Abstract
Pseudomonas aeruginosa (Pa) and Aspergillus fumigatus (Af) colonize cystic fibrosis (CF) patient airways. Pa culture filtrates inhibit Af biofilms, and Pa non-CF, mucoid (Muc-CF) and nonmucoid CF (NMuc-CF) isolates form an ascending inhibitory hierarchy. We hypothesized this activity is mediated through apoptosis induction. One Af and three Pa (non-CF, Muc-CF, NMuc-CF) reference isolates were studied. Af biofilm was formed in 96 well plates for 16 h ± Pa biofilm filtrates. After 24 h, apoptosis was characterized by viability dye DiBAc, reactive oxygen species (ROS) generation, mitochondrial membrane depolarization, DNA fragmentation and metacaspase activity. Muc-CF and NMuc-CF filtrates inhibited and damaged Af biofilm (p<0.0001). Intracellular ROS levels were elevated (p<0.001) in NMuc-CF-treated Af biofilms (3.7- fold) compared to treatment with filtrates from Muc-CF- (2.5- fold) or non-CF Pa (1.7- fold). Depolarization of mitochondrial potential was greater upon exposure to NMuc-CF (2.4-fold) compared to Muc-CF (1.8-fold) or non-CF (1.25-fold) (p<0.0001) filtrates. Exposure to filtrates resulted in more DNA fragmentation in Af biofilm, compared to control, mediated by metacaspase activation. In conclusion, filtrates from CF-Pa isolates were more inhibitory against Af biofilms than from non-CF. The apoptotic effect involves mitochondrial membrane damage associated with metacaspase activation.
Introduction
Cystic fibrosis (CF) is an inherited disorder in which a mutation of the CF transmembrane conductance regulator gene (CFTR) leads to defective salt and water channels [1]. Despite significant progress in antimicrobial therapy and supportive care, chronic pulmonary infections and destructive inflammatory processes are still the major causes of morbidity and mortality of CF patients. CF patients have persistent colonization or infection with various opportunistic bacterial and fungal pathogens, of which Pseudomonas aeruginosa and Aspergillus fumigatus are the most prominent.
Both A. fumigatus and P. aeruginosa have been shown to form complex aggregates of microorganisms in vivo, growing as biofilms with an extracellular matrix. Biofilms form a physical barrier to host defenses, and their formation often results in resistance to antimicrobials [2–4]. Long-term colonization of CF patients with P. aeruginosa is associated with changes to the bacterium, including an increased mutation frequency with the temporal development of genotypic and phenotypic variants, such as a mucoid type, in the CF lungs [5]. Concurrent colonization with both P. aeruginosa and A. fumigatus in CF patients leads to further decline in pulmonary function compared to mono-colonization with either microbe [5]. Interestingly, in a murine pulmonary model, mice co-infected with P. aeruginosa and A. fumigatus had a higher survival rate than mice infected by A. fumigatus alone, suggesting that P. aeruginosa may secrete antifungal compounds [6]. Small molecules secreted by Pseudomonas can inhibit biofilm formation by Aspergillus [5]. We have previously reported, in a study with 26 isolates of P. aeruginosa, that non-mucoid CF isolates (NMuc-CF) were more inhibitory to formation of Aspergillus biofilms or to preformed biofilms, than mucoid isolates (Muc-CF), and that mucoid isolates were more inhibitory than non-CF isolates [7]. In addition, it was noted that P. aeruginosa biofilm culture filtrates were more inhibitory against A. fumigatus biofilm formation than were P. aeruginosa filtrates from planktonic cultures.
In this study, our goal was to assess whether the inhibitory effects of biofilm culture filtrates from 3 different P. aeruginosa variants (i.e., Muc-CF, NMuc-CF, and non-CF), obtained from CF and non-CF patients, on A. fumigatus biofilms are mediated through induction of apoptosis.
Materials and Methods
Isolates and growth conditions
Representative P. aeruginosa isolates, Muc-CF, NMuc-CF, and non-CF types, were selected from homogeneous phenotype groups, as studied previously [7]. P. aeruginosa stocks were maintained at -80°C in Microbank microbial storage vials (Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada). P. aeruginosa stock culture was initially inoculated on Trypticase Soy Agar plates containing 5% sheep blood agar (TSA; BBL, Becton Dickinson) and incubated overnight at 37°C.
To obtain the P. aeruginosa biofilm culture filtrates, a suspension of 104 cells/mL of P. aeruginosa, were prepared in fresh RPMI 1640 medium without serum and allowed to adhere to tissue culture flasks (BD Biosciences, San Diego) for 2 h at 37°C on a 100 rpm shaker incubator (attachment phase). The medium was then removed and the flasks were rinsed gently 3 times with sterile saline. After washing, 20 ml of fresh RPMI-1640 was added to the flask and adhered cells formed P. aeruginosa biofilms in 24 h at 37°C. The medium was removed, transferred to a 50 ml conical tube, and centrifuged for 30 min at 2,000 x g to remove any suspended cells or debris. The biofilm supernatant was gently removed, filter sterilized (0.22 μm filter) (Fisher, Pittsburgh, PA) and used immediately.
A. fumigatus (reference strain 10Af) [7, 8] from frozen stock cultures was inoculated on potato dextrose agar plates and incubated for 72 hours at 37°C. The A. fumigatus conidia were harvested by flooding the surface of the agar plates with saline containing 0.05% (v/v) Tween 80. The conidia suspension was recovered and dispensed into a 50-mL conical centrifuge tube and stored at 4°C. Before use, the suspension was centrifuged for 15 minutes at 3000 x g at 4°C. The pellet was washed twice by centrifugation with sterile phosphate-buffered saline (PBS) and finally suspended in RPMI-1640.
