Abstract
Two-photon excitation of fluorescent proteins is an attractive approach for imaging living systems. Today researchers are eager to know which proteins are the brightest, and what the best excitation wavelengths are. Here we review the two-photon absorption properties of a wide variety of fluorescent proteins, including new far-red variants, to produce a comprehensive guide to choosing the right FP and excitation wavelength for two-photon applications.
Two-photon laser scanning microscopy (2PLSM)1,2 of cells and tissues expressing fluorescent proteins is becoming a powerful tool for biological studies at different levels of organization2-4. The advantages of two-photon excitation (2PE) include reduced out-of-focus photobleaching, less autofluorescence, deeper tissue penetration, and intrinsically high three-dimensional resolution1,2. 2PLSM should make it possible to obtain even better optical recordings of ion concentration and cell signaling with genetically targeted sensors 5,6. Two-photon excitation of fluorescent proteins can also be considered as potentially advantageous in the contexts of genetically targeted deep photodynamic therapy or chromophore-assisted light inactivation6, three-dimensional optical memory7, as well as superresolution (sub-diffraction limited) imaging techniques, such as stimulated emission depletion8, photo-activated localization microscopy, and stochastic optical reconstruction microscopy9.
To fully realize the potential of 2PE of the fluorescent proteins, it is important to know their two-photon absorption (2PA) spectra, cross sections, σ2, and 2PE action cross sections, or brightness, σ2’, (σ2’ = σ2 × φ, where φ is the fluorescence quantum yield). The linear, one-photon absorption (1PA) spectra and extinction coefficients of many fluorescent proteins have been described and reviewed5,6,10 (Supplementary Table 1 online), but the 1PA properties are not sufficient to predict the key 2PA properties such as optimum excitation wavelength and maximum brightness (Box 2).
Here we present a systematic characterization of the 2PA properties of 48 different fluorescent proteins using the same experimental setup, common 2PA reference standards, and an all-optical method for measuring mature chromophore concentration11 (Supplementary Methods online). Briefly, we use a relative fluorescence method with femtosecond excitation and coumarin 485 (Exciton), coumarin 540A (Exciton), rhodamine 610 (Exciton), fluorescein (Aldrich), and styryl 9M (Aldrich) as 2PA standards12, which eliminates the necessity to calibrate the laser parameters. The power dependence of the fluorescence signal was quadratic for all data presented, assuring that the spectra represent pure 2PA. Further, the spectra were collected in a much broader range (550 – 1,400 nm) than commonly reported, which reveals new optimal wavelengths for excitation and provides new insights into the relation of 2PA properties with protein structure.
To sample broadly, and include the proteins commonly used with one-photon excitation, we combine previously reported spectra of some blue (EBFP series), cyan (ECFP and mCerulean), teal (mTFP series), green (EGFP, mWasabi, mAmetrine), orange and red (DsRed, mRFP, and Fruits series) fluorescent proteins11,13 with newly obtained results for a number of other fluorescent proteins, including the most promising far-red variants, such as mRaspberry, E2-Crimson, mKate, mKate2, tdKatushka2, mGrape3, mNeptune, eqFP650, and eqFP670. The 2PA spectrum (presented in absolute cross section values per mature chromophore) represents the fundamental molecular property of a fluorescent protein, property that could strongly constrain its utility in any two-photon application. The data presented here is an important first step in determining which proteins are brightest upon two-photon excitation and at what wavelengths, but there are other factors, such as expression rate, photostability, photoswitching efficiency, or ability to generate singlet oxygen, that need to be considered while selecting the right mutant for a particular two-photon application.
