1016/j.gaitpost.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Courtney C, Rine RM, Kroll P. Central somatosensory changes and altered muscle synergies in subjects with anterior cruciate ligament deficiency. Gait Posture. 2005;22(1):69–74. doi: 10.1016/j.gaitpost.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Nyland J, Brand E, Fisher B. Update on rehabilitation following ACL reconstruction. Open Access J Sports Med. 2010;1:151–166. doi: 10.2147/oajsm.s9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyland J, Wera J, Klein S, Caborn DN. Lower extremity neuromuscular compensations during instrumented single leg hop testing 2–10 years following ACL reconstruction. Knee. 2014;21(6):1191–1197. doi: 10.1016/j.knee.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Nyland J, Klein S, Caborn DN. Lower extremity compensatory neuromuscular and biomechanical adaptations 2 to 11 years after anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(9):1212–1225. doi: 10.1016/j.arthro.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Nyland JA. Redirecting the thrust to put “therapeutic” back into therapeutic exercise. J Orthop Sports Phys Ther. 2015;45(3):148–150. doi: 10.2519/jospt.2015.0103. [DOI] [PubMed] [Google Scholar]
- 28.Nyland J, Kuzemchek S, Parks M, Caborn DN. Femoral anteversion influences vastus medialis and gluteus medius EMG amplitude: composite hip abductor EMG amplitude ratios during isometric combined hip abduction-external rotation. J Electromyogr Kinesiol. 2004;14(2):255–261. doi: 10.1016/S1050-6411(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 29.Hewett TE, Myer GD. The mechanistic connection between the trunk, hip, knee, and anterior cruciate ligament injury. Exerc Sport Sci Rev. 2011;39(4):161–166. doi: 10.1097/JES.0b013e3182297439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenehan EA, Payne WB, Askam BM, Grana WA, Farrow LD. Long-term outcomes of allograft reconstruction of the anterior cruciate ligament. Am J Orthop (Belle Mead NJ) 2015;44(5):217–222. [PubMed] [Google Scholar]
- 31.Nyland J, Lee YH, McGinnis M, Kibbe S, Kocabey Y, Caborn DN. ACL double bundle linked cortical-aperture tibial fixation: a technical note. Arch Orthop Trauma Surg. 2014;134(6):835–842. doi: 10.1007/s00402-014-1989-5. [DOI] [PubMed] [Google Scholar]
- 32.Giedraitis A, Arnoczky SP, Bedi A. Allografts in soft tissue reconstructive procedures: important considerations. Sports Health. 2014;6(3):256–264. doi: 10.1177/1941738113503442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiefenboeck TM, Thurmaier E, Tiefenboeck MM, et al. Clinical and functional outcome after anterior cruciate ligament reconstruction using the LARS™ system at a minimum follow-up of 10 years. Knee. 2015;22(6):565–568. doi: 10.1016/j.knee.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Stone KR, Walgenbach AW, Turek TJ, Somers DL, Wicomb W, Galili U. Anterior cruciate ligament reconstruction with a porcine xenograft: a serologic, histologic, and biomechanical study in primates. Arthroscopy. 2007;23(4):411–419. doi: 10.1016/j.arthro.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Mahalingam VD, Behbahani-Nejad N, Ronan EA, et al. Fresh versus frozen engineered bone-ligament-bone grafts for sheep anterior cruciate ligament repair. Tissue Eng Part C Methods. 2015;21(6):548–556. doi: 10.1089/ten.tec.2014.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahalingam VD, Behbahani-Nejad N, Horine SV, et al. Allogeneic versus autologous derived cell sources for use in engineered bone-ligament-bone grafts in sheep anterior cruciate ligament repair. Tissue Eng Part A. 2015;21(5–6):1047–1054. doi: 10.1089/ten.tea.2014.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25:255–260. doi: 10.1055/s-0032-1313742. [DOI] [PubMed] [Google Scholar]
- 38.Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239–255. [PubMed] [Google Scholar]
- 39.Lubowitz JH, MacKay G, Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech. 2014;3(4):e505–e508. doi: 10.1016/j.eats.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anoka N, Nyland J, McGinnis M, Lee D, Doral MN, Caborn DN. Consideration of growth factors and bio-scaffolds for treatment of combined grade II MCL and ACL injury. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):878–888. doi: 10.1007/s00167-011-1641-7. [DOI] [PubMed] [Google Scholar]
- 41.Dhillon MS, Ball K, Prabhakar S. Differences among mechanoreceptors in healthy and injured anterior cruciate ligaments and their clinical importance. Muscles Ligaments Tendons J. 2012;2:38–43. [PMC free article] [PubMed] [Google Scholar]
- 42.Andriolo L, Di Matteo B, Kon E, Filardo G, Venieri G, Marcacci M. PRP augmentation for ACL reconstruction. Biomed Res Int. 2015;2015:371746. doi: 10.1155/2015/371746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnoczky SP, Sheibani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180–185. doi: 10.1097/JSA.0b013e3182999712. [DOI] [PubMed] [Google Scholar]
- 44.Russell RP, Apostolakos J, Hirose T, Cote MP, Mazzocca AD. Variability of platelet-rich plasma preparations. Sports Med Arthrosc. 2013;21(4):186–190. doi: 10.1097/JSA.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 45.Delzell E. Cell games: clinical use of stem cell therapy is starting to outpace the evidence. Lower Extremity Review. May, 2014. [Accessed January 4, 2016]. Available from: http://lermagazine.com/cover_story/cell-games-clinical-use-of-stem-cell-therapy-is-starting-to-outpace-the-evidence.