To initiate biofilm formation, 100 μl of a suspension of A. fumigatus conidia was placed into each well (105 conidia/well) of flat-bottom 96-well plates. When the wells were seeded, the entire microtiter plate was sealed with para-film and incubated for 16 h at 37°C in a shaker incubator at 100 rpm to allow the fungi to form biofilm. After biofilm formation, the medium was carefully aspirated, minimizing contact with the biofilm. The wells were washed 3 times with PBS, and 150 μl of fresh RPMI-1640 was added to each well. The plate was further incubated for 24 h at 37°C in a shaker incubator at 100 rpm to allow the fungi to form mature biofilms.
Effect of P. aeruginosa culture filtrates on preformed A. fumigatus biofilm
A) XTT assay
To measure the activity of P. aeruginosa biofilm culture filtrate against A. fumigatus biofilms, the Aspergillus biofilm was grown for 16 h and the preformed biofilm was challenged with Pseudomonas filtrates (1:1 vol./vol. of Pseudomonas filtrate and RPMI) obtained from the non- CF, Muc-CF or NMuc-CF strains respectively, and were further incubated for 24 h at 37°C. After incubation, plates were washed with PBS, and metabolic activity was assessed with the use of the 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) colorimetric formazan reduction assay. Briefly, 100 μl of XTT (1 mg) /menadione (0.17 mg) solution was added to each well. Plates were covered in aluminum foil and incubated in the dark for 2 h at 37°C. After incubation, 80 μl of the resulting colored supernatant from each well was removed and transferred into the corresponding wells of a new microtiter plate. Absorbance was recorded at 490 nm [9].
B) Viability assay
After 16 hours of incubation at 37°C, preformed A. fumigatus biofilm was exposed to P. aeruginosa non-CF, Muc-CF, or NMuc-CF biofilm filtrates. After 24 h of incubation with P. aeruginosa biofilm filtrates, the A. fumigatus biofilms were stained with the viability dye bis-[1,3-dibutylbarbituric acid] trimethine oxonol (DiBAC; Molecular Probes), which enters depolarized cells, as described previously [10, 11].
C) Detection of intracellular reactive oxygen species (ROS) in Aspergillus biofilms
After 16 h of A. fumigatus biofilm formation, intracellular reactive oxygen species (ROS) levels were measured in A. fumigatus biofilm exposed to P. aeruginosa filtrates, as previously described [11–16]. A. fumigatus biofilms were scraped from the plate and spiked with dihydrorhodamine (DHR) 123 (5.0 μg/ml). After 2 h at room temperature, the cells were washed with PBS, centrifuged at 13 000 x g for 5 minutes, harvested, and observed with a Nikon Microphot SA fluorescence microscope (excitation, 488 nm; emission, 520 nm). For quantitative assays, fluorescence intensity values were recorded by using a POLARstar Galaxy microplate reader (excitation, 488 nm; emission, 520 nm; BMG LABTECH.
D) Mitochondrial membrane potential measurement in A. fumigatus biofilms
Mitochondrial membrane depolarization was assessed by staining with rhodamine 123, a fluorescent dye that is distributed in the mitochondrial matrix, as described previously [11–16]. Briefly, preformed A. fumigatus biofilm scraped from a 96-well plate pre-exposed to P. aeruginosa filtrates (non-CF, Muc-CF, or NMuc-CF) for 24 h at 37°C, was harvested via centrifugation, washed twice, and resuspended in PBS. Rhodamine 123 was added to the final concentration of 10 μM, and the cells were incubated for 30 min in the dark at room temperature. Fluorescence intensity was recorded as described above (excitation, 488 nm; emission, 520 nm).
E) Measurement of DNA damage in A. fumigatus biofilms
DNA fragmentation, a characteristic of apoptosis, was detected using a terminal deoxynucelotidyltransferase-mediated dUTP nick end-labeling (TUNEL) assay [11–16]. After incubation for 16 h in RPMI-1640 medium, A. fumigatus biofilm was exposed to P. aeruginosa filtrate (non-CF, Muc-CF, or NMuc-CF) for 24 h at 37°C, fixed with 3.7% formaldehyde for 30 min on ice, and digested with a lysing enzyme mixture (0.25 mg/ml of chitinase, 15 U of lyticase, and 20 mg/ml lysing enzyme; Sigma-Aldrich) for 3 h at 30°C. The enzyme-digested cells were subjected to the TUNEL assay, as described in detail elsewhere [11–17]. The cells were observed for fluorescence as described above, with excitation and emission wavelengths of 488 nm and 520 nm, respectively.
F) Detection of metacaspase activity in Aspergillus biofilms
Activation of metacaspases was detected with the CaspACE FITC-VAD-FMK In Situ Marker (Promega), which fluoresces green when it binds to active metacaspases [14]. After 16 h of biofilm formation in RPMI-1640 medium, A. fumigatus biofilm was exposed to P. aeruginosa biofilm culture filtrates (non-CF, Muc-CF, or NMuc-CF) for 24 h at 37°C. The cells were then harvested, washed in PBS, and suspended in 10 μM CaspACE FITC-VAD-FMK solution. After 2 hours of incubation at room temperature, cells were washed twice and suspended in PBS. Samples were mounted and viewed in a fluorescence microscope as described above (emission, 488 nm; excitation, 520 nm).
Statistical analysis
For all assays, three independent experiments were performed three times on three different days in triplicate. Multiple treatment groups were compared using a Kruskal-Wallis test and post-hoc paired comparisons were done using Dunnett’s tests. Calculations were made with InStat (GraphPad Software). All results are expressed as means ± standard deviations. Two-tailed P values of less than 0.05 were considered statistically significant.