Two-photon versus one-photon absorption properties
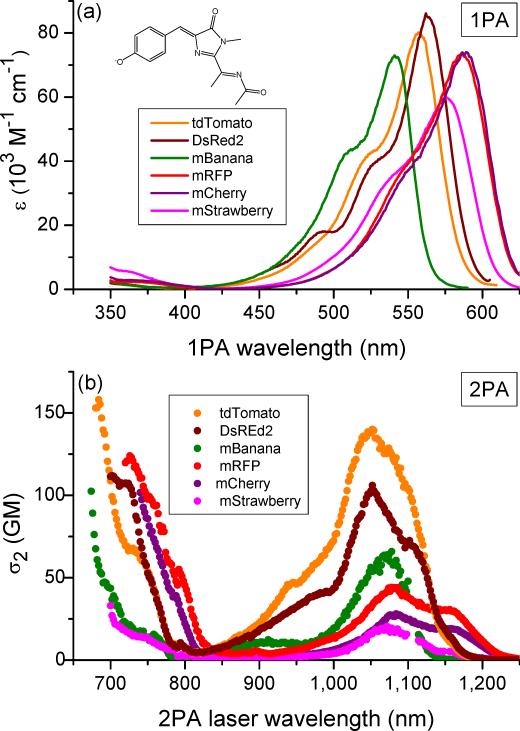
There are several different classes of fluorescent proteins that are usually grouped according to their chromophore structure. These classes vary tremendously in terms of their absorption and fluorescence properties. Similarly, large variations are observed in 2PA spectra (Fig. 1 and Supplementary Figs. 1 - 12 online). Because the chromophore in fluorescent proteins is not centrosymmetric, the parity selection rules for one- and two-photon transitions are relaxed14. As a result, the same bands should appear in both one- and two-photon absorption spectra, although with different relative intensities. An overlap between 1PA and 2PA spectra is generally apparent for the longest-wavelength absorption band, corresponding to the first electronic, S0 → S1, transition (Box 2 and Fig. 2). For the neutral chromophores found in EBFP2.0, mAmetrine, ECFP, and mBlueberry1, the corresponding 1PA and 2PA peaks coincide rather well. In the anionic chromophores of EGFP, Citrine, mOrange, DsRed2, and TagRFP, however, there is a distinct blue shift of the 2PA band relative to the 1PA band. This observation, previously ascribed to an enhancement of certain vibronic transitions in 2PA spectrum11,15, illustrates why the optimal 2PA excitation wavelength cannot in general be deduced from the 1PA peak wavelength.
Figure 1.
Comparison of one-photon and two-photon absorption spectra of fluorescent proteins with different chromophores. Two-photon absorption spectra (red symbols, left axis) are presented versus laser wavelength, used for excitation. For comparison purposes, in one-photon absorption spectra (blue lines, right axis) the actual excitation wavelength is multiplied by a factor of two. All spectra are presented in absolute values determined per mature chromophore. Reproduced in part from Ref.11. Copyright 2009 American Chemical Society.
Figure 2.
(Should be placed in Box 2). Upper part: One-photon absorption and two-photon absorption spectra of TagRFP. Lower part: Jablonski diagram of 1PA and 2PA transitions.
The most striking difference between the 2PA and 1PA spectra is however the appearance of a strong two-photon absorption in the region of wavelengths much shorter than those of the S0 → S1 transition, where the 1PA is extremely weak. These recently observed 2PA features of fluorescent proteins14 belong to higher electronic S0 → Sn transitions, which have been predicted theoretically for various types of chromophores16 (Supplementary Fig. 13 online). The S0 → Sn shorter-wavelength 2PA transitions can be very intense due to the resonance enhancement effect14 (Box 2). Indeed, in some chromophores, including those of Citrine, mOrange, mBlueberry1, DsRed2, and TagRFP, this enhancement is so strong that optimal excitation is found at wavelengths much shorter than what one would expect based on the 1PA properties alone (Fig. 1). In the spectra of red and far-red proteins these S0 → Sn transitions occur at 700 - 770 nm, matching well with the tuning range of femtosecond Ti:sapphire lasers and thus offering new opportunities with already existing commercial 2PLSM systems13,17. For example, excitation of the higher S0 → Sn transition of TagRFP simultaneously with the first, S0 → S1, transition of mKalama1 makes dual-color two-photon imaging possible with a single excitation laser wavelength13. Alternatively, two proteins with very different Stokes shifts can be used for this purpose18.
Compared to a regular dye in solution, the chromophore of a fluorescent protein is buried within a complex, spatially organized protein environment that influences the optical properties of the chromophore through electrostatic interactions19. Recently, it has been shown that different hues in a series of mutants that contain the same (DsRed-type) chromophore are caused by variations in the local electric field inside the beta barrel20. Interestingly, the shifts of the 1PA peak wavelength are accompanied by only small changes in the extinction coefficient (Fig. 3a). The local electrostatic environment also influences the 2PA properties, but with the important difference that the shift of the peak absorbance is accompanied by large changes in 2PA cross section (Fig. 3b). This is because the 2PA cross section is much more sensitive to the local electric field variations (Box 3, Fig. 4). For example, Citrine is as bright as EGFP upon one-photon excitation, but it is much dimmer than EGFP upon two-photon excitation. This effect could be due to the change of the local field at the chromophore site caused by the high polarizability of a nearby stacking tyrosine 203 residue21.
Figure 3.
1PA (a) and 2PA (b) spectra of the Fruit series of fluorescent proteins. Adopted from Ref.11. Copyright 2009 American Chemical Society.
Figure 4.
(Should be placed in Box 3). Dependence of 2PA peak cross section and 1PA peak extinction on the change of permanent dipole moment of chromophore upon excitation, |Δμ10| in the of Fruit series.
