Abstract
The inner ear vestibular system has numerous projections on central brain centers that regulate sympathetic outflow, and skeletal sympathetic projections affect bone remodeling by inhibiting bone formation by osteoblasts and promoting bone resorption by osteoclasts. In this study, we show that bilateral vestibular lesions in mice cause a low bone mass phenotype associated with decreased bone formation and increased bone resorption. This reduction in bone mass is most pronounced in lower limbs, is not associated with reduced locomotor activity or chronic inflammation, and could be prevented by the administration of the β-blocker propranolol and by genetic deletion of the β2-adrenergic receptor, globally or specifically in osteoblasts. These results provide novel experimental evidence supporting a functional autonomic link between central proprioceptive vestibular structures and the skeleton. Because vestibular dysfunction often affects the elderly, these results also suggest that age-related bone loss might have a vestibular component and that patients with inner ear pathologies might be at risk for fracture. Lastly, these data might have relevance to the bone loss observed in microgravity, as vestibular function is altered in this condition as well.
Keywords: VESTIBULAR SYSTEM, INNER EAR, BONE, SYMPATHETIC NERVOUS SYSTEM, B2-ADRENERGIC RECEPTOR, MICROGRAVITY, AGING, OSTEOPOROSIS
Introduction
The skeleton is richly innervated by sympathetic neurons, which are found in close vicinity to osteoblasts, the bone-forming cells.(1–6) The beta 2-adrenergic receptor (β2AR) is expressed by osteoblasts, and β2AR stimulation by pharmacological β1/β2 nonselective agonists such as isoproterenol causes bone loss, owing to reduced bone formation and increased bone resorption triggered by stimulation of Rankl expression.(7,8) In contrast, βAR blockade by propranolol or genetic lack of the β2AR in Adrβ2-deficient mice leads to a high bone mass phenotype caused by an increase in bone formation and a decrease in bone resorption.(8,9) These studies in mice indicated that sympathetic nerve activity had important repercussions on bone homeostasis. Clinical observational evidence also suggested that this regulatory pathway was conserved between mice and humans, based on the observation that β-blocker use was associated with increased bone mineral density (BMD) and reduced fracture risk in humans (although some studies did not detect association).(10–12) In addition, aging is associated with an elevation in sympathetic outflow and a reduction in trabecular bone volume in humans.(13)
Another condition in which sympathetic outflow and bone homeostasis are altered together is microgravity, during which sympathetic neuronal outflow is elevated.(14) Mechanical unloading contributes to bone loss in microgravity, as shown by models of bed rest in humans(15) and of tail suspension in rodents.(16) However, increased mechanical load by daily exercise in microgravity improved muscular parameters but did not preserve bone density without additional pharmacological intervention.(17) These observations suggested that countermeasures based on bone mechanical stimulation only are not optimal and/or that physiological or external factors other than mechanical unloading and muscular atrophy contribute to bone loss in space.
We recently showed that bilateral vestibular lesions induce bone loss in the lower limbs of rats.(18) The vestibular system is known for its crucial role in spatial orientation and postural balance, but it is also involved in the regulation of other important neurophysiological systems, including the respiratory and cardiovascular systems,(19,20) circadian regulation,(21) and cognitive function.(22) Central neural circuits enable the integration of vestibular and autonomic information. Stimulation of the vestibular nerve, either through natural stimulation induced by head movements on a stationary body or via selective stimulation of vestibular afferents, produces large effects on sympathetic neural reflexes and blood pressure.(23,24) These relationships between vestibular function, sympathetic activation, and bone loss highlighted by these independent studies led us to the hypothesis that vestibular alterations may alter bone homeostasis and bone mass via activation of sympathetic outflow.
We show here, in mice, that alterations in vestibular function have a negative influence on bone mass via activation of autonomic nerves and the β2AR in osteoblasts. These findings suggest that bone loss triggered by aging and perhaps microgravity might have, in part, a vestibular origin that may initiate or exacerbate the effects of reduced mechanical and muscular loading on the skeleton.
Materials and Methods
General procedures
All procedures were approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center. Mice were group-housed in plastic cages (n = 5/cage) in an AAALAC-approved facility, under standard laboratory conditions with a 12-hour dark, 12-hour light cycle, a constant temperature of 20 °C, and humidity of 48%. Mice were fed a standard rodent diet (Pharma Serv, Purina Rodent Laboratory Chow 5001; Framingham, MA, USA). β2AR2.3col1−/− mice were generated by crossing the 2.3-kb Col1a1-cre mice and Adrβ2flox/flox mice.(1,25)
Drug administration
Propranolol (0.5 g/L; Sigma-Aldrich, St. Louis, MO, USA) was given ad libitum in drinking water. Calcein was administered 6 and 2 days before euthanization for dynamic histomorphometry.
Bilateral vestibular lesion procedure and vestibular syndrome clinical scale
Two-month-old female mice were anesthetized in an isoflurane chamber at 3.5% oxygen (flow rate 2 L/min) and maintained under a nosecone at 2% oxygen (flow rate 0.8 L/min). Each mouse received a bilateral transtympanic injection of 0.05 mL sodium arsanilate solution (D-Arsenilic, Sigma-Aldrich) at a dose of 25 mg/mL (diluted in saline solution at 0.9%, 2.5 mg/ear). Sham mice received bilateral transtympanic injections of 0.05 mL of saline solution. Female mice were studied because the clinical burden of osteoporosis is higher in females than in males.