Results
P. aeruginosa culture filtrates depresses metabolism and damages membranes of A. fumigatus preformed biofilm
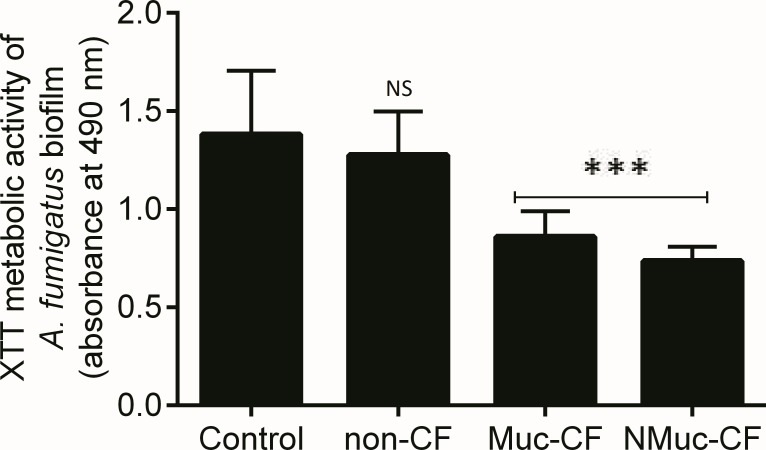
Using the XTT reduction assay, we found that the biofilm culture filtrates of P. aeruginosa strains recovered from CF patients (Muc-CF and NMuc-CF) resulted in significant inhibition of growth of A. fumigatus preformed biofilm (Fig 1). The NMuc-CF- and Muc-CF-Pa biofilm filtrates- treated Aspergillus biofilms showed significantly greater inhibition than the untreated controls (p <0.0001) and the non-CF-Pa treated biofilms (p <0.0001). The non-CF P. aeruginosa culture filtrate was not inhibitory compared to control (p>0.05). P. aeruginosa culture filtrate from NMuc-CF was more inhibitory than that from Muc-CF (p>0.05).
Fig 1. Inhibition of A. fumigatus biofilm formation of P. aeruginosa (Pa) biofilm culture filtrates: non-CF Pa isolate, mucoid CF Pa isolate (Muc-CF), and nonmucoid CF Pa isolate (NMuc-CF).
Bars represent metabolic activity as measured by the XTT assay and by spectrophotometry at 490 nm. Assays were performed in triplicate and results are presented as the mean of three replicates. Error bars represent the standard deviation of the mean. ***p <0.0001 (compared with untreated controls).
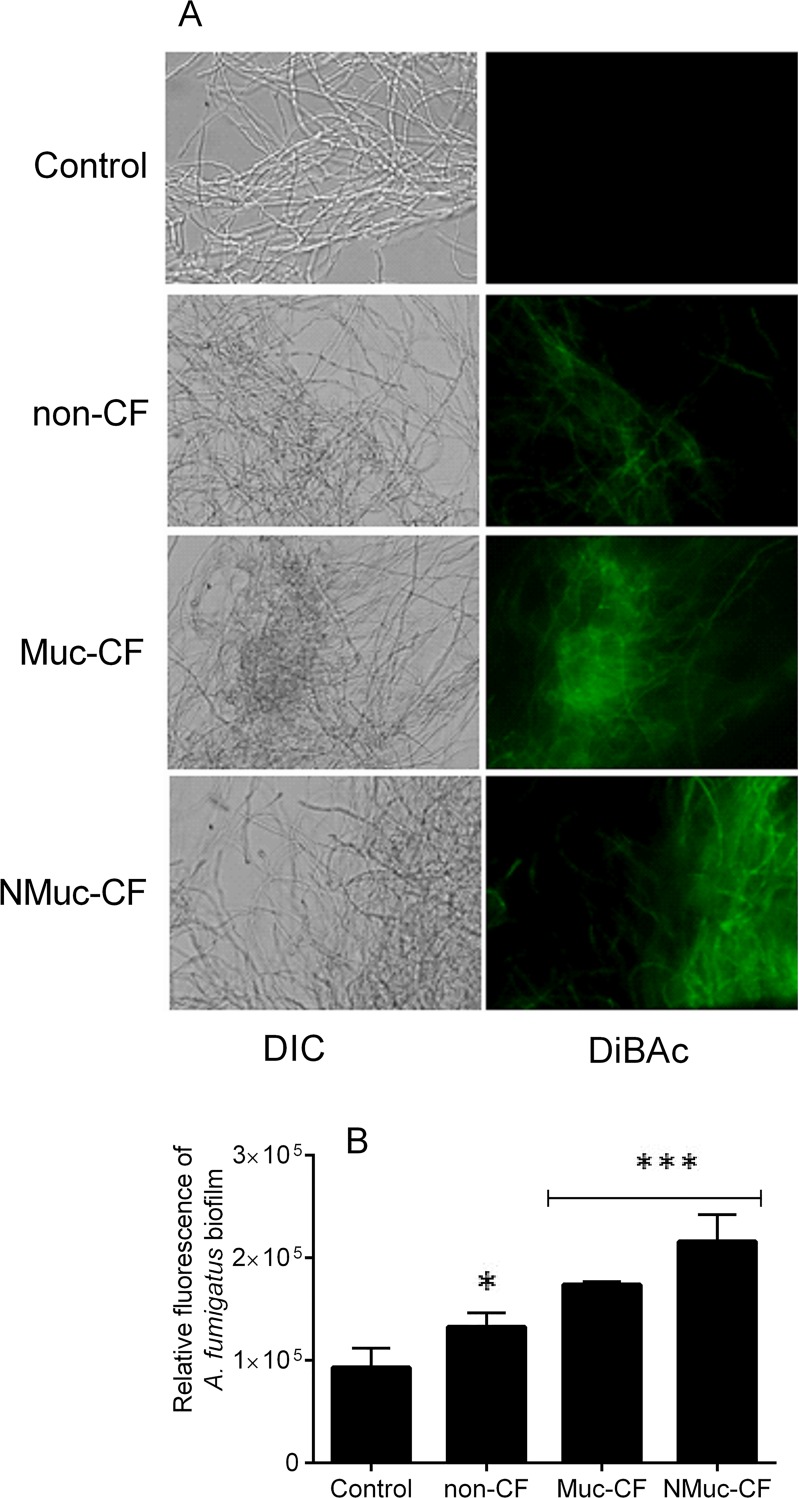
Furthermore, we observed that DiBAC uptake, a marker of membrane damage, was higher in A. fumigatus preformed biofilms treated with biofilm culture filtrates from NMuc-CF or Muc-CF P. aeruginosa strains than in A fumigatus biofilms biofilms exposed to non-CF P. aeruginosa biofilm culture filtrates or in untreated control A. fumigatus biofilms. Specifically, this effect was more prominent in preformed A. fumigatus biofilms treated with NMuc-CF culture filtrates (2.4-fold higher DiBAC uptake) than in biofilms treated with Muc-CF culture filtrates (Fig 2A and 2B, p <0.001) or non-CF filtrates (1.4- to 1.8-fold higher) (p<0.0001) (Fig 2B). Non-CF P. aeruginosa filtrates were significantly different than controls
Fig 2. Membrane damaging action of P. aeruginosa biofilm culture filtrates (non-CF, Muc-CF and NMuc-CF) on preformed A. fumigatus biofilms, as shown by the morbidity stain DiBAC.