Choosing the protein and laser for two-photon excitation
Table 1 summarizes the most relevant 2PA properties of a representative set of fluorescent proteins (see Supplementary Table 2 online for all the proteins studied). Comparison of the data reveals a remarkable variability: the maximum 2PA cross section (σ2) and brightness (σ2’) change over two orders of magnitude (Fig. 1 and Table 1). These are much larger differences than those observed for one-photon extinction (ε) which changes at most by eight fold (from 10,800 (mBlueberry1) to 85,600 M−1 cm−1 (DsRed2)). This means that identifying the right fluorescent protein for a particular 2PA application is crucial for success.
Table 1.
Summary of the 2PA properties of representative fluorescent proteins. Two-photon absorption cross section (σ2), two-photon brightness (σ2’), both per mature protein are presented in two spectral regions, corresponding to the short- and long-wavelength transitions.
| Short-wavelength band | Long-wavelength band | |||||
|---|---|---|---|---|---|---|
| Protein | λ2PA (nm) | σ2 (GM) | σ2 × φ (GM) | λ2PA (nm) | σ2 (GM) | σ2 × φ (GM) |
| EBFP2.041 | 552 | 16 | 11 | 750 | 13 | 9.2 |
| ECFP (Clontech) | 550 | 15 | 7.8 | 857 | 23 | 12 |
| Cerulean41 | 550 | 27 | 16 | 858 | 23 | 13 |
| mTFP1.042 | 667 | 6.4 | 5.4 | 875 | 70 | 59 |
| EGFP (Clontech) | 660 | 22 | 17 | 927 | 39 | 30 |
| mAmetrine43 | 655 | 19 | 13 | 809 | 56 | 40 |
| Citrine44 | 590 | 34 | 23 | 968 | 10 | 6.7 |
| mKok45 | 686 | 93 | 67 | 1044 | 41 | 30 |
| mOrange31 | 640 | 200 | 140 | 1080 | 67 | 47 |
| TagRFP46 | 759 | 300 | 130 | 1050 | 95 | 42 |
| tdTomato31 | 684 | 316 | 228 | 1050 | 278 | 200 |
| DsRed247 | 700 | 112 | 79 | 1050 | 103 | 73 |
| mCherry31 | 740 | 101 | 24 | 1080 | 27 | 6.4 |
| mRaspberry48 | 704 | 346 | 65 | 1118 | 31 | 5.8 |
| mPlum48 | 724 | 114 | 15 | 1105 | 22 | 2.9 |
| tdKatushka249 | 710 | 645 | 285 | 1100 | 143 | 63 |
| mKate249 | 712 | 216 | 91 | 1140 | 72 | 30 |
| mNeptune50 | 750 | 335 | 57 | 1105 | 70 | 12 |
Note: To obtain σ2 and σ2’ per chromophore in the case of dimers, one has to take one-half of the corresponding values presented in the Table.
If we concentrate only on two-photon brightness, several proteins stand out as good potential two-photon probes in the Ti:sapphire tuning range. Among the proteins fluorescing in the blue-green region, the members of mTFP series are twice as bright as EGFP when excited at 870 - 920 nm. In the yellow-orange region of fluorescence, mOrange can be efficiently excited near 730 nm, while TagRFP, tdTomato, DsRed, mKate, mKate2, mKate S158A, mKate S158C, tdKatushka2, and mNeptune show quite bright red fluorescence when excited at 730 – 770 nm.
Several proteins offer useful 2PA properties beyond the common range of Ti:sapphire lasers. In particular, those with orange to far-red fluorescence can be excited efficiently at wavelengths between 1,000 and 1,200 nm, where there is relatively little tissue or water absorption, weak tissue scattering, and vanishing small amounts of tissue autofluorescence1 (Fig. 5). There is a good match between the 2PA peak of tdTomato and tdKatushka2 and minimum attenuation of the tissue in the spectral range from 1,000 to 1,150 nm. While tdKatushka2 is not as bright as tdTomato, its fluorescence spectrum is red shifted with respect to that of tdTomato (Fig. 5), fitting better to the highest transparency range. Although the region of 1,000 – 1,150 nm is inaccessible to standard Ti:sapphire lasers, there are other laser solutions currently available, such as femto- and picosecond Nd- and Yb-doped fiber and glass lasers.
Figure 5.
Matching of two-photon excitation spectra of red fluorescent proteins with the optimum tissue transparency and with the wavelengths of some short-pulse laser systems. (a) Typical tissue transparency window (Ref.39, filled gray) presented as attenuation coefficient, left y-axis; 2PE brightness spectra per protein chain of tdTomato and tdKatushka2, right y-axis; and their corresponding normalized fluorescence spectra. (b) Effective laser efficiency relevant to two-photon excitation (average power squared divided by repetition rate and pulse duration) of several commercial femtosecond lasers.