The behavioral effects of chemical vestibular lesions were evaluated with a previously validated clinical vestibular scale.(26) This scale assesses six static or dynamic locomotor points, with a value ranging from 0 to 4 for each criterion. Three categories of spontaneous motor behavior were evaluated, including circular walking, retropulsive movement, and abnormal head bobbing, alongside three reflex behaviors, including tail-hang, contact inhibition of righting, and air righting. Clinical score was recorded for each mouse 3, 15, and 30 days after vestibular lesion. Bobbing is an abnormal head movement characterized by the periodic backward extension of the neck, circular walking is circular locomotor activity, and rearing is defined as retropulsive movement (backward walking of the animal). When the animal is lifted from the table, a successful tail-hang reflex results in the forelimbs reaching out in anticipation of contacting a surface; vestibular lesion (VBX) mice often bend themselves ventrally and occasionally attempt to climb their own tails, which can lead them to fall on the back of their skulls. Contact inhibition related to the righting reflex reflects the ability and speed to go from a supine to a prone position on a table. The mouse is turned over into a supine position with a grid placed to touch its feet while its back maintains contact with the table. Sham-treated mice turn over to the physiological prone position, whereas VBX mice stay in a supine position, attempting to walk with their feet up on the grid. The air-righting reflex is tested by holding animals supine and dropping them onto a padded cushion from a height of 30 cm. Sham-treated mice are able to right themselves, whereas VBX mice fail to right and land on their backs.
Locomotor activity
Ambulatory distance was recorded 3 and 27 days post-VBX. Mice (1 per cage, 8 per group) were placed in a 27.3 cm × 27.3 cm open-field cage equipped with three 16-beam I/R arrays (Med Associates, St Albans, VT, USA) for 90 minutes.
Micro-computed tomography (μCT) analysis
The right femur and L3 to L4 vertebrae from each animal were dissected and fixed overnight in 4% phosphate-buffered formalin and then transferred to 70% ethanol, loaded into 12.3-mm-diameter scanning tubes, and imaged using a μCT 40 (Scanco Medical, Bassersdorf, Switzerland), according to JBMR guidelines for micro-CT analysis of rodent specimens.(27) The scans were integrated into three-dimensional (3D) voxel images. A Gaussian filter (sigma = 0.5, support 2) was used to reduce signal noise, and a threshold of 400 was applied to segment mineralized bone from soft tissue. Scans were acquired with 12-μm isotropic voxels (E = 55 kVp, I = 145 μA). The regions of interest were 1) cortex of the femur at the midpoint (2 mm in length); 2) cancellous bone of the tibial proximal metaphysis (0.24 to 1.20 mm below the growth plate); and 3) cancellous bone of the L3 to L4 vertebral bodies (1.2 mm between end plates).
Histomorphometry
The right femur from each animal was fixed in 4% phosphate-buffered formalin, dehydrated, and embedded, undecalcified, in methyl methacrylate using standard procedures. Longitudinal 5- or 7-μm sections were cut on a rotary microtome and stained by van Gieson/von Kossa. Histomorphometry measurements were performed using the Bioquant Image Analysis System (R&M Biometrics, Nashville, TN, USA). Distal femur cancellous bone measurements (osteoblast surface per bone surface [ObS/BS], osteoclast surface per bone surface [OcS/BS], trabeculae number [Tb.N], trabeculae thickness [Tb.Th], trabeculae space [Tb.Sp.]) were restricted to the secondary spongiosa, excluding primary spongiosa and cortical tissue.
Gene expression
Total RNA was extracted from snap-frozen brown adipose tissue pulverized into frozen powder, using the TRIzol reagent (Invitrogen, Grand Island, NY, USA), as recommended by the manufacturer. cDNAs were synthesized after DNase I treatment using the high-capacity cDNA reverse-transcription kit (Applied Biosystems, Carlsbad, CA, USA). Quantitative PCR (qPCR) was performed using TaqMan gene expression assays. The probe and primer sets for Hprt (Mm00446968_m1, housekeeping gene) and Ucp1 (Mm00494069_m1) were obtained from Applied Biosystems.
Serum assay
Blood was collected by intracardiac puncture and centrifuged at 3000g for 10 minutes to obtain serum. TNFα level was measured using a mouse ELISA kit (Abcam, Cambridge, MA, USA).
Statistics
All data are presented as means ± SD. Statistical analyses were performed using one-way ANOVA for multiple comparisons followed by pair-wise comparison with Tukey’s post hoc test or unpaired two-tailed Student’s t tests for two group comparisons. For all analyses, p < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism v5.04 (GraphPad, La Jolla, CA, USA).