(A) Fluorescence images of A. fumigatus preformed biofilms stained with DiBAC. (B) Relative fluorescence of preformed A. fumigatus biofilms stained with DiBAC. Bars represent fungicidal action as measured by DiBAC staining using spectrophotometry at 490 nm. Assays were performed in triplicate and results are presented as the mean of three replicates. Error bars represent the standard deviation of the mean. DIC: differential interference contrast. *p <0.05, ***p <0.0001 (compared with untreated controls).
Non-mucoid P. aeruginosa culture filtrates induce more pronounced apoptosis in preformed A. fumigatus biofilm
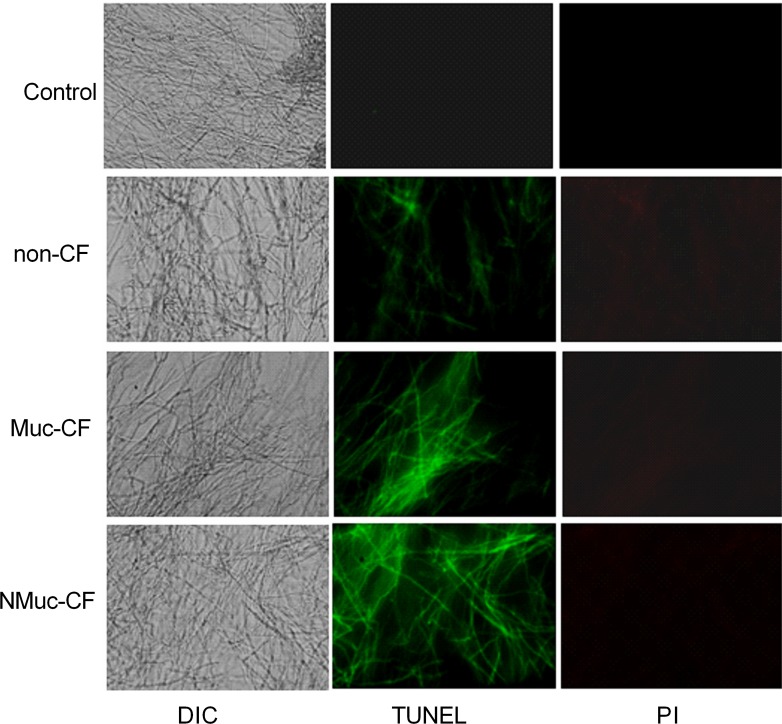
To assess whether cell death in A. fumigatus biofilms following exposure to culture filtrate of P. aeruginosa biofilms (non-CF, Muc-CF, and NMuc-CF) occurs through induction of apoptosis, we performed TUNEL assays to detect DNA fragmentation. Compared with untreated control, exposure to non-CF, Muc-CF, or NMuc-CF P. aeruginosa culture filtrates resulted in increasing degrees of DNA fragmentation in preformed A. fumigatus biofilms (Fig 3).
Fig 3. Culture filtrates of P. aeruginosa leads to DNA fragmentation and membrane disruption in preformed A. fumigatus biofilms, as shown by TUNEL assay and membrane integrity by propidium staining (PI).
Fluorescence images of DNA fragmentation of preformed A. fumigatus biofilms treated with biofilm culture filtrates of non-CF, Muc-CF, and NMuc-CF P. aeruginosa. DIC: differential interference contrast.