Why do the published 2PE cross sections vary so much?
An accurate measurement of the absolute 2PA cross section remains a technically demanding task requiring careful independent experimental evaluation of a number of parameters, each of which has a particular experimental error that contributes to uncertainty in the cross section value. The pioneering works by the Webb1,2,22,23, Schmidt15, and Kleinfeld3 groups reported the 2PE action cross section spectra of ECFP, EGFP, EYFP, and DsRed, measured within the typical spectral range of a Ti:sapphire laser. More 2PE4,11,13,14,18,24-26 and 2PA27 spectra followed, but the absolute 2PE cross sections obtained even for the same protein varied dramatically. For example, the peak σ2’ value of EGFP has been reported to be 1.527, 2024, 4015, 6022, 18018, 1802, and 300 (for mEGFP)26 GM. Such discrepancies may be attributed to several potential sources of experimental errors. First, measurements based on two-photon excited fluorescence are usually more accurate than nonlinear transmission techniques (NLT, including Z-scan, pump-probe, etc.) because they rely on zero-background detection and require much less excitation power and lower concentration of chromophores. The high levels of excitation used in NLT can initiate other spurious nonlinear optical processes such as thermal lensing, stimulated emission and scattering, resulting in underestimated 2PA cross sections28.
Most of the reported cross sections values were obtained by using 2PE fluorescence in combination with 2PA standards. In several works these measurements were performed relative to fluorescein or rhodamine B, but the actual values for these reference dyes vary in the literature12,29. This can explain the difference between the EGFP peak cross section, σ2’ = 29 GM, obtained here using the fluorescein value of σ2 = 16 ± 2 GM at 920 nm12, and σ2’ = 41 GM reported by Blab et al.15 that was based upon a higher value (σ2 = 26 ± 8 GM) for fluorescein at the same wavelength29.
Finally, most measurements have been complicated by an uncertainty over the actual concentration of the mature chromophore in a protein sample. Typically the concentration is measured by Bicinchoninic Acid protein assay (BCA) or alkaline denaturation methods. Both can introduce errors, especially if the protein folds/matures poorly or/and the chromophore is unstable under alkaline denaturating conditions30. This can be circumvented by using an all-optical approach11 that relates the fluorescence lifetime and quantum yield to the extinction coefficient (Supplementary Methods and Supplementary Fig. 14 online). Comparison of our measurements of mBanana11 and those reported previously31 reveals that this protein has an order of magnitude higher extinction coefficient than previously thought because it appears that only a small portion of the protein matures to form the chromophore.
While the last 15 years of work have produced a remarkably wide range of reported cross sections values for EGFP and other commonly used proteins, there is an emerging consensus for some of the proteins. The cross section of EGFP at 850 nm recently obtained by Herz et al.4 is in perfect agreement with our result at the same wavelength, and recent data by Hashimoto et al.24 on EGFP, BFP, and ECFP are also consistent with our measurements (Supplementary Table 2 online).
Remaining challenges
A great deal of effort has been devoted to optimizing one-photon properties of fluorescent proteins, but surprisingly little attention has been paid to creating brighter mutants for two-photon applications. The recently developed model of the effect of local protein environment on 2PA (Box 3) should make it possible to increase the 2PA cross section by manipulating the strength and direction of the electric field inside the barrel. New generations of mutants with even brighter two-photon fluorescence will inevitably be created. Large 2PA cross sections and quantum yields are only part of the solution however. In 2PLSM, the photostability of the probe is a serious constraint. To date, there is very limited information on the nonlinear multiphoton photobleaching of fluorescent proteins, and it is often difficult to compare measurements directly because the bleaching rate depends upon several laser parameters in a complex way4,32-37 and can vary dramatically between fluorescent proteins38.
At the rather high excitation intensities usually required for 2PLSM (105 – 107 W/cm2 at the sample), the photobleaching rate rises with laser intensity more steeply than the expected quadratic function 4,32,33. This means that lowering the peak power, while raising the repetition rate, can reduce total photodamage while producing the same fluorescence signal34. One possible explanation for the very steep power dependence of the photobleaching could be a further (stepwise) absorption from a long-lived triplet state35. This is consistent with the observation that lowering the repetition rate, while maintaining the same peak power, can be beneficial35, although the improvement comes with the cost of longer acquisition times.