Results
Bilateral arsanilate vestibular injections induce a vestibular syndrome and bone loss in mice
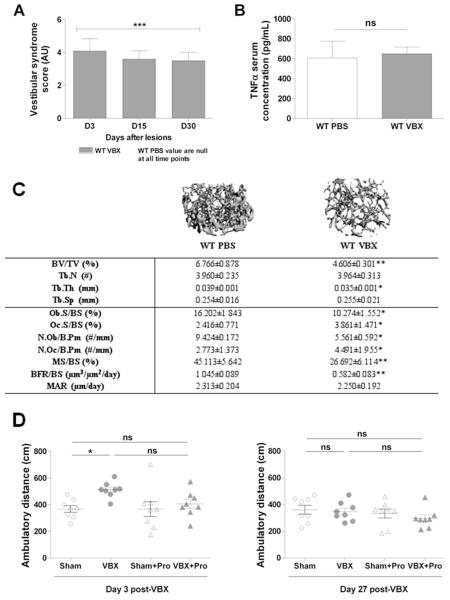
We previously reported that bilateral sodium arsanilate injections in rats cause bone loss in hindlimbs,(28) but further investigations of the mechanism by which vestibular signals control bone remodeling were hampered by limitations in generating loss-of-function studies in this species. We, therefore, sought to establish a model of vestibular dysfunction in mice, based on a similar approach. For that purpose, 2-month-old wild-type (WT) C57BL6/J female mice were administered PBS (control) or sodium arsanilate via transtympanic injections (VBX), and behaviors indicative of a vestibular-like syndrome were assessed 3, 15, and 30 days post-injections using an established scoring system (see Boadas-Vaello and colleagues(26) and Materials and Methods). At all time points, mice subjected to VBX exhibited a significant vestibular syndrome (score >3) compared with sham animals (score = 0) (Fig. 1A). Such lesions, however, did not have any effect on body weight and fat pad weight at death, 30 days post-VBX (body weight 19.8 ± 1.0 g versus 19.9 ± 0.9 g, fat pad weight 0.2 ± 0.1 g versus 0.2 ± 0.1 g for sham and VBX, respectively) and did not elevate serum levels of the inflammation marker TNFα (Fig. 1B).
Fig. 1.
VBX-induced bone loss in mice 1 month after VBX. (A) Vestibular syndrome score (in arbitrary unit), 3, 15, and 30 days after VBX. (B) Serum TNFα level 1 month after sham operation or VBX. (C) Trabecular bone volume ratio (BV/TV), trabeculae number (Tb.N), thickness (Tb.Th), and space (Tb.Sp), osteoblast and osteoclast surface per bone surface (Ob.S/BS, Oc.S/BS), osteoblasts and osteoclasts number per bone perimeter (N.Ob/B.Pm, N.Oc/B.Pm), mineralized surface per bone surface (MS/BS), bone formation rate per bone surface (BFR/BS), and mineral apposition rate (MAR) 1 month after sham operation or VBX. (D) Ambulatory distance 3 and 27 days after sham operation or VBX. (A–D) n = 8–10/group; error bars are standard deviations; *p < 0.05, **p < 0.01, ***p < 0.005 versus WT PBS.
To assess the effect of vestibular lesions on the skeleton, distal femoral metaphyses, diaphysis, and L3 to L4 vertebrae were analyzed 30 days post-VBX by high-resolution 3D microcomputed tomography. Metaphyseal femoral trabecular bone analyses revealed a significant decrease in bone volume/tissue volume (BV/TV) and trabecular thickness (Tb.Th) in mice subjected to VBX compared with sham animals (Fig. 1C), whereas diaphyseal cortical thickness (Ct.Th) and cortical tissue mineral density (TMD) remained unchanged (Supplementary Fig. S1A, B). Histological analyses on undecalcified metaphyseal femoral bone sections showed that this VBX-induced bone loss was associated with a significant decrease in trabecular osteoblast surface per bone surface (Ob.S/BS) and osteoblast number per bone perimeter (N.Ob/B.Pm). VBX also caused a significant increase in osteoclast surface per bone surface (Oc.S/BS) and osteoclast number per bone perimeter (Fig. 1C). Dynamic histomorphometric analyses indicated that mineralized surface per bone surface (MS/BS) and bone formation rate per bone surface (BFR/BS) were decreased upon vestibular lesion, whereas mineral apposition rate (MAR) remained unchanged (Fig. 1C). Vertebral trabecular bone volume per tissue volume (Supplementary Fig. S1C), as well as body size and femoral bone size, were not affected by VBX (Supplementary Table S1). No effect of vestibular lesions on locomotor activity was observed 27 days post-lesion, although increased locomotor activity was observed 3 days post-lesion in the VBX group compared with sham-operated controls (Fig. 1D). Collectively, these results suggest that intact vestibular outputs are required for normal bone remodeling.
The bone loss induced by bilateral vestibular lesion is compensated with time
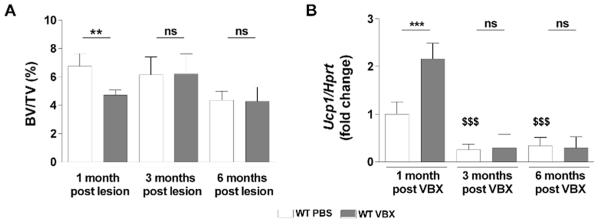
Animal studies showed that bilateral vestibular dysfunction entails postural, muscular, visual, cognitive, and autonomic alterations. Although postural stability, hypermetria in limb muscle, blood flow, and pressure were compensated within a week,(29–35) other parameters including balance, eye movements in response to head rotations in the dark, navigational abilities, and spatial memory remained permanently altered.(29,32,36–38) The long-term consequences of bilateral vestibular lesions on bone mass, on the other hand, remain unknown. We thus asked whether the bone loss observed 1 month post-VBX lesions was sustained with time, or reversible. To address this question, bilateral vestibular lesions were performed at age 2 months and bone mass was measured 1, 3, and 6 months later. In contrast to the 1-month time point, no significant reduction in femoral trabecular BV/TV could be detected at 3 and 6 months post-VBX compared with aged-matched sham groups (Fig. 2A, Table 1). Similarly, Ucp1 expression in brown adipose tissue (BAT), used as a surrogate marker of sympathetic peripheral outflow, was not significantly different between sham and VBX mice at 3 and 6 months, whereas it was upregulated 1 month post-VBX (Fig. 2B). These results indicate that the bone loss triggered by bilateral vestibular dysfunction in young adult mice is compensated with time.