- 46.Kristjansson B, Honsawek S. Current perspectives in mesenchymal stem cell therapies for osteoarthritis. Stem Cells Int. 2014;2014:194318. doi: 10.1155/2014/194318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grassel S, Lorenz J. Tissue-engineering strategies to repair chondral and osteochondral tissue in osteoarthritis: use of mesenchymal stem cells. Curr Rheumatol Rep. 2014;16:452. doi: 10.1007/s11926-014-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff’s Law: mechanical regulation of the cells that make and maintain bone. J Biomech. 2010;43(1):108–118. doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Driscoll M, Blyum L. The presence of physiological stress shielding in the degenerative cycle of musculoskeletal disorders. J Bodyw Mov Ther. 2011;15(3):335–342. doi: 10.1016/j.jbmt.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Smith SD, Laprade RF, Jansson KS, Arøen A, Wijdicks CA. Functional bracing of ACL injuries: current state and future directions. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1131–1141. doi: 10.1007/s00167-013-2514-z. [DOI] [PubMed] [Google Scholar]
- 51.Giotis D, Zampeli F, Pappas E, Mitsionis G, Papadopoulos P, Georgoulis AD. Effects of knee bracing on tibial rotation during high loading activities in anterior cruciate ligament-reconstructed knees. Arthroscopy. 2013;29(10):1644–1652. doi: 10.1016/j.arthro.2013.07.258. [DOI] [PubMed] [Google Scholar]
- 52.Strutzenberger G, Braig M, Sell S, Boes K, Schwameder H. Effect of brace design on patients with ACL-ruptures. Int J Sports Med. 2012;33(11):934–939. doi: 10.1055/s-0032-1304634. [DOI] [PubMed] [Google Scholar]
- 53.Pierrat B, Oullion R, Molimard J, et al. Characterisation of in-vivo mechanical action of knee braces regarding their anti-drawer effect. Knee. 2015;22(2):80–87. doi: 10.1016/j.knee.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Palm HG, Brattinger F, Stegmueller B, Achatz G, Riesner HJ, Friemert B. Effects of knee bracing on postural control after anterior cruciate ligament rupture. Knee. 2012;19(5):664–671. doi: 10.1016/j.knee.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 55.Wera JC, Nyland J, Ghazi C, et al. International knee documentation committee knee survey use after anterior cruciate ligament reconstruction: a 2005–2012 systematic review and world region comparison. Arthroscopy. 2014;30(11):1505–1512. doi: 10.1016/j.arthro.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 56.Nyland J, Mauser N, Caborn DN. Sports involvement following ACL reconstruction is related to lower extremity neuromuscular adaptations, subjective knee function and health locus of control. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2019–2028. doi: 10.1007/s00167-013-2366-6. [DOI] [PubMed] [Google Scholar]
- 57.Nyland J, Fisher B, Brand E, Krupp R, Caborn DN. Osseous deficits after anterior cruciate ligament injury and reconstruction: a systematic literature review with suggestions to improve osseous homeostasis. Arthroscopy. 2010;26(9):1248–1257. doi: 10.1016/j.arthro.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Brand E, Nyland J. Patient outcomes following anterior cruciate ligament reconstruction: the influence of psychological factors. Orthopedics. 2009;32(5):335. doi: 10.3928/01477447-20090502-01. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez-Roiz JM, Caballero M, Ares O, Sastre S, Lozano L, Popescu D. Return to recreational sports activity after anterior cruciate ligament reconstruction: a one- to six-year follow-up study. Arch Orthop Trauma Surg. 2015;135(8):1117–1122. doi: 10.1007/s00402-015-2240-8. [DOI] [PubMed] [Google Scholar]
- 60.Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48:1543–1552. doi: 10.1136/bjsports-2013-093398. [DOI] [PubMed] [Google Scholar]
- 61.Tjong VK, Murnaghan L, Nyhof-Young JM, Ogilvie-Harris DJ. A qualitative investigation of the decision to return to sport after anterior cruciate ligament reconstruction: to play or not to play. Am J Sports Med. 2014;42:336–342. doi: 10.1177/0363546513508762. [DOI] [PubMed] [Google Scholar]
- 62.Czuppon S, Racette BA, Klein SE, Harris-Hayes M. Variables associated with return to sport following anterior cruciate ligament reconstruction: a systematic review. Br J Sports Med. 2014;48(5):356–364. doi: 10.1136/bjsports-2012-091786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731–738. doi: 10.1016/j.arthro.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 64.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: part 1, outcomes. Am J Sports Med. 2008;36(1):40–47. doi: 10.1177/0363546507308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: part 2, determinants of dynamic knee stability. Am J Sports Med. 2008;36(1):48–56. doi: 10.1177/0363546507308191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Månsson O, Sernert N, Ejerhed L, Kartus J. Long-term examination of bone mineral density in the calcanei after anterior cruciate ligament reconstruction in adolescents and matched adult controls. Arthroscopy. 2015 doi: 10.1016/j.arthro.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 67.Nyland J. Coming to terms with early sports specialization and athletic injuries. J Orthop Sports Phys Ther. 2014;44(6):389–390. doi: 10.2519/jospt.2014.0109. [DOI] [PubMed] [Google Scholar]
- 68.Myer GD, Martin L, Jr, Ford KR, et al. No association of time from surgery with functional deficits in athletes after anterior cruciate ligament reconstruction: evidence for objective return-to-sport criteria. Am J Sports Med. 2012;40(10):2256–2263. doi: 10.1177/0363546512454656. [DOI] [PMC free article] [PubMed] [Google Scholar]