The photophysical mechanisms of nonlinear photobleaching remain unclear. The first step will be to carefully characterize the multiphoton photobleaching rates of a broad set of fluorescent proteins, which should then lead to a structure – property model that can be used to develop new variants more suitable for 2PLSM and other challenging applications. There is empirical evidence that reducing the photon energy (i.e. increasing wavelength) often provides better photostability in 2PLSM. The photobleaching of DsRed slows down by an order of magnitude when the excitation wavelength is shifted to the red, from 750 to 950 nm32. Similarly, excitation of EGFP at 920 nm provides the two-fold improvement compared to 850 nm4, and the red protein (tdRFP) is much more stable upon 1,100-nm excitation than the green protein (EGFP) excited at 850 or 920 nm4. These observations, and the high 2PE brightness near 1,050 – 1,150 nm of mOrange, TagRFP, tdTomato, DsRed, tdKatushka2, and mKate2 indicate that these bright 2PE probes can offer new opportunities for deep tissue imaging.
Supplementary Material
Box 1. Glossary of terms.
Electronic transitions: The absorption and fluorescence emission of photons by fluorescent protein is due to the system of π-conjugated electrons of the chromophore. Upon excitation, this system moves into an excited, higher energy state. This movement from the initial, or ground, state (S0) to an excited state (S1 or Sn) is called an electronic transition, whose frequency is equal to the energy difference between the two states divided by the Planck constant. Higher-energy transitions (S0 → Sn) correspond to absorption at shorter (more energetic) wavelengths compared to lowest-energy transition (S0 → S1) which corresponds to the longest wavelength.
Vavilov-Kasha's rule states that fluorescence properties are independent of the mode (one- or two-photon) or wavelength of excitation and thus exciting any state Sn (n = 1, 2, 3,...) will yield the same fluorescence spectrum. When exciting a higher-energy sate Sn (n = 2, 3,..) fluorescence does not occur from that state. Instead, some of the energy is lost in the form of heat (see dashed arrows in the Fig. 2) and emission occurs from the lowest excited state S1.
Stokes shift is the frequency difference between the excitation maximum of the S0 → S1 transition and fluorescence emission maximum of the S1 → S0 transition.
Vibronic transitions: In addition to a change of electronic states upon optical excitation, a vibrational state of a molecule can also change. While in the vibrational ground state atoms mostly occupy their near-equilibrium positions. In a vibrational excited state these atoms start to oscillate (acquire vibration energy) along certain normal coordinates. If both electronic and vibrational states change upon excitation, then this transition corresponds to the molecule acquiring a quantum (or several quanta) of vibrational energy in the electronically excited state that sum to form vibronic transition. This transition always occurs higher in energy (at shorter wavelength) than the pure electronic transition in the absorption spectrum.
Box 2. Anatomy of two-photon absorption spectrum.
Two-photon absorption cross section, σ2, (measured in Goeppert-Mayer units, 1 GM = 10−50 cm4 s) characterizes the probability of the simultaneous absorption of two photons whose energies add up to match the molecular transition energy. Two-photon absorption is governed by different quantum-mechanical rules than one-photon absorption, and, as a result, the 2PA spectra are often much different in shape than their one-photon counterparts, as exemplified in the Fig. 2 for TagRFP. The 1PA (extinction) spectrum in the region of the S0 → S1 transition (right hand side of the Fig. 2 and right Jablonski diagram) is described by
| (1) |
where A is a constant, ν is the frequency, μ10 is the matrix element of the electronic transition dipole moment between states S0 and S1, g1(ν) is the 1PA lineshape function, which includes the distribution of intensity due to vibronic transitions. The 2PA spectrum in the same region is represented, within the two-level approximation, by14:
| (2) |
where B is another constant, Δμ10 is the difference between permanent dipole moments of the excited (S1) and ground (S0) states, g2(ν) is the 2PA lineshape function. The shape of the 2PA spectrum, g2(ν), is different from the shape of the 1PA spectrum, g1(ν), because some vibronic transitions become enhanced in the two-photon process, whereas the pure electronic transition is the strongest in one-photon process. In the S0 → Sn transition region (left hand side of the Fig. 2), the 2PA is surprisingly strong. The 1PA in this region is described by
| (3) |
where g1’(ν) is the 1PA lineshape function for this higher transition, μn0 is the transition dipole moment connecting states S0 and Sn. The 1PA is very weak (see Fig. 2) because of small absolute value of μn0. The 2PA, in the three-level approximation14, reads:
| (4) |
where μ1n is the transition dipole moment between states S1 and Sn, g2’(ν) is the 2PA lineshape function, ν10 is the (peak) frequency of the S0 → S1 transition, νL is the laser frequency (νL = ν/2). The 2PA becomes very strong in this region due to large absolute values of μ10, μ1n, and quantum-mechanical effect known as resonant enhancement14. Resonant enhancement occurs when the laser frequency, νL, approaches from below the energy of the lowest S1 state, ν10, resulting in fast reduction of denominator in (4).
