Abstract
Obesity increases the risk of numerous poor health outcomes, including cancer. Obesity is especially problematic in women because both they and their offspring may be at increased risk of cancer. Studying transmission of obesity-induced cancer risk is challenging in humans, but animal studies are beginning to reveal the underlying mechanisms.
Nearly 70% of Americans are overweight or obese (1). Additionally, the prevalence of obesity has doubled in children and quadrupled in adolescents in the United States over the past three decades (2); in 2012, one third of children and adolescents were overweight or obese (3). Overweight and obesity put both adults and children at increased risk for poor health outcomes. For example, adults are affected by obesity-related morbidities, such as heart disease, cardiovascular disease, stroke, and diabetes. In women, 37% of whom are obese in the United States (3), an elevated body mass index (BMI) increases the risk of anovulation, polycystic ovarian syndrome, infertility, and pregnancy complications (4). Even in young children, obesity is associated with risk factors for cardiovascular disease, such as elevated cholesterol and high blood pressure, and prediabetes with intermittent hyperglycemia. Obese adolescents also have higher rates of depression, sleep apnea, and joint and bone problems than their normal-weight peers.
In addition to these health effects of obesity, numerous studies have reported that overweight and obesity are associated with increased risk of cancer (5). Obese women are at increased risk of endometrial cancer, and obesity in early life is associated with increased risk for multiple myeloma and non-Hodgkin lymphoma (6), as well as cancer of the colon, kidney, and liver (5). In direct contrast, childhood and adolescent adiposity is inversely related to breast cancer risk across the life course (7). Adult obesity is also related to increased risk for cancers of the thyroid, ovary, cervix, esophagus, pancreas, gall bladder, and prostate (8). In this Perspective, we highlight recent clinical and translational data from studies seeking to understand the developmental windows during which obesity has the greatest impact on cancer risk and the mechanisms by which risk is conferred.
IN UTERO AND EARLY-LIFE EXPOSURES
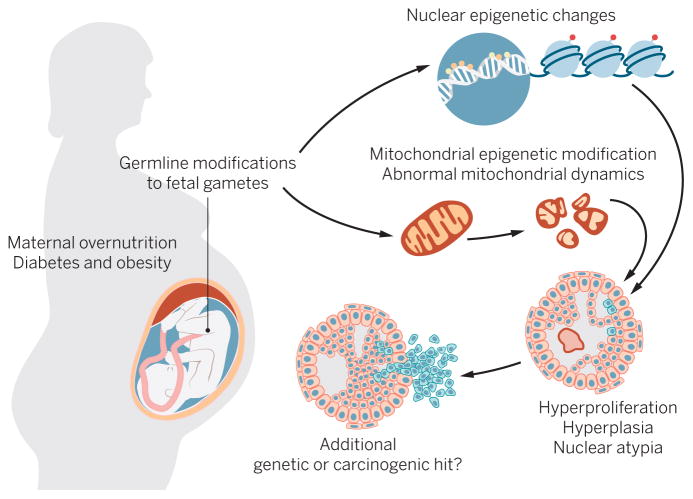
Among the obesity statistics, one that is particularly alarming is that 37% of reproductive-age (25 to 54 years old) women are obese (1). This is alarming because maternal obesity dramatically affects the health of a pregnancy, and rodent and human studies demonstrate that the effects manifest in a variety of conditions in offspring (4, 9, 10). According to the Developmental Origins of Health and Disease Hypothesis, exposures occurring during embryonic and fetal life are critical to the developmental patterning of tissues in offspring (11). This has been best exemplified by studies reporting cardiometabolic defects in children of mothers who are obese during gestation [extensively reviewed in (12)]. Additionally, deregulated maternal energetics has been linked to mammary tumor development, reproductive and pituitary tumors, and prostate hyperplasia (13). Notably, a recent study from the Helsinki Birth Cohort found that maternal obesity is positively associated with all cancer outcomes in human offspring (14). In Fig. 1, we summarize possible maternal influences on cancer risk in offspring through malfunctioning mitochondria and/or deregulated epigenetic signatures.
Fig. 1. A schematic of possible maternal influences on cancer risk in offspring.
In a maternal obesogenic environment, offspring cells could inherit malfunctioning mitochondria and/or deregulated epigenetic signatures. These alterations contribute to a tissue environment characterized by hyperplasia and nuclear atypia. When combined with additional genetic or carcinogenic hits, this predisposed phenotype may result in cancer in exposed offspring.