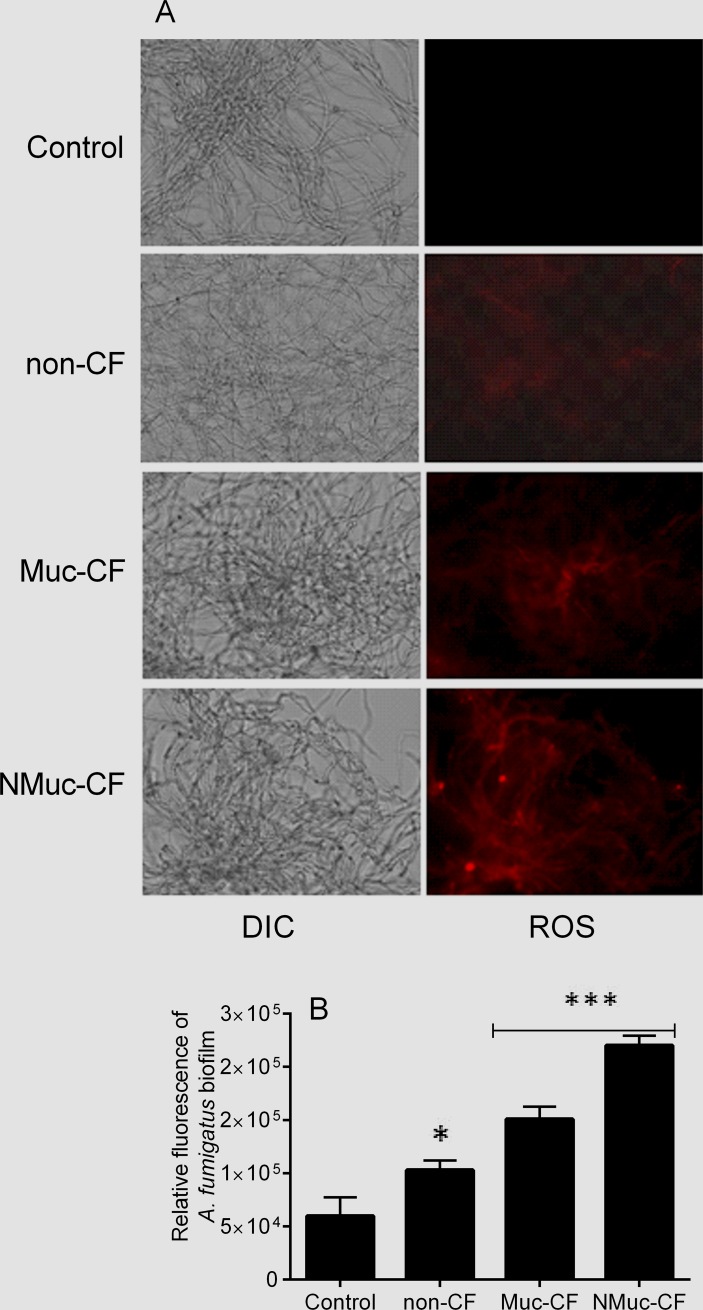
P. aeruginosa biofilm culture filtrate increase intracellular ROS accumulation in preformed A. fumigatus biofilms
ROS play an important role as early initiators of apoptosis in yeasts and other filamentous fungi [11–16]. Using a fluorometric assay with DHR-123 staining, we found significantly higher intracellular ROS levels in preformed A. fumigatus biofilms treated with P. aeruginosa culture filtrate compared with untreated controls. Specifically, in preformed A. fumigatus biofilms treated with NMuc-CF, the relative fluorescence was 3.7-fold (p<0.0001) compared with Muc-CF (2.5-fold) and non-CF (1.7-fold) treated and untreated controls (Fig 4A and 4B). Muc-CF-treated biofilms showed significantly higher relative fluorescence than did non-CF treated biofilms (p <0.0001), as did non-CF treated biofilms compared to untreated controls (p <0.0001).
Fig 4. Intracellular reactive oxygen species (ROS) accumulation detected in preformed A. fumigatus biofilms treated with culture filtrates of P. aeruginosa biofilm, as shown by staining with dihydrorhodamine (DHR)-123.
(A) Fluorescence images of preformed A. fumigatus treated with biofilm culture filtrates of non-CF, Muc-CF, and NMuc-CF P. aeruginosa stained with (DHR)-123. (B) Relative fluorescence of preformed A. fumigatus biofilms treated with P. aeruginosa biofilm culture filtrates stained with DHR 123. Bars represent ROS accumulation as shown by DHR 123 staining and by spectrophotometry at 490 nm. Assays were performed in triplicate and results are presented as the mean of three replicates. Error bars represent the standard deviation of the mean. DIC: differential interference contrast. *p<0.05, ***p<0.0001 (compared with untreated controls).
P. aeruginosa biofilm culture filtrate decreases mitochondrial membrane potential in preformed A. fumigatus biofilms
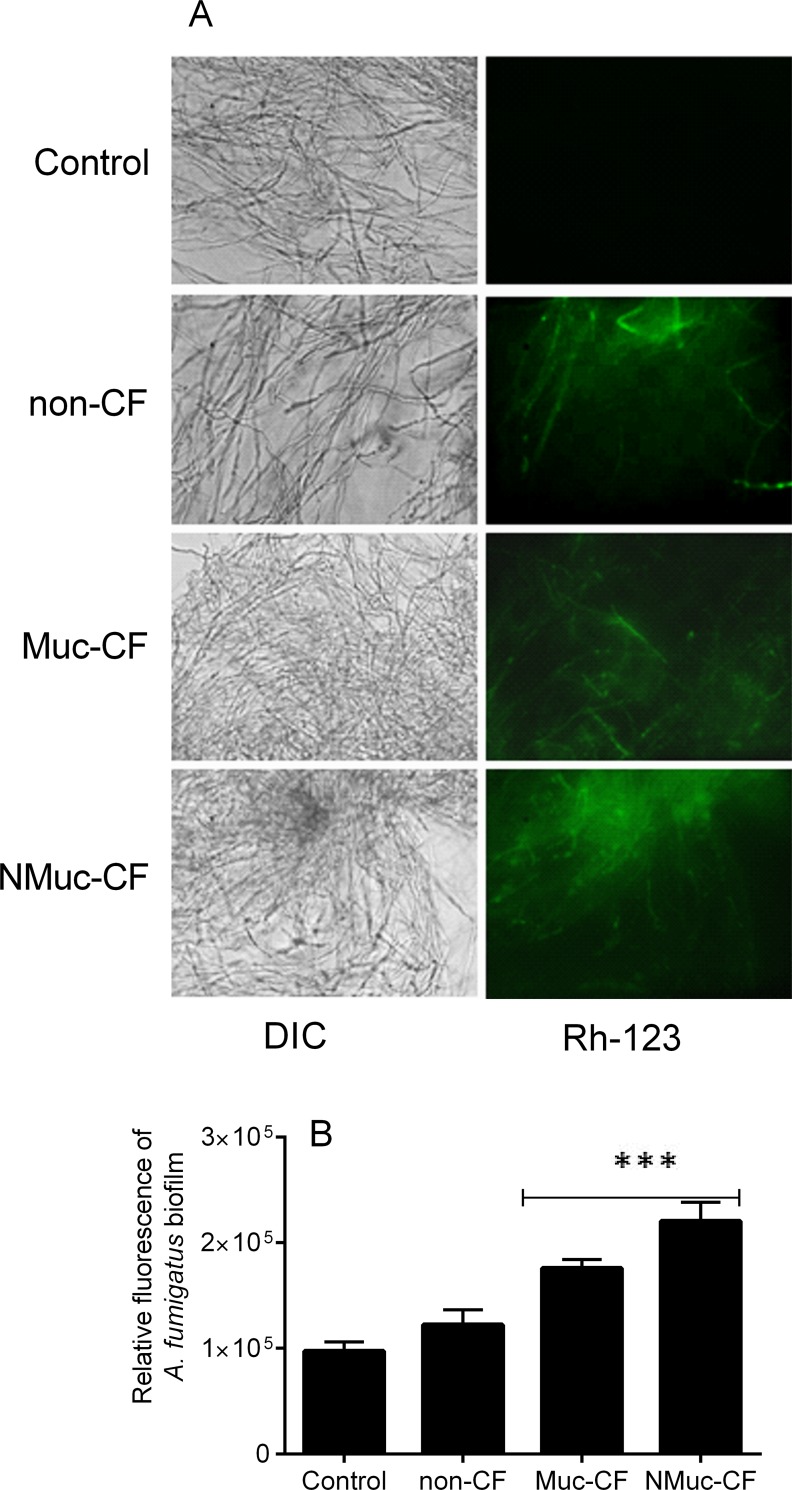