Box 3. Effect of local internal electric field on optical properties of fluorescent proteins.
Because two-photon absorption is governed by different quantum mechanical rules, it reveals different aspects of the chromophore structure and chromophore – protein interactions. Unlike linear extinction ε, which depends only on the transition dipole moment squared, |μ10|2, 2PA cross section σ2 is proportional to both |μ10|2 and |Δμ10|2 (cf. Eq. (2) in Box 2). The consequence of this is that σ2 is sensitive to the electric field present within the protein barrel. Therefore, σ2 can be manipulated by changing the electrostatic environment of the chromophore. For example, in a series of Fruits, all possessing the same chromophore but different environment, 1PA extinction coefficient vary only very slightly, but 2PA cross section increases very fast when |Δμ10| increases (Fig. 5).
The chromophore of a fluorescent protein has a permanent dipole moment in both the ground (μ0) and excited (μ1) states. Because of the high mobility of the π-electronic cloud, the S0 → S1 transition has a certain charge-transfer character, resulting in a difference between permanent dipoles (Δμ10 = μ1 − μ0 ≠ 0). Another important property of the liable π-electron system of the chromophore is that the charge density easily redistributes upon application of external electric field E, thus resulting in an additional, induced component of the dipole moment μind. This induced portion of the dipole is related to the electric field through polarizability coefficient (more generally, tensor) α: μind = α E. If the polarizabilities in the ground (α0) and excited (α1) states are different, the change of induced dipole moment Δμind10 will contribute to the total change of dipole moment: Δμ1 = Δμ010 + ½ Δμind10 = Δμ010 + ½ (α1 − α0) E, where index 0 in Δμ010 corresponds to “zero field” situation. This dependence makes Δμ10 sensitive to the electric field. This mechanistic picture of how the chromophore electronic structure is tuned by its environment makes strong predictions for creating better fluorescent proteins for two-photon applications: One can obtain a strong gain in peak 2PA cross section upon increasing of |Δμ10| (which follows the changes of E, or, more accurately, projection of E on Δμ10). In fluorescent proteins, E can be altered by changing certain charged amino acids, or the hydrogen bonding network, on the axis of the chromophore dipole.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences, grant R01 GM086198. We thank Mr. B. H. Davis for technical help and Drs. R. Campbell (University of Alberta, Edmonton, Canada), D. M. Chudakov (Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Moscow Russia), M. Lin (Stanford University, Stanford, California, USA), and R. Y. Tsien (University of California, San Diego, La Jolla, California, USA) for kindly providing us cDNA of different fluorescent proteins.
References
- 1.Xu C, Zipfel W, Shear JB, Williams RM, Webb WW. Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA. 1996;93:10763–10768. doi: 10.1073/pnas.93.20.10763. [An important presentation of the 2PLSM method with an emphasis on 2PA spectroscopy of biologically relevant fluorescent dyes. Here, the two-photon absorption spectra of two GFPs (wt-GFP and S65T mutant) were presented for the first time.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotech. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 3.Tsai PS, et al. All-optical histology using ultrashort laser pulses. Neuron. 2003;39:27–41. doi: 10.1016/s0896-6273(03)00370-2. [DOI] [PubMed] [Google Scholar]
- 4.Herz J, et al. Expanding two-photon intravital microscopy to the infrared by means of optical parametric oscillator. Biophys. J. 2010;98:715–723. doi: 10.1016/j.bpj.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsien RY. The green fluorescent protein. Ann. Rev. Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. and references therein. [This early review of the fluorescent proteins describes 1PA properties of GFPs with different chromophores and identifies the need of more studies of their 2PA properties.] [DOI] [PubMed] [Google Scholar]
- 6.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 2010;90:1103–1163. doi: 10.1152/physrev.00038.2009. and references therein. [DOI] [PubMed] [Google Scholar]
- 7.Adam V, et al. Data storage based on photochromic and photoconvertible fluorescent proteins. J. Biotech. 2010;149:289–298. doi: 10.1016/j.jbiotec.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Moneron G, Hell SW. Two-photon excitation STED microscopy. Opt. Express. 2009;17:14567–14573. doi: 10.1364/oe.17.014567. [DOI] [PubMed] [Google Scholar]