CREDIT: H. MCDONALD/SCIENCE TRANSLATIONAL MEDICINE
Breast cancer incidence has long been associated with maternal exposures (15), but the data regarding maternal energetics and generationally transmitted breast cancer risk in humans are mixed. For example, a study combining two small population data sets showed that pregnancy weight gain, but not prepregnancy BMI, was positively associated with breast cancer risk in offspring (16). Prospective data from the Nurses’ Mothers study showed no evidence of association between maternal prepregnancy BMI or gestational weight gain and breast cancer risk in daughters (17). Other U.S.-based reports indicate that higher prepregnancy BMI is related to lower risk of subsequent breast cancer (18) but higher birth weight for offspring. The inverse association for prepregnancy BMI is consistent with the overall inverse association for higher BMI at age 18. Past studies, however, do not reflect the current prevalence of obesity among mothers, thus limiting the inferences. Conversely, numerous studies reviewed and combined quantitatively (19) show a positive linear association between increased birth weight and breast cancer risk, suggesting that other conditions influenced by gestational obesity, such as fluctuations in hormone concentrations, cytokines, or lipids, promote development of breast cancer. Thus, the role of maternal obesity as a risk factor for breast cancer in humans is somewhat contradictory, but maternal obesity may indirectly influence breast cancer risk by affecting offspring health factors such as birth weight.
One challenge to studying generational transmission of cancer risk is the long latency between perinatal exposures and cancer development, which makes prospective studies difficult and costly. Likewise, retrospective studies are challenged by the fact that at the time of cancer diagnosis, a patient’s recall of maternal weight during pregnancy is unreliable. Thus, many have turned to animal models, such as mice and rats, which offer shorter generation times, ability to control the time window of exposure to obesity, and the ability to strictly control diet composition, thus allowing assessment of specific dietary components.
Rats are a very useful model for breast cancer because they both develop tumors spontaneously after 18 months and readily develop them earlier in genetic models or when treated with carcinogens such as 7,12-dimethylbenz[a]anthracene (DMBA) (20). In these models, the additive effects of maternal obesity and tumor induction on transmission of mammary tumor risk have been extensively evaluated (21). For example, Hilakivi-Clarke et al. demonstrated that consumption of a high-fat diet [consisting of 46% calories from the ω-6 polyunsaturated fatty acid (PUFA) linoleic acid] increased circulating estradiol concentrations in the dams and caused a twofold increase in DMBA-induced mammary tumor incidence in offspring, despite the fact that the offspring consumed control chow after weaning (22). Additional studies have determined that generational transmission of mammary tumor risk extends to the next two generations (23) and that perinatal exposure to a high-fat diet is more important to the phenotype than post-natal exposure (24). Interestingly, the effect of maternal high-fat diet exposure may be specific to plant-derived ω-6 PUFAs, because the tumorigenic effects of maternal ω-6 PUFA exposure were not seen when the rats were fed an animal fat-based diet and could be abrogated by coexposure to ω-3 PUFAs (25). Thus, during the perinatal time window, patterning of adult breast tissue may be exquisitely sensitive to exposure to certain fatty acids.
The mechanism by which mammary tumor risk is transmitted to offspring may involve deregulation of expression of structural and functional genes. For example, in one transgenic mammary tumor model, maternal high-fat diet exposure increased tumor number and size and affected insulin concentrations and expression of oxidative stress markers (26). Changes were also observed in expression of tumor suppressors and oncogenes, and studies indicate that increased insulin sensitivity is associated with changes in expression of tumor-regulating genes. Another study found that tumors derived from rats whose mothers were obese overexpressed the DNA methyltransferases DNMT1, DNMT3a, and DNMT3b, suggesting that the DNA methylation machinery was impaired (23). This is important because other studies have demonstrated that the estrogen receptor gene of obesity-exposed animals is hypomethylated and overexpressed, whereas the estrogen receptor in low-fat diet–exposed animals is nearly completely methylated and underexpressed (20). Because impaired estrogen receptor signaling is a critical factor regulating development of breast tissue and adenocarcinomas in rodents and humans (27), deregulated epigenetic patterning is a direct mechanism by which maternal diet could influence breast cancer development in offspring.