Mitochondrial membrane depolarization was assessed by staining with rhodamine (Rh)-123, a fluorescent dye that is distributed in the mitochondrial matrix, as previously described [11–16]. A. fumigatus preformed biofilm treated with NMuc-CF or Muc-CF P. aeruginosa culture filtrates showed sharp decreases in mitochondrial potential (1.8 to 2.4-fold increase in fluorescence compared with untreated controls; p<0.0001), whereas the mitochondrial potential in the non-CF-treated A. fumigatus biofilm increased fluorescence by 1.25-fold c compared to untreated control (p<0.0001) (Fig 5A and 5B). The difference observed in the NMuc-CF-treated biofilm compared with control was significantly greater than that seen in the Muc-CF-treated biofilm (p <0.0001).
Fig 5. Depolarization of mitochondrial membrane potential as shown by rhodamine 123 staining in A. fumigatus preformed biofilms treated with P. aeruginosa biofilm culture filtrates.
(A) Fluorescence images of preformed A. fumigatus biofilms treated with biofilm culture filtrates of non-CF, Muc-CF, and NMuc-CF P. aeruginosa stained with rhodamine 123. (B) Relative fluorescence of preformed A. fumigatus biofilms treated with P. aeruginosa biofilm culture filtrates stained with rhodamine 123. Bars represent mitochondrial membrane depolarization as shown by rhodamine 123 staining and by spectrophotometry at 490 nm. Assays were performed in triplicate and results are presented as the mean of three replicates. Error bars represent the standard deviation of the mean. ***p<0.0001 (compared with untreated controls).
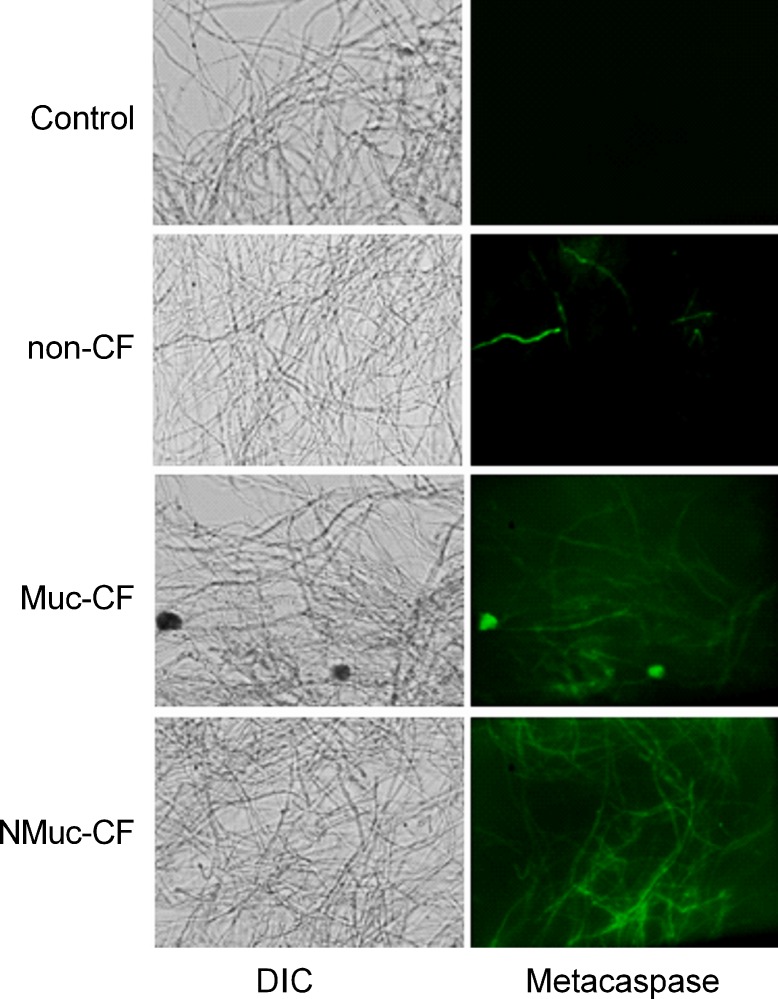
Biofilm culture filtrates of P. aeruginosa activate caspase-like activity in A. fumigatus preformed biofilms
Metacaspases, orthologs of mammalian caspases that are found in fungi and plants, are activated in the early stages of apoptosis and play a central role in the apoptotic cascade [18–20]. We detected metacaspase activation using CaspACE FITC-VAD-FMK In Situ Marker. The cells of the preformed A. fumigatus biofilm treated with biofilm culture filtrates of P. aeruginosa showed green fluorescence, indicating activation of the marker and suggesting that exposure to P. aeruginosa biofilms may induce apoptosis in A. fumigatus by activating metacaspases (Fig 6). Again, the inhibitory effect of biofilm filtrates from NMuc-CF and Muc-CF Pa was greater compared to that of non-CF Pa filtrates.