- 9.Vaziri A, Tang J, Shroff H, Shank CV. Multilayer three-dimensional super resolution imaging of thick biological samples. Proc. Natl. Acad. Sci. USA. 2008;105:20221–20226. doi: 10.1073/pnas.0810636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat. Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 11.Drobizhev M, Tillo S, Makarov NS, Hughes TE, Rebane A. Absolute two-photon absorption spectra and two-photon brightness of orange and red fluorescent proteins. J. Phys. Chem. B. 2009;113:855–859. doi: 10.1021/jp8087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makarov NS, Drobizhev M, Rebane A. Two-photon absorption standards in the 550--1600 nm excitation wavelength range. Opt. Express. 2008;16:4029–4047. doi: 10.1364/oe.16.004029. [This paper presents a large number of 2PA standards that can be used to measure the spectra of new fluorophores with high accuracy in a very wide spectral range.] [DOI] [PubMed] [Google Scholar]
- 13.Tillo SE, Hughes TE, Makarov NS, Rebane A, Drobizhev M. A new approach to dual-color two-photon microscopy with fluorescent proteins. BMC Biotech. 2010;10 doi: 10.1186/1472-6750-10-6. Art. # 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drobizhev M, Makarov NS, Hughes T, Rebane A. Resonance enhancement of two-photon absorption in fluorescent proteins. J. Phys. Chem. B. 2007;111:14051–14054. doi: 10.1021/jp075879k. [DOI] [PubMed] [Google Scholar]
- 15.Blab GA, Lommerse PHM, Cognet L, Harms GS, Schmidt T. Two-photon excitation action cross-sections of the autofluorescent proteins. Chem. Phys. Lett. 2001;350:71–77. [Google Scholar]
- 16.Nifosi R, Luo Y. Predictions of novel two-photon absorption bands in fluorescent proteins. J. Phys. Chem. B. 2007;111:14043–14050. doi: 10.1021/jp075545v. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen QT, et al. An in vivo biosensor for neurotransmitter release and in situ receptor activity. Nat. Neurosci. 2010;13:127–132. doi: 10.1038/nn.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawano H, Kogure T, Abe Y, Mizuno H, Miyawaki A. Two-photon dual-color imaging using fluorescent proteins. Nat. Methods. 2008;5:373–374. doi: 10.1038/nmeth0508-373. [DOI] [PubMed] [Google Scholar]
- 19.Shu X, Shaner NC, Yarbrough CA, Tsien RY, Remington SJ. Novel chromophores and buried charges control color in mFruits. Biochem. 2006;45:9639–9647. doi: 10.1021/bi060773l. [This paper suggests the effect of local electrostatic fields on the different absorption and fluorescence properties of red fluorescent proteins, an effect that was later quantitatively demonstrated with 2PA spectroscopy in Ref. 20.] [DOI] [PubMed] [Google Scholar]
- 20.Drobizhev M, Tillo S, Makarov NS, Hughes TE, Rebane A. Color hues in red fluorescent proteins are due to internal quadratic Stark effect. J. Phys. Chem. B. 2009;113:12860–12864. doi: 10.1021/jp907085p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wachter RM, Elsliger M-A, Kallio K, Hanson GT, Remington SJ. Structural basis of spectral shifts in the yellow-emission variants of green fluorescent protein. Struct. 1998;6:1267–1277. doi: 10.1016/s0969-2126(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 22.Heikal AA, Hess ST, Webb WW. Multiphoton molecular spectroscopy and excited-state dynamics of enhanced green fluorescent protein (EGFP): Acid-base specificity. Chem. Phys. 2001;274:37–45. [Google Scholar]
- 23.Heikal AA, Hess ST, Baird GS, Tsien RY, Webb WW. Molecular spectroscopy and dynamics of intrinsically fluorescent proteins: Coral red (dsRed) and yellow (Citrine). Proc. Nat. Acad. Sci. USA. 2000;97:11996–12001. doi: 10.1073/pnas.97.22.11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto H, et al. Measurement of two-photon excitation spectra of fluorescent proteins with nonlinear Fourier-transform spectroscopy. Appl. Opt. 2010;49:3323–3329. doi: 10.1364/AO.49.003323. [DOI] [PubMed] [Google Scholar]
- 25.Piatkevich KD, et al. Monomeric red fluorescent proteins with a large Stokes shift. Proc. Natl. Acad. Sci. USA. 2010;107:5369–5374. doi: 10.1073/pnas.0914365107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzo MA, Springer G, Segawa K, Zipfel WR, Piston DW. Optimization of pairings and detection conditions for measurements of FRET between cyan and yellow fluorescent proteins. Microsc. Microanal. 2006;12:238–254. doi: 10.1017/S1431927606060235. [DOI] [PubMed] [Google Scholar]
- 27.Hosoi H, Yamaguchi S, Mizuno H, Miyawaki A, Tahara T. Hidden electronic excited state of enhanced green fluorescent protein. J. Phys. Chem. B. 2008;112:2761–2763. doi: 10.1021/jp711628u. [DOI] [PubMed] [Google Scholar]