Another strong possibility is that maternal obesity causes alterations in tissue metabolism. For example, several animal studies have suggested that maternal consumption of an obesogenic diet, during or just before gestation, directly alters the health of both oocytes and early embryos (28). Additionally, marked alterations in mitochondrial structure and metabolic function of oocytes were reported in female mice after just 6 weeks of consuming a high-fat/high-sucrose diet (28, 29). These maternal mitochondrial differences could be carried over to the next generation, either by mitochondrial inheritance or as nuclear or mitochondrial genome epigenetic modifications. These germline modifications could then subsequently affect every tissue in the offspring, predisposing the offspring to abnormal mitochondrial performance and, perhaps, to the development of cancer in susceptible tissues.
Whereas some studies have addressed transmission of risk of breast and prostate cancer (13, 22), little work has been done to assess the mechanisms by which maternal obesity increases the risk of other forms of cancer. All the current data suggest that maternal obesity is linked to other cancer outcomes through secondary mechanisms. For example, a recent review proposed that maternal obesity influences obesity and weight gain in offspring and therefore predisposes offspring to colorectal cancer (30). This is founded on the observation that obesity of the patient at diagnosis and weight gain in adult life are risk factors for colorectal cancer (30). The same authors also suggested a similar mechanism for hepatic carcinoma. Last, Walker and colleagues have demonstrated that mice whose mothers consumed a high-fat diet had an increased risk of developing reproductive and pituitary tumors (31), but no mechanism has yet been determined.
CHILDHOOD AND ADOLESCENT OBESITY AND OUTCOMES
In utero and early life exposures may drive subsequent tumor growth, or exposures during childhood and adolescence may themselves confer a risk of future cancer. Increasing evidence from prospective cohorts shows that adiposity in childhood, typically assessed between ages 5 and 10 years, is inversely related to subsequent risk of breast cancer (7). This association is strongest for estrogen receptor–negative (ER−) disease, including basal-like breast cancer (32), although the mechanism for this association is not understood. There is also an inverse association for increasing adolescent adiposity and breast cancer risk, and a meta-analysis shows that this association is of comparable magnitude among women of Caucasian and African heritage (33). How adiposity early in life lowers the risk of breast cancer across the life course has been examined in a number of ways. Childhood adiposity has dual opposing effects on breast cancer risk. Although it is associated with earlier menarche (increasing breast cancer risk), childhood adiposity also results in lower peak height growth velocity (34), which is associated with lower cancer risk in some studies (35). Some studies use intermediate markers of cancer risk, including benign breast disease and mammographic density. Childhood adiposity is inversely related to the risk of benign breast lesions, and more rapid height growth is directly related to risk (34). Moreover, some studies found that childhood adiposity was associated with lower risk of adult mammographic density. This reduced density may mediate the influence of childhood growth rate and adiposity on breast cancer risk (36), although a meta-analysis of 19 studies shows that mammographic breast density is related with comparable magnitude with that of ER− and ER+ breast cancer (37).
OBESITY DURING THE REPRODUCTIVE YEARS
It is generally accepted that higher adiposity in early adult and premenopausal years is related to a lower risk of premenopausal breast cancer (38). However, recent more-detailed studies (39–41) of weight change across the life course point to the interplay of the protection by childhood adiposity and the adverse effect of subsequent adult weight gain. European prospective data showed that weight gain during age of 40 to 50 years was associated with subsequent risk of breast cancer after the age of 50 years (39). Other studies of women in their 40s have related weight gain to the risk of pre- and postmenopausal breast cancer (40). In the Nurses’ Health Study, recent weight gain over 4 years was associated with increased risk of premenopausal breast cancer, and the risk was greater for ER− progesterone receptor–negative (PR−) and ER+PR− disease than for ER+PR+ disease (41). Moreover, obesity increases free insulin-like growth factor 1 (IGF1) among both normal-weight and overweight women and is associated with increased risk of premenopausal and postmenopausal breast cancer (42).