Fig. 2.
VBX-induced bone loss in young adult mice is transient. (A) Trabecular bone volume ratio (BV/TV) 1, 3, and 6 months after VBX lesion (μCT measurements). (B) Ucp1 expression in brown adipose tissue 1, 3, and 6 months after VBX lesion. Values are normalized to WT PBS 1 month after lesion in B; n = 10/group; error bars are standard deviations; **p < 0.01, ***p < 0.005; $$$p < 0.005 versus WT PBS 1 month after lesion.
Table 1.
Bone Microarchitecture Parameters 1, 3, and 6 Months After Sham Operation or VBX (μCT Measurements)
| 1 month post-VBX |
3 months post-VBX |
6 months post-VBX |
||||
|---|---|---|---|---|---|---|
| WT PBS | WT VBX | WT PBS | WT VBX | WT PBS | WT VBX | |
| Tb.N | 3.960±0.235 | 3.964±0.313 | 3.608±0.261 | 3.615±0.199 | 2.831±0.241 | 2.988±0.136 |
| Tb.Th (mm) | 0.039±0.001 | 0.035±0.001* | 0.041±0.004 | 0.047±0.006 | 0.045±0.004 | 0.044±0.007 |
| Tb.Sp (mm) | 0.254±0.016 | 0.255±0.021 | 0.279±0.022 | 0.279±0.013 | 0.360±0.033 | 0.335±0.013 |
Tb.N, trabeculae number; Tb.Th, trabeculae thickness; Tb.Sp, trabeculae space; n = 10/group; mean±standard deviations.
p < 0.05 versus WT PBS 1 month after lesion.
Beta-2 adrenergic signaling is required for VBX-induced bone loss
The vestibular system has autonomic projections(39) and pharmacological agonists used as a surrogate of sympathetic activation induce bone loss.(8,40,41) In addition, we have shown that the nonselective β1/β2-adrenergic receptor antagonist propranolol mitigates the bone loss induced by bilateral vestibular lesions in rats.(18) These observations, along with the aforementioned correlation between bone loss and Ucp1 BAT expression in mice subjected to VBX, suggested that activation of the sympathetic nervous system (SNS) mediates the bone loss caused by vestibular lesions. To address this hypothesis, bilateral vestibular lesions were performed in mice in which sympathetic signals were blocked, either genetically in Adrβ2-deficient mice or pharmacologically by propranolol.
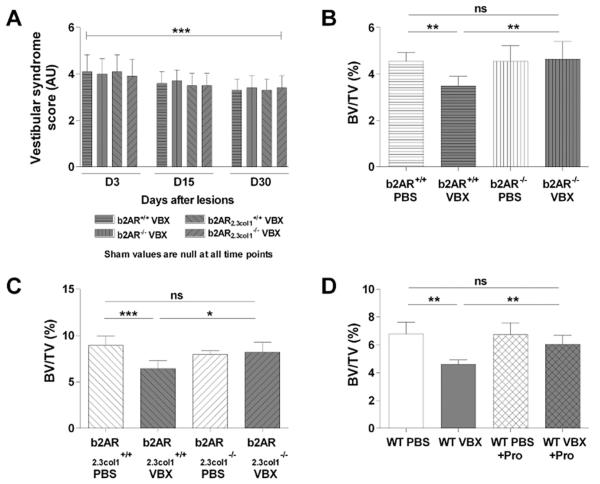
The effect of vestibular lesions was first investigated in mice deficient for Adrβ2 globally (β2AR−/− mice)(42) or in mature osteoblasts (β2AR2.3col1−/− mice). β2AR−/−, β2AR2.3col1−/−, and respective WT littermates were subjected to bilateral vestibular lesions at age 2 months, and their skeletal response was analyzed 1 month later. Vestibular lesions induced a significant vestibular syndrome in all genotypes (Fig. 3A), but only WT mice subjected to VBX lost bone, whereas β2AR−/− and β2AR2.3col1−/− mice were resistant to VBX-induced bone loss (Fig. 3B, C). VBX-induced structural and cellular bone alterations, including decreases in Tb.Th, Ob.S/BS, N.Ob/B.Pm, MS/BS, and BFR/BS and increases in Oc.S/BS and N.Oc/B.Pm, were also blunted by lack of the β2AR (Table 2).
Fig. 3.
βAR blockade prevents VBX-induced bone loss. (A) Vestibular syndrome score (in arbitrary unit) 3, 15, and 30 days after VBX (***p < 0.005 versus sham). (B) Trabecular bone volume ratio (BV/TV) in 3-month-old β2AR+/+ and β2AR−/− mice subjected to sham or VBX (μCT measurements). (C) Trabecular bone volume ratio (BV/TV) in 3-month-old β2AR2.3col1+/+ and β2AR2.3col1−/− mice subjected to sham or VBX (μCT measurements). (D) Trabecular bone volume ratio (BV/TV) in mice subjected to sham or VBX, treated or not with propranolol (Pro., 0.5 g/L). (A–D) n = 10/group; error bars are standard deviations; *p < 0.05, **p < 0.01, ***p < 0.005.
Table 2.