Fig 6. A. fumigatus preformed biofilms treated with P. aeruginosa biofilm culture filtrates activates caspase-like (metacaspase) activity.
Fluorescent images depicting metacaspase activation of preformed A. fumigatus biofilm treated with culture filtrates of non-CF, Muc-CF, and NMuc-CF P. aeruginosa, as detected by staining with CaspACE FITC-VAD-FMK. DIC: differential interference contrast.
Discussion
Our study confirms that P. aeruginosa biofilm culture filtrates significantly compromise A. fumigatus preformed biofilm and that this effect is associated with apoptosis induction in preformed biofilms. Although studying more strains of each phenotype would have augmented the correlation between apoptosis and the different groups studied. In a previous study, each of the 3 phenotypic groups formed into tight cohorts with respect to effect on A. fumigatus metabolism [7], thus making it rational to pick representative isolates for extensive studies, as detailed here.
Apoptosis is, in general, initiated by multiple extracellular and intracellular stimuli. In filamentous fungi, apoptosis is associated with mitochondria depolarization, ROS production, and metacaspase activity [11–16, 21]. Many microbes produce virulence factors that induce apoptosis in host cells [22], and several antimicrobial agents have been observed to be proapoptotic agents in a number of filamentous fungi [22]. However, the nature of the signaling and effector pathways that regulate fungal apoptosis still remains unclear [21].
Previous investigations have suggested that secondary metabolites of P. aeruginosa are responsible for its inhibitory effect on A. fumigatus biofilms [5]. Secondary metabolites from Pseudomonas, such as phenazines, join a growing list of compounds (e.g., H2O2, amphotericin B, the antifungal protein PAF, sphingoid bases, itraconazole, and posaconazole) that are known to trigger an apoptotic response in fungi [11–16, 23, 24–27]. Briard et al. [28] showed that shift from vegetative growth to conidiation in A. fumigatus is associated with phenazine radicals and ROS accumulation during phenazine redox cycling, which showed a dual role of phenazine as toxic at higher levels and as sporulation signal at moderate level. Phenazine 1-carboxamide, a Pseudomonas metabolite, has been reported to induce apoptosis in the non-pathogenic Zygomycete Benjaminiella poitrasii [23], and phenazine-like compounds have been shown to eliminate other fungal competitors in mammalian host environments [23, 29]. P. aeruginosa has been shown to produce phenazines, present in CF sputum at concentrations between 1 and 100 μM, which are important in regulating Aspergillus development [30, 31]. Zheng et al. [31] have demonstrated that bacterial metabolites can act as sporulation signals affecting fungal development in mixed-species communities and that bacterial phenazine production modulates P. aeruginosa-A. fumigatus co-culture biofilm formation. Denial of iron appears to be another important mechanism of inhibition of Aspergillus by Pseudomonas [8].
Intracellular accumulation of ROS is another major stimulus for the induction of apoptosis in eukaryotes; in fact, it is considered one of the important hallmarks of apoptosis [24, 25]. ROS accumulation is understood to be a converging pathway underlying cellular damage induced by different types of stress, including oxidative stress [13, 21]. Antifungal agents primarily act through stimulating ROS accumulation in yeast and filamentous fungi [24, 32, 33, 34]. Quorum-sensing molecules are also thought to play an important role in determining pathogens’ ability to compete with each other for space and nutrients, and may contribute to persistent infections in the airways of CF patients [4]. The quorum-sensing molecule farnesol has been reported to induce apoptosis in fungi by promoting intracellular ROS generation [17, 35–38]. For example, Semighini et al. [17] reported that farnesol produced by Candida albicans, when co-cultured with Aspergillus, caused nuclear condensation and cell death of Aspergillus. Also, 3-oxo-C12 homoserine lactone, a quorum-sensing molecule from P. aeruginosa, which has structural similarity to farnesol, was shown to mimic the action of farnesol in C. albicans [38].
Instead of caspases, fungi have related proteases called metacaspases [20]. Apoptotic pathways in fungi can be either metacaspase-dependent or metacaspase-independent [39]. In our study, we show that P. aeruginosa culture filtrates inhibited growth of A. fumigatus preformed biofilm and triggered apoptosis via the induction of metacaspase activity, although the specific role of metacaspases needs to be investigated further.
Our results add to the growing evidence regarding the antagonistic interactions between fungal and bacterial biofilm communities. This study confirms that the inhibiting capacity of NMuc-CF P. aeruginosa filtrates was greater than that of Muc-CF and non-CF filtrates, and suggests correlation in the relative abilities of each to act by inducing apoptotic cell death. Dose correlation between the concentrations of specific metabolites and apoptosis has not been made, however concentration dependent dose responsiveness of Pseudomonas supernatants and metabolic inhibition of A. fumigatus has been previously detailed [7]. Future studies are needed to determine how A. fumigatus senses and responds to secondary metabolites from other microbial communities. Strategies targeting Pseudomonas with antibiotics in CF lungs need to be further explored in regards to the elimination of Aspergillus from respiratory secretions in these patients [40]. Research is also needed to discern the molecular mechanisms that govern apoptosis in A. fumigatus after P. aeruginosa culture filtrate treatment and to evaluate the potential of the metabolic products of P. aeruginosa as antifungal agents against A. fumigatus.