OBESITY AFTER MENOPAUSE
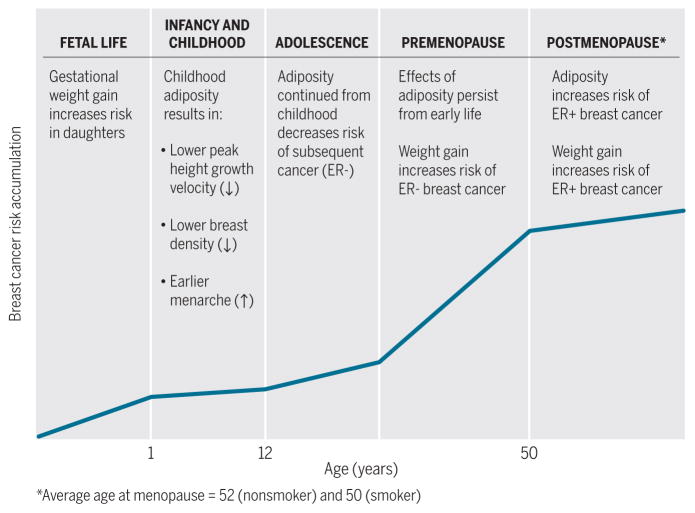
Obesity after menopause (occurring as a result of weight gain during either the premenopausal or postmenopausal years) is directly related to risk of breast and endometrial cancer (43). In a meta-analysis summarizing some 50 studies, adult weight gain was associated with increased risk of postmenopausal breast cancer [among nonhormone therapy users, the relative risk (RR) per 5 kg increase in weight gain was 1.11, 95% confidence interval (CI) 1.08 to 1.13], postmenopausal endometrial cancer (RR = 1.39; 9%% CI 1.29 to 1.49), and postmenopausal ovarian cancer (RR = 1.13; 95% CI 1.03 to 1.23) (44). Adult weight gain is more strongly related to ER+ breast cancer than ER− breast cancer (40). Additionally, a report from Mexico that used trajectories of estimated weight gain showed that increasing adiposity from age 8 was positively related to increased risk of breast cancer among women aged 39 to 69 years (45). Importantly, the connection between adult weight gain and postmenopausal breast cancer risk appears to be modified by use of hormone therapy, and the excess risk associated with weight gain was largely eliminated among women who used hormone therapy because they were at a greater risk regardless of weight (44). This finding is consistent with an analysis of prospective cohorts in which plasma hormone concentrations were measured. This study found that the association of obesity with postmenopausal breast cancer was largely explained by circulating free estradiol; the RR per 5 kg increase in BMI was 1.19 (95% CI 1.05 to 1.34) but was reduced to 1.02 (0.89 to 1.17) after controlling for free estradiol (46). These studies all suggest that the detrimental effects of obesity after menopause are clearest among women not using hormone therapy and are largely mediated through circulating estrogens. The use of hormone therapy raises estrogen concentrations and thereby increases cancer risk in all women, obscuring the effect of adiposity on breast cancer risk. The directions of the associations between adiposity during life and breast cancer risk are summarized in Fig. 2. For example, the effects of adiposity and weight gain in premenopausal years reflect persisting protection from earlier adiposity and an opposite or adverse effect of weight gain increasing cancer risk compared with that of normal BMI and no weight gain.
Fig. 2. Life course relationship of adiposity and weight gain with breast cancer risk.
Breast cancer risk accumulates across the life course, summarized here by time periods labeled as fetal life, infancy and childhood, adolescence, premenopause, and postmenopause because the demarcation of these periods varies modestly among women. The relationship of adiposity with future breast cancer risk is shown relative to the risk of cancer for women with BMI in the normal range.
CREDIT: H. MCDONALD/SCIENCE TRANSLATIONAL MEDICINE
Obesity also is related to recurrence of breast cancer and death after breast cancer (47). A meta-analysis of 82 studies shows that obesity correlates with recurrence (increasing risk with increasing BMI) (47). This systematic review and meta-analysis included more than 200,000 women with breast cancer and more than 40,000 deaths. For obese women compared with normal-weight women, the relative risk of breast cancer death was 1.75 for women with premenopausal and 1.34 for postmenopausal breast cancer. Importantly, there was no protective effect of obesity on survival among premenopausal women. The increase in mortality may be mediated through higher estrogen and higher insulin concentrations among obese women because both of these hormones have been linked to poor prognosis in women with breast cancer (48).
WEIGHT LOSS
Evidence as to whether sustained weight loss reduces the incidence of cancer is limited, in large part because of low long-term success of weight loss interventions. However, weight loss after bariatric surgery is more sustained than after other interventions and is protective against endometrial cancer (49). In addition, weight loss after menopause has been related to reduced risk of postmenopausal breast cancer (50), and one study suggested that weight loss of more than 5 kg after age 18 reduced the risk of premenopausal breast cancer (51). Numerous studies examining the effects of weight loss interventions have evaluated intermediate markers such as hormone concentrations (52, 53), but thus far, no studies have been conducted to investigate the effects of sustained weight loss interventions on cancer incidence.