Bone Microarchitecture Parameters 1 Month After Sham Operation or VBX (μCT Measurements) in Control, β−/−, or β2.3col1−/− Mice
| β2AR−/− mice | β2AR+/+ PBS | β2AR+/+ VBX | β2AR−/− PBS | β2AR−/− VBX |
|---|---|---|---|---|
| Tb.N | 3.900±0.269 | 3.834±0.144 | 4.099±0.356 | 3.827±0.231 |
| Tb.Th (mm) | 0.032±0.002 | 0.028±0.002* | 0.032±0.002 | 0.032±0.002 |
| Tb.Sp (mm) | 0.259±0.019 | 0.258±0.016 | 0.245±0.022 | 0.265±0.016 |
| Ob.S/BS (%) | 16.313±1.334 | 10.222±1.394*** | 15.510±1.195 | 16.128±0.835 |
| Oc.S/BS (%) | 2.293±0.517 | 4.645±0.923*** | 2.292±0.461 | 2.275±0.641 |
| N.Ob/B.Pm (Nb/mm) | 9.560±0.152 | 5.422±0.178*** | 9.743±0.182 | 2.292±0.461 |
| N.Oc/B.Pm (Nb/mm) | 2.151±0.685 | 5.034±1.713** | 2.197±1.145 | 2.700±1.390 |
| MS/BS (%) | 45.535±6.999 | 26.075±6.840* | 46.204±7.210 | 45.871±6.544 |
| βBFR/BS (μm3/μm2/d) | 1.055±0.082 | 0.6135±0.424* | 1.105±0.076 | 1.092±0.0875 |
| MAR (μm/d) | 2.317±0.331 | 2.353±0.379 | 2.392±0.283 | 2.382±0.443 |
| β2AR2.3col1−/− mice | β2AR2.3col1+/+ PBS | β2AR2.3col1+/+ VBX | β2AR2.3col1−/− PBS | β2AR2.3col1−/− VBX |
| Tb.N | 4.237±0.243 | 4.133±0.254 | 4.402±0.063 | 4.464±0.332 |
| Tb.Th (mm) | 0.040±0.003 | 0.036±0.001* | 0.040±0.003 | 0.040±0.003 |
| Tb.Sp (mm) | 0.231±0.018 | 0.251±0.026 | 0.226±0.005 | 0.222±0.028 |
| Ob.S/BS (%) | 16.874±0.835 | 10.623±1.682*** | 16.622±1.070 | 16.542±1.310 |
| Oc.S/BS (%) | 2.252±0.707 | 5.258±1.389*** | 2.375±1.061 | 2.756±1.282 |
| N.Ob/B.Pm (Nb/mm) | 9.871±1.246 | 5.625±0.744*** | 9.623±1.401 | 9.520±1.773 |
| N.Oc/B.Pm (Nb/mm) | 2.162±0.723 | 5.320±1.581** | 2.288±1.137 | 2.835±1.342 |
| MS/BS (%) | 46.472±7.436 | 27.429±7.035* | 46.774±6.860 | 45.873±7.280 |
| βBFR/BS (μm3/μm2/d) | 1.098±0.084 | 0.655±0.418* | 1.112±0.074 | 1.097±0.0827 |
| MAR (μm/d) | 2.363±0.367 | 2.388±0.402 | 2.378±0.362 | 2.392±0.381 |
Tb.N, trabeculae number; Tb.Th, trabeculae thickness; Tb.Sp, trabeculae space; Ob.S/BS, osteoblast surface per bone surface; Oc.S/BS, osteoclast surface per bone surface; N.Ob/B.Pm, osteoblasts number per bone perimeter; N.Oc/B.Pm, osteoclasts number per bone perimeter; MS/BS, mineralized surface per bone surface; BFR/BS, bone formation rate per bone surface; MAR, mineral apposition rate. n = 10/group; mean±standard deviations.
p < 0.05,
p < 0.01,
p < 0.005 versus control.
Another approach to block sympathetic activation in response to VBX, without potential developmental phenotypes caused by genetic alterations during development, is to use propranolol, a nonselective β1/β2AR antagonist, in WT adult mice, from the time of VBX lesion (2-month-old mice) until death, 1 month later. This short duration treatment had no bone anabolic effect in sham mice but prevented bone loss and the reduction in trabecular thickness observed in VBX mice (Fig. 3D, Table 3) without affecting locomotor activity (Fig. 1D), body and fat pad weight (body weight 19.8 ± 1.0 g versus 19.5 ± 1.1 g, fat pad weight 0.2 ± 0.1 g versus 0.2 ± 0.1 g at day 30 for sham and VBX + Pro, respectively), or body and femur size (Supplementary Table S1). Collectively, these results support the hypothesis that loss of normal vestibular signals induces bone loss through the activation of the sympathetic nervous system and stimulation of the β2AR in osteoblasts.
Table 3.
Bone Microarchitecture Parameters 1 Month After Sham Operation or VBX (μCT Measurements)+Propranolol (Pro) Treatment in WT Mice
| WT PBS | WT VBX | WT PBS+Pro | WT VBX+Pro | |
|---|---|---|---|---|
| Tb.N | 3.960±0.235 | 3.964±0.313 | 4.076±0.235 | 3.697±0.161 |
| Tb.Th (mm) | 0.039±0.001 | 0.035±0.001* | 0.038±0.001 | 0.041±0.005 |
| Tb.Sp (mm) | 0.254±0.016 | 0.255±0.021 | 0.246±0.015 | 0.273±0.012 |
Tb.N, trabeculae number; Tb.Th, trabeculae thickness; Tb.Sp, trabeculae space; n = 10/group; mean±standard deviations.
p < 0.05 versus WT PBS.