- 28.Oulianov DA, Tomov IV, Dvornikov AS, Rentzepis PM. Observations on the measurement of two-photon absorption cross-section. Opt. Comm. 2001;191:235–243. [Google Scholar]
- 29.Albota MA, Xu C, Webb WW. Two-photon fluorescence excitation cross sections of biomolecular probes from 690 to 960 nm. Appl. Opt. 1998;37:7352–7356. doi: 10.1364/ao.37.007352. [DOI] [PubMed] [Google Scholar]
- 30.Kredel S, et al. Optimized and far-red-emitting variants of fluorescent protein eqFP611. Chem. Biol. 2008;15:224–233. doi: 10.1016/j.chembiol.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotech. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 32.Marchant JS, Stutzmann GE, Leissring MA, LaFerla FM, Parker I. Multiphoton-evoked color change of DsRed as an optical highlighter for cellular and subcellular labeling. Nat. Biotech. 2001;19:645–649. doi: 10.1038/90249. [DOI] [PubMed] [Google Scholar]
- 33.Chen T-S, Zeng S-Q, Luo Q-M, Zhang Z-H, Zhou W. High-order photobleaching of Green Fluorescent Protein inside live cells in two-photon excitation microscopy. Biochem. Biophys. Res. Comm. 2002;291:1272–1275. doi: 10.1006/bbrc.2002.6587. [DOI] [PubMed] [Google Scholar]
- 34.Ji N, Magee JC, Betzig E. High-speed, low-photodamage nonlinear imaging using passive pulse splitters. Nat. Methods. 2008;5:197–202. doi: 10.1038/nmeth.1175. [Currently, one of the greatest constraints in two-photon imaging with fluorescent proteins is rapid photobleaching. This paper describes a novel optical solution to at least part of the problem.] [DOI] [PubMed] [Google Scholar]
- 35.Donnert G, Eggeling C, Hell SW. Major signal increase in fluorescence microscopy through dark-state relaxation. Nat. Methods. 2007;4:81–86. doi: 10.1038/nmeth986. [DOI] [PubMed] [Google Scholar]
- 36.Kawano H, et al. Attenuation of photobleaching in two-photon excitation fluorescence from green fluorescent protein with shaped excitation pulses. Biochem. Biophys. Res. Comm. 2003;311:592–596. doi: 10.1016/j.bbrc.2003.09.236. [DOI] [PubMed] [Google Scholar]
- 37.Field JJ, et al. Optimizing the fluorescent yield in two-photon laser scanning microscopy with dispersion compensation. Opt. Express. 2010;18:13661–13672. doi: 10.1364/OE.18.013661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson GH, Knobel SM, Sharif WD, Kain SR, Piston DW. Use of the Green Fluorescent Protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 1997;73:2782–2790. doi: 10.1016/S0006-3495(97)78307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritz J-P, et al. Optical properties of native and coagulated porcine liver tissue between 400 and 2400 nm. Lasers Surg. Med. 2001;29:205–212. doi: 10.1002/lsm.1134. [DOI] [PubMed] [Google Scholar]
- 40.Ai HW, Shaner NC, Cheng Z, Tsien RY, Campbell RE. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochem. 2007;46:5904–5910. doi: 10.1021/bi700199g. [DOI] [PubMed] [Google Scholar]
- 41.Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotech. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 42.Ai HW, Henderson JN, Remington SJ, Campbell RE. Directed evolution of a monomeric, bright and photostable version of Clavularia cyan fluorescent protein: structural characterization and applications in fluorescence imaging. Biochem. J. 2006;400:531–540. doi: 10.1042/BJ20060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ai HW, Hazelwood KL, Davidson MW, Campbell RE. Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat. Methods. 2008;5:401–403. doi: 10.1038/nmeth.1207. [DOI] [PubMed] [Google Scholar]
- 44.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. J. Biol. Chem. 2001;31:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 45.Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat. Methods. 2008;5:683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- 46.Merzlyak EM, et al. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods. 2007;4:555–557. doi: 10.1038/nmeth1062. [DOI] [PubMed] [Google Scholar]
- 47.Yanushevich YG, et al. A strategy for the generation of non-aggregating mutants of Anthozoa fluorescent proteins. FEBS Lett. 2002;511:11–14. doi: 10.1016/s0014-5793(01)03263-x. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Jackson WC, Steinbach PA, Tsien RY. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc. Nat. Acad. Sci. USA. 2004;101:16745–16749. doi: 10.1073/pnas.0407752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shcherbo D, et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 2009;418:567–574. doi: 10.1042/BJ20081949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin MZ, et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem. Biol. 2009;16:1169–1179. doi: 10.1016/j.chembiol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.