In wild-type mice, weight and metabolic phenotype normalize with exercise and/or weight loss, but thus far, no studies have directly looked at reversal of tumor progression (54). In a recent study, investigators fed an obesogenic diet to a mouse model predisposed to develop basal-like breast cancer and found that tumor growth was prevented when obese mice were induced to lose weight by switching to a control low-fat diet before tumor onset (55).
TRANSLATIONAL IMPLICATIONS.
Excess energy intake relative to expenditure results in positive energy balance, excess weight gain, and numerous chronic conditions. Disentangling the timing of exposure to excess adiposity in relation to transmission of cancer risk across generations is a difficult task, and thus this topic remains understudied. However, this issue is important for future generations as the global increase in obesity results in more pregnancies and deliveries among overweight and obese mothers. The current evidence indicates that with the exception of childhood and adolescent adiposity reducing breast cancer risk, obesity generally increases cancer risk. Because long-term human studies are so challenging, future studies that bridge animal models and humans will accelerate our understanding of these connections. Priorities for study include better understanding of timing of adiposity in relation to conception and cancer risk; the mechanisms by which childhood adiposity, growth velocity, and breast density affect breast cancer risk; and the mechanisms underlying connections between birth weight and breast cancer.
Acknowledgments
Funding: NIH U54 CA155496 and 1R01HD083895.
Footnotes
Competing interests: The authors declare they have no competing interests.
References and notes
- 1.Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007–2012. JAMA Intern Med. 2015;175:1412–1413. doi: 10.1001/jamainternmed.2015.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312:189–190. doi: 10.1001/jama.2014.6228. [DOI] [PubMed] [Google Scholar]
- 4.Jungheim ES, Moley KH. Current knowledge of obesity’s effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol. 2010;203:525–530. doi: 10.1016/j.ajog.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-67360860269-X. [DOI] [PubMed] [Google Scholar]
- 6.Troy JD, Hartge P, Weissfeld JL, Oken MM, Colditz GA, Mechanic LE, Morton LM. Associations between anthropometry, cigarette smoking, alcohol consumption, and non-Hodgkin lymphoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Epidemiol. 2010;171:1270–1281. doi: 10.1093/aje/kwq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–1194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes Rev. 2015;17:56–67. doi: 10.1111/obr.12316. [DOI] [PubMed] [Google Scholar]
- 9.Jungheim ES, Travieso JL, Carson KR, Moley KH. Obesity and reproductive function. Obstet Gynecol Clin North Am. 2012;39:479–493. doi: 10.1016/j.ogc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: Abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benesh E, Moley K. In: Energy Balance and Cancer. Berger N, editor. Springer International Publishing; 2015. pp. XXX–XXX. [Google Scholar]
- 13.Benesh EC, Humphrey PA, Wang Q, Moley KH. Maternal high-fat diet induces hyperproliferation and alters Pten/Akt signaling in prostates of offspring. Sci Rep. 2013;3:3466. doi: 10.1038/srep03466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: Findings from the Helsinki Birth Cohort Study. Ann Med. 2014;46:434–438. doi: 10.3109/07853890.2014.919728. [DOI] [PubMed] [Google Scholar]
- 15.Sanderson M, Williams MA, Malone KE, Stanford JL, Emanuel I, White E, Daling JR. Perinatal factors and risk of breast cancer. Epidemiology. 1996;7:34–37. doi: 10.1097/00001648-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson M, Williams MA, Daling JR, Holt VL, Malone KE, Self SG, Moore DE. Maternal factors and breast cancer risk among young women. Paediatr Perinat Epidemiol. 1998;12:397–407. doi: 10.1046/j.1365-3016.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson KM, Willett WC, Michels KB. Mothers’ pre-pregnancy BMI and weight gain during pregnancy and risk of breast cancer in daughters. Breast Cancer Res Treat. 2011;130:273–279. doi: 10.1007/s10549-011-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troisi R, Doody DR, Mueller BA. A linked-registry study of gestational factors and subsequent breast cancer risk in the mother. Cancer Epidemiol Biomarkers Prev. 2013;22:835–847. doi: 10.1158/1055-9965.EPI-12-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SK, Kang D, McGlynn KA, Garcia-Closas M, Kim Y, Yoo KY, Brinton LA. Intrauterine environments and breast cancer risk: Meta-analysis and systematic review. Breast Cancer Res. 2008;10:R8. doi: 10.1186/bcr1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yenbutr P, Hilakivi-Clarke L, Passaniti A. Hypomethylation of an exon I estrogen receptor CpG island in spontaneous and carcinogen-induced mammary tumorigenesis in the rat. Mech Ageing Dev. 1998;106:93–102. doi: 10.1016/S0047-6374(98)00093-1. [DOI] [PubMed] [Google Scholar]