Discussion
Immunological reactivity to various neuropeptides and retrograde neuronal labeling showed that sympathetic projections in bone are anatomically linked to the central nervous system (CNS)(1,2) and receive inputs from leptin-sensitive hypothalamic and brainstem neurons.(43) In this study, we show that alterations in vestibular neuronal outputs by single bilateral arsanilate injections influence skeletal homeostasis in adult mice, independently of changes in locomotor activity, and that this effect requires activation of the β2AR in osteoblasts. These results raise the hypothesis that vestibular dysfunctions caused by aging, diseases, or microgravity might affect bone remodeling and bone mass via alterations in sympathetic outflow.
A number of results from this study support the contribution of sympathetic activation as the main mechanism by which bilateral vestibular lesions induce bone loss in mice. Those include the similarity of changes in structural and cellular bone parameters triggered by VBX and the β1/β2AR agonist isoproterenol,(44) the observed association between bone loss and peripheral sympathetic activation after VBX, and perhaps most convincingly, the absence of VBX-induced bone loss in mice lacking the β2AR globally or specifically in osteoblasts. Because blood vessels are innervated and responsive to sympathetic outflow, the lack of bone loss upon VBX in global β2AR-deficient could have been explained by possible alterations in vascular parameters. However, the blunting of VBX-induced bone loss in mice lacking the β2AR specifically in osteoblasts excluded this putative mechanism and supports the notion that VBX affects bone homeostasis independently of indirect effects on the vasculature or other organ systems whose function is under regulation by β2AR signaling. The observed reduction in bone mass observed after VBX appears to arise from changes in vestibular neuronal signals, independently of systemic inflammation (as measured by normal TNFα levels) or significant structural lesions in the vestibular nerve.(28) In addition, the bone loss induced by vestibular lesions did not reduce locomotor activity, indicating that a reduction in mechanical loading is unlikely to explain the observed bone loss.
Although neuroanatomical studies provide a preliminary blueprint of the CNS circuitry linking vestibular neurons and central autonomic structures, the precise CNS pathways and neurotransmitters involved in the observed bone loss induced by VBX remain unknown. Electrophysiological studies indicated the existence of a predominant indirect pathway linking the vestibular nucleus (directly innervated by the vestibular nerve) and the rostro-ventro-lateral medullary structure (RVLM) through the lateral medullary reticular formation,(45,46) and neuroanatomical studies reported an indirect connection through the tractus solitary nucleus,(47) with the RVLM being directly connected to the sympathetic medullary tractus innervating arterioles and possibly bone tissues.(48)
Studies in cats indicated that electrical vestibular stimulations lead to a decrease in SNS activity in hindlimbs and opposite effects in upper and lower body parts with a decrease in femoral vasoconstriction and an increase in brachial vascular tone.(49) Interestingly, this pattern matches the pattern of space-induced bone loss, which is more pronounced in lower limbs.(15,18) These observations suggest that vestibulosympathetic reflexes may mainly impact lower body tissues. The occurrence of bone loss in the trabecular compartment observed after VBX is consistent with previous studies in which adrenergic-mediated effects on bone mass were restricted to cancellous bone.(50,51) This regionalization of bone loss in the trabecular bone compartment and in appendicular bones thus further supports the contribution of increased sympathetic output as the mechanism by which bilateral vestibular lesions induce bone loss. It is unknown why the appendicular skeleton, but not the axial skeleton, is affected by VBX. A differential innervation between cancellous versus cortical and appendicular versus axial bone might explain these differences, but further studies will be necessary to address this question.
Although bone unloading in microgravity conditions is viewed as the major contributing factor explaining bone loss in space, additional factors including nutritional deficiencies, decreased calcium intestinal absorption, radiation exposure, changes in blood volume distribution, and psychological chronic stress may contribute to bone loss in space.(52) It is currently unknown to what extent alterations in vestibular outputs and subsequent SNS and hypothalamic-pituitary-adrenal axis activation caused by spaceflight may affect bone homeostasis. Results of this study suggest that such alterations might contribute and add up to the known deleterious effects of spaceflight on the skeleton.
Vestibular dysfunctions are also prevalent in aging individuals on earth. Interestingly, low BMD was associated with hearing and postural balance impairments among adults older than 40 years,(53) leading the authors of this study to conclude that osteoporosis negatively affects vestibular function and balance/hearing. In light of our results in rodent models, we propose an alternative interpretation of these results, being that vestibular abnormalities caused by aging contribute, in part and via sympathetic activation, to the osteoporosis and increased fracture risk observed in aging individuals.
Although the transient effect of VBX on bone mass observed in mice would argue against this hypothesis, it has to be emphasized that the duration and extent of the vestibular impairments induced by a unique injection of arsanilate is unknown, and alternative models may need to be generated to guarantee a long-term vestibular deficit. In addition, these studies in mice were conducted at a relatively young age, before peak bone mass was reached. Hence, the normal bone anabolism occurring in mice up to 4 to 6 months of age could have contributed to the recovery of VBX-induced bone loss. In contrast, peak bone mass is achieved in humans by approximately age 30 years. Therefore, is it possible that the bone loss triggered by vestibular abnormalities in aging adults would not be recovered over time without pharmacological intervention. This idea is in line with the observation that astronauts, despite their higher than average resistance training, never fully recover the bone loss caused by microgravity after return to earth.
Lastly, these results may also have implications for the care of patients with a history of vestibular pathologies, such as labyrinthectomy, antibiotic treatment (aminoglycosides and platinum-based chemotherapy), vestibular neuritis, or Ménière’s disease, and further studies are warranted to investigate fracture risk in these conditions.
Supplementary Material
Acknowledgments
This work was supported by grant NNX12AL35G from the National Aeronautics and Space Administration (FE), a postdoctoral fellowship from the National Space Biomedical Research Institute (GV, NASA NCC 9-58), and grant number S10 RR027631 from the National Institutes of Health (DP). The locomotor activity experiment was performed in part through the use of the Murine Neurobehavior Core lab (Vanderbilt University Medical Center). The authors thank Dr S Besnard, Prof P Denise (University of Caen, France), and Dr J Allison (Vanderbilt Murine Neurobehavioral Laboratory) for their valuable inputs. This work was also supported in part by Career Development Award # 1IK2 BX001634 from the United States (U.S.) Department of Veterans Affairs, Biomedical Laboratory Research and Development Program.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Elefteriou F. Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci. 2005;62(19–20):2339–49. doi: 10.1007/s00018-005-5175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dénes A, Boldogkoi Z, Uhereczky G, et al. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience. 2005;134(3):947–63. doi: 10.1016/j.neuroscience.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 3.Imai S, Tokunaga Y, Maeda T, Kikkawa M, Hukuda S. Calcitonin gene-related peptide, substance P, and tyrosine hydroxylase-immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res. 1997;15(1):133–40. doi: 10.1002/jor.1100150120. [DOI] [PubMed] [Google Scholar]
- 4.Mach DB, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–66. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 5.Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25(6):623–9. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda T, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497(7450):490–3. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet N, et al. Alteration of trabecular bone under chronic beta2 agonists treatment. Med Sci Sports Exerc. 2005;37(9):1493–501. doi: 10.1249/01.mss.0000177592.82507.95. [DOI] [PubMed] [Google Scholar]
- 8.Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008;42(5):837–40. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Pasco JA, et al. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res. 2004;19(1):19–24. doi: 10.1359/JBMR.0301214. [DOI] [PubMed] [Google Scholar]
- 11.Schlienger RG, Kraenzlin ME, Jick SS, Meier CR. Use of beta-blockers risk of fractures. JAMA. 2004;292(11):1326–32. doi: 10.1001/jama.292.11.1326. [DOI] [PubMed] [Google Scholar]
- 12.Reid IR, et al. Beta-blocker use, BMD, and fractures in the study of osteoporotic fractures. J Bone Miner Res. 2005;20(4):613–8. doi: 10.1359/JBMR.041202. [DOI] [PubMed] [Google Scholar]
- 13.Farr JN, et al. Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab. 2012;97(11):4219–27. doi: 10.1210/jc.2012-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ertl AC, et al. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J Physiol. 2002;538(Pt 1):321–9. doi: 10.1113/jphysiol.2001.012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact. 2007;7(1):33–47. [PubMed] [Google Scholar]
- 16.Vico L, Novikov VE, Very JM, Alexandre C. Bone histomorphometric comparison of rat tibial metaphysis after 7-day tail suspension vs. 7-day spaceflight. Aviat Space Environ Med. 1991;62(1):26–31. [PubMed] [Google Scholar]
- 17.Cavanagh PR, Licata AA, Rice AJ. Exercise and pharmacological countermeasures for bone loss during long-duration space flight. Gravitat Space Biol Bull. 2005;18(2):39–58. [PubMed] [Google Scholar]
- 18.Vignaux G, et al. Bone remodeling is regulated by inner ear vestibular signals. J Bone Miner Res. 2013;28(10):2136–44. doi: 10.1002/jbmr.1940. [DOI] [PubMed] [Google Scholar]
- 19.Normand H, Etard O, Denise P. Otolithic and tonic neck receptors control of limb blood flow in humans. J Appl Physiol. 1997;82(6):1734–8. doi: 10.1152/jappl.1997.82.6.1734. [DOI] [PubMed] [Google Scholar]
- 20.Yates BJ, Billig I, Cotter LA, Mori RL, Card JP. Role of the vestibular system in regulating respiratory muscle activity during movement. Clin Exp Pharmacol Physiol. 2002;29(1–2):112–7. doi: 10.1046/j.1440-1681.2002.03612.x. [DOI] [PubMed] [Google Scholar]
- 21.Fuller PM, Jones TA, Jones SM, Fuller CA. Neurovestibular modulation of circadian homeostatic regulation: vestibulohypothalamic connection. Proc Natl Acad Sci USA. 2002;99(24):15723–8. doi: 10.1073/pnas.242251499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besnard S, et al. Influence of vestibular input on spatial and nonspatial memory and on hippocampal NMDA receptors. Hippocampus. 2012;22(4):814–26. doi: 10.1002/hipo.20942. [DOI] [PubMed] [Google Scholar]
- 23.Yates BJ, Miller AD. Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J Neurophysiol. 1994;71(6):2087–92. doi: 10.1152/jn.1994.71.6.2087. [DOI] [PubMed] [Google Scholar]
- 24.Kerman IA, McAllen RM, Yates BJ. Patterning of sympathetic nerve activity in response to vestibular stimulation. Brain Res Bull. 2000;53(1):11–6. doi: 10.1016/s0361-9230(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 25.Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224(2):245–51. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 26.Boadas-Vaello P, Riera J, Llorens J. Behavioral and pathological effects in the rat define two groups of neurotoxic nitriles. Toxicol Sci. 2005;88(2):456–66. doi: 10.1093/toxsci/kfi314. [DOI] [PubMed] [Google Scholar]