- 21.La Merrill M, Harper R, Birnbaum LS, Cardiff RD, Threadgill DW. Maternal dioxin exposure combined with a diet high in fat increases mammary cancer incidence in mice. Environ Health Perspect. 2010;118:596–601. doi: 10.1289/ehp.0901047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman M. A maternal diet high in n - 6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci USA. 1997;94:9372–9377. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Assis S, Warri A, Cruz MI, Laja O, Tian Y, Zhang B, Wang Y, Huang TH, Hilakivi-Clarke L. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun. 2012;3:1053. doi: 10.1038/ncomms2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo CY, Hsieh PH, Chen HF, Su HM. A maternal high-fat diet during pregnancy in rats results in a greater risk of carcinogen-induced mammary tumors in the female offspring than exposure to a high-fat diet in postnatal life. Int J Cancer. 2009;125:767–773. doi: 10.1002/ijc.24464. [DOI] [PubMed] [Google Scholar]
- 25.Su HM, Hsieh PH, Chen HF. A maternal high n-6 fat diet with fish oil supplementation during pregnancy and lactation in rats decreases breast cancer risk in the female offspring. J Nutr Biochem. 2010;21:1033–1037. doi: 10.1016/j.jnutbio.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Montales MT, Melnyk SB, Simmen FA, Simmen RC. Maternal metabolic perturbations elicited by high-fat diet promote Wnt-1-induced mammary tumor risk in adult female offspring via long-term effects on mammary and systemic phenotypes. Carcinogenesis. 2014;35:2102–2112. doi: 10.1093/carcin/bgu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerdivel G, Flouriot G, Pakdel F. Modulation of estrogen receptor alpha activity and expression during breast cancer progression. Vitam Horm. 2013;93:135–160. doi: 10.1016/B978-0-12-416673-8.00004-6. [DOI] [PubMed] [Google Scholar]
- 28.Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: Oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLOS ONE. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Han L, Ma R, Hou X, Yu Y, Sun S, Xu Y, Schedl T, Moley KH, Wang Q. Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle. 2015;14:2959–2968. doi: 10.1080/15384101.2015.1026517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmen FA, Simmen RC. The maternal womb: A novel target for cancer prevention in the era of the obesity pandemic? Eur J Cancer Prev. 2011;20:539–548. doi: 10.1097/CEJ.0b013e328348fc21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker BE. Tumors in female offspring of control and diethylstilbestrol-exposed mice fed high-fat diets. J Natl Cancer Inst. 1990;82:50–54. doi: 10.1093/jnci/82.1.50. [DOI] [PubMed] [Google Scholar]
- 32.Tamimi RM, Colditz GA, Hazra A, Baer HJ, Hankinson SE, Rosner B, Marotti J, Connolly JL, Schnitt SJ, Collins LC. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131:159–167. doi: 10.1007/s10549-011-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, Hainaut P. Overweight obesity and risk of premenopausal breast cancer according to ethnicity: A systematic review and dose-response meta-analysis. Obes Rev. 2013;14:665–678. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- 34.Berkey CS, Gardner JD, Frazier AL, Colditz GA. Relation of childhood diet and body size to menarche and adolescent growth in girls. Am J Epidemiol. 2000;152:446–452. doi: 10.1093/aje/152.5.446. [DOI] [PubMed] [Google Scholar]
- 35.Ahlgren M, Melbye M, Wohlfahrt J, Sørensen TI. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 36.Andersen ZJ, Baker JL, Bihrmann K, Vejborg I, Sørensen TI, Lynge E. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: A register-based cohort study. Breast Cancer Res. 2014;16:R4. doi: 10.1186/bcr3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antoni S, Sasco AJ, dos Santos Silva I, McCormack V. Is mammographic density differentially associated with breast cancer according to receptor status? A meta-analysis. Breast Cancer Res Treat. 2013;137:337–347. doi: 10.1007/s10549-012-2362-4. [DOI] [PubMed] [Google Scholar]