- 27.Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 28.Vignaux G, et al. Evaluation of the chemical model of vestibular lesions induced by arsanilate in rats. Toxicol Appl Pharmacol. 2012;258(1):61–71. doi: 10.1016/j.taap.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Thomson DB, Inglis JT, Schor RH, Macpherson JM. Bilateral labyrinthectomy in the cat: motor behaviour and quiet stance parameters. Exp Brain Res. 1991;85(2):364–372. doi: 10.1007/BF00229414. [DOI] [PubMed] [Google Scholar]
- 30.Inglis JT, Macpherson JM. Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J Neurophysiol. 1995;73(3):1181–91. doi: 10.1152/jn.1995.73.3.1181. [DOI] [PubMed] [Google Scholar]
- 31.Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol (1985) 1999;86(5):1552–60. doi: 10.1152/jappl.1999.86.5.1552. [DOI] [PubMed] [Google Scholar]
- 32.Stapley PJ, Ting LH, Kuifu C, Everaert DG, Macpherson JM. Bilateral vestibular loss leads to active destabilization of balance during voluntary head turns in the standing cat. J Neurophysiol. 2006;95(6):3783–97. doi: 10.1152/jn.00034.2006. [DOI] [PubMed] [Google Scholar]
- 33.Wilson TD, et al. Vestibular inputs elicit patterned changes in limb blood flow in conscious cats. J Physiol. 2006;575(Pt 2):671–84. doi: 10.1113/jphysiol.2006.112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macpherson JM, Everaert DG, Stapley PJ, Ting LH. Bilateral vestibular loss in cats leads to active destabilization of balance during pitch and roll rotations of the support surface. J Neurophysiol. 2007;97(6):4357–67. doi: 10.1152/jn.01338.2006. [DOI] [PubMed] [Google Scholar]
- 35.Yavorcik KJ, et al. Effects of postural changes and removal of vestibular inputs on blood flow to and from the hindlimb of conscious felines. Am J Physiol Regul Integr Comp Physiol. 2009;297(6):R1777–84. doi: 10.1152/ajpregu.00551.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barmack NH, Pettorossi VE, Erickson RG. The influence of bilateral labyrinthectomy on horizontal and vertical optokinetic reflexes in the rabbit. Brain Res. 1980;196(2):520–4. doi: 10.1016/0006-8993(80)90418-7. [DOI] [PubMed] [Google Scholar]
- 37.Waespe W, Wolfensberger M. Optokinetic nystagmus (OKN) and optokinetic after-responses after bilateral vestibular neurectomy in the monkey. Exp Brain Res. 1985;60(2):263–9. doi: 10.1007/BF00235920. [DOI] [PubMed] [Google Scholar]
- 38.Baek JH, Zheng Y, Darlington CL, Smith PF. Evidence that spatial memory deficits following bilateral vestibular deafferentation in rats are probably permanent. Neurobiol Learn Mem. 2010;94(3):402–13. doi: 10.1016/j.nlm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Yates BJ, Siniaia MS, Miller AD. Descending pathways necessary for vestibular influences on sympathetic and inspiratory outflow. Am J Physiol. 1995;268(6 Pt 2):R1381–5. doi: 10.1152/ajpregu.1995.268.6.R1381. [DOI] [PubMed] [Google Scholar]
- 40.Hinoi E, et al. An osteoblast-dependent mechanism contributes to the leptin regulation of insulin secretion. Ann NY Acad Sci. 2009;1173(Suppl 1):E20–30. doi: 10.1111/j.1749-6632.2009.05061.x. [DOI] [PubMed] [Google Scholar]
- 41.Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys. 2008;473(2):231–6. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem. 1999;274(24):16701–8. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- 43.Yadav VK, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–89. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 45.Kerman IA, Yates BJ. Regional and functional differences in the distribution of vestibulosympathetic reflexes. Am J Physiol. 1998;275(3 Pt 2):R824–35. doi: 10.1152/ajpregu.1998.275.3.R824. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson KA, Maurer AP, Sadacca BF, Yates BJ. Responses of feline medial medullary reticular formation neurons with projections to the C5-C6 ventral horn to vestibular stimulation. Brain Res. 2004;1018(2):247–56. doi: 10.1016/j.brainres.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 47.Cai Y-L, Ma W-L, Wang J-Q, Li Y-Q, Li M. Excitatory pathways from the vestibular nuclei to the NTS and the PBN and indirect vestibulo-cardiovascular pathway from the vestibular nuclei to the RVLM relayed by the NTS. Brain Res. 2008;1240:96–104. doi: 10.1016/j.brainres.2008.08.093. [DOI] [PubMed] [Google Scholar]
- 48.Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232(4752):868–71. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 49.Kerman IA, Emanuel BA, Yates BJ. Vestibular stimulation leads to distinct hemodynamic patterning. Am J Physiol Regul Integr Comp Physiol. 2000;279(1):R118–25. doi: 10.1152/ajpregu.2000.279.1.R118. [DOI] [PubMed] [Google Scholar]
- 50.De Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res. 2005;20(12):2159–68. doi: 10.1359/JBMR.050812. [DOI] [PubMed] [Google Scholar]
- 51.Levasseur R, et al. Sympathetic nervous system as transmitter of mechanical loading in bone. Joint Bone Spine. 2003;70(6):515–519. doi: 10.1016/j.jbspin.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Tavassoli M. Medical problems of space flight. Am J Med. 1986;81(5):850–4. doi: 10.1016/0002-9343(86)90357-8. [DOI] [PubMed] [Google Scholar]
- 53.Mendy A, et al. Low bone mineral density is associated with balance and hearing impairments. Ann Epidemiol. 2014;24(1):58–62. doi: 10.1016/j.annepidem.2013.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.