- 38.World Cancer Research Fund. Continous update project. American Institute for Cancer Research; 2010. Continous Update Project. Keeping the science current. Breast Cancer 2010 Report. [Google Scholar]
- 39.Emaus MJ, van Gils CH, Bakker MF, Bisschop CN, Monninkhof EM, Bueno-de-Mesquita HB, Travier N, Berentzen TL, Overvad K, Tjønneland A, Romieu I, Rinaldi S, Chajes V, Gunter MJ, Clavel-Chapelon F, Fagherazzi G, Mesrine S, Chang-Claude J, Kaaks R, Boeing H, Aleksandrova K, Trichopoulou A, Naska A, Orfanos P, Palli D, Agnoli C, Tumino R, Vineis P, Mattiello A, Braaten T, Borch KB, Lund E, Menéndez V, Sánchez MJ, Navarro C, Barricarte A, Amiano P, Sund M, Andersson A, Borgquist S, Olsson A, Khaw KT, Wareham N, Travis RC, Riboli E, Peeters PH, May AM. Weight change in middle adulthood and breast cancer risk in the EPIC-PANACEA study. Int J Cancer. 2014;135:2887–2899. doi: 10.1002/ijc.28926. [DOI] [PubMed] [Google Scholar]
- 40.Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: A meta-analysis. Breast Cancer Res Treat. 2010;123:641–649. doi: 10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- 41.Rosner B, Eliassen AH, Toriola AT, Hankinson SE, Willett WC, Natarajan L, Colditz GA. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res Treat. 2015;150:643–653. doi: 10.1007/s10549-015-3344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Key TJ, Appleby PN, Reeves GK, Roddam; AW. Endogenous Hormones and Breast Cancer Collaborative Group, Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.International Agency for Research on Cancer. Weight Control and Physical Activity. Vol. 6. IARC Handbook on Cancer Prevention (International Agency for Research on Cancer; 2002. p. 315. [Google Scholar]
- 44.Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: A dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107:dju428. doi: 10.1093/jnci/dju428. [DOI] [PubMed] [Google Scholar]
- 45.Amadou A, Torres Mejia G, Fagherazzi G, Ortega C, Angeles-Llerenas A, Chajes V, Biessy C, Sighoko D, Hainaut P, Romieu I. Anthropometry, silhouette trajectory, and risk of breast cancer in Mexican women. Am J Prev Med. 2014;46(Suppl 1):S52–S64. doi: 10.1016/j.amepre.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Jr, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Longcope C Endogenous Hormones Breast Cancer Collaborative Group. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 47.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodwin PJ, Ennis M, Bahl M, Fantus IG, Pritchard KI, Trudeau ME, Koo J, Hood N. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. 2009;114:517–525. doi: 10.1007/s10549-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 49.Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. Effect of bariatric surgery on oncologic outcomes: A systematic review and meta-analysis. Surg Endosc. 2013;27:4449–4456. doi: 10.1007/s00464-013-3127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 51.Michels KB, Terry KL, Eliassen AH, Hankinson SE, Willett WC. Adult weight change and incidence of premenopausal breast cancer. Int J Cancer. 2012;130:902–909. doi: 10.1002/ijc.26069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rock CL, Pande C, Flatt SW, Ying C, Pakiz B, Parker BA, Williams K, Bardwell WA, Heath DD, Nichols JF. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13:188–195. doi: 10.1016/j.clbc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mason C, Xiao L, Imayama I, Duggan CR, Campbell KL, Kong A, Wang CY, Alfano CM, Blackburn GL, Foster-Schubert KE, McTiernan A. The effects of separate and combined dietary weight loss and exercise on fasting ghrelin concentrations in overweight and obese women: A randomized controlled trial. Clin Endocrinol (Oxf) 2015;82:369–376. doi: 10.1111/cen.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, Giri D, Hudis CA, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila) 2013;6:282–289. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Sundaram S, Le TL, Essaid L, Freemerman AJ, Huang MJ, Galanko JA, McNaughton KK, Bendt KM, Darr DB, Troester MA, Makowski L. Weight loss reversed obesity-induced HGF/c-Met pathway and basal-like breast cancer progression. Front Oncol. 2014;4:175. doi: 10.3389/fonc.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]