Acknowledgments
D.P.K. acknowledges the Frances King Black Endowed Professorship for Cancer Research.
Data Availability
All data is available in the paper.
Funding Statement
These studies were partially supported by a grant from the Child Health Research Institute, Stanford Transdisciplinary Initiatives Program (to DAS) and a gift from Mr. John Flatley (to DAS). JAGF was partially supported by a grant from the Brazilian National Council for Scientific and Technological Development (CNPq).
References
- 1.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006; 173:475–482. [DOI] [PubMed] [Google Scholar]
- 2.Muller FC, Seidler M, Beauvais A. Aspergillus fumigatus biofilms in the clinical setting. Med Mycol. 2011; 49: S96–S100. 10.3109/13693786.2010.502190 [DOI] [PubMed] [Google Scholar]
- 3.Hoiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010; 1:53–61. [DOI] [PubMed] [Google Scholar]
- 4.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000; 407:762–764. [DOI] [PubMed] [Google Scholar]
- 5.Mowat E, Rajendran R, Williams C, McCulloch E, Jones B, Lang S, et al. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol Lett. 2010; 313:96–102. 10.1111/j.1574-6968.2010.02130.x [DOI] [PubMed] [Google Scholar]
- 6.Yonezawa M, Sugiyama H, Kizawa K, Hori R, Mitsuyama J, Araki H, Shimakura M, et al. A new model of pulmonary superinfection with Aspergillus fumigatus and Pseudomonas aeruginosa in mice. J Infect Chemother. 2000; 6(3):155–161. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira JAG, Penner JC, Moss RB, Haagensen JA, Clemons KV, Spormann AM, et al. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa on the source, phenotype, and growth condition of the bacterium. PLoS One 2015; 10 (8): e0134692 10.1371/journal.pone.0134692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nazik H, Penner JC, Ferreira JAG, Haagensen JAJ, Cohen K, Spormann AM, et al. Effect of iron chelators on the formation and development of Aspergillus fumigatus biofilm. Antimicrob Agents Chemother. 2015; 59: 6514–6520. 10.1128/AAC.01684-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira JA, Carr JH, Starling CEF, de Resende MA, Donlan RM. Biofilm formation and effect of caspofungin on biofilm structure of Candida species blood stream isolates. Antimicrob Agents Chemother. 2009; 53: 4377–4384. 10.1128/AAC.00316-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Ami R, Lewis RE, Tarrand J, Leventakos K, Kontoyiannis DP. Antifungal activity of colistin against Mucorales species in vitro and in a murine model of Rhizopus oryzae pulmonary infection. Antimicrob Agents Chemother. 2010; 54:484–490. 10.1128/AAC.00956-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirazi F, Kontoyiannis DP. Mitochondrial respiratory pathways inhibition in Rhizopus oryzae potentiates activity of posaconazole and itraconazole via apoptosis. PLoS One 2013; 8:e63393 10.1371/journal.pone.0063393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbu EM, Shirazi F, McGrath DM, Albert N, Sidman RL, Pasqualini R. An antimicrobial peptidomimetic induces Mucorales cell death through mitochondria-mediated apoptosis. PloS One 2013; 8 (10):e76981 10.1371/journal.pone.0076981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirazi F, Pontikos MA, Walsh TJ, Albert N, Lewis RE, Kontoyiannis DP. Hyperthermia sensitizes Rhizopus oryzae to posaconazole and itraconazole action through apoptosis. Antimicrob Agents Chemother. 2013; 57:4360–4368. 10.1128/AAC.00571-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirazi F, Kontoyiannis DP. The calcineurin pathway inhibitor tacrolimus enhances the in vitro activity of azoles against Mucorales via apoptosis. Eukaryot Cell. 2013; 12 (9): 1225–1234. 10.1128/EC.00138-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farmakiotis D, Shirazi F, Zhao Y, Saad PJ, Albert ND, Roilides E, et al. Methylprednisolone enhances the growth of Exserohilum rostratum in-vitro, attenuates spontaneous apoptosis, and increases mortality rates in immunocompetent Drosophila flies. J Infect Dis. 2014; 210:1471–1475. 10.1093/infdis/jiu289 [DOI] [PubMed] [Google Scholar]
- 16.Shirazi F, Kontoyiannis DP. Heat shock protein 90 and calcineurin pathway inhibitors enhance the efficacy of triazoles against Scedosporium prolificans via induction of apoptosis. Microbial Cell 2014; 1:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semighini CP, Hornby M, Dumitru R, Nickerson KW, Harris SD. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol Microbiol. 2006; 59 (3):753–764. [DOI] [PubMed] [Google Scholar]
- 18.Cho J, Lee DG. The antimicrobial peptide arenicin-1 promotes generation of reactive oxygen species and induction of apoptosis. Biochim Biophys Acta. 2011; 1810: 1246–1251. 10.1016/j.bbagen.2011.08.011 [DOI] [PubMed] [Google Scholar]
- 19.Hwang In-S, Lee J, Hwang JH, Kim KJ, Lee DG. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012; 279:1327–1338. 10.1111/j.1742-4658.2012.08527.x [DOI] [PubMed] [Google Scholar]
- 20.Uren AG, O'Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, et al. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell. 2000; 6:961–967. [DOI] [PubMed] [Google Scholar]
- 21.Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, Frohlich KU. Apoptosis in yeast. Curr Opin Microbiol. 2004; 7: 655–660. [DOI] [PubMed] [Google Scholar]
- 22.Moss JE, Idanpaan-Heikkila I, Zychlinsky A. Induction of apoptosis by microbial pathogens In Cellular Microbiology. Cossart, P.E.A. (ed.). Washington, DC: American Society for Microbiology Press, 2005; pp. 409–423. [Google Scholar]
- 23.Tupe SG, Kulkarni RR, Shirazi F, Sant DG, Joshi SP, Deshpande MV. Possible mechanism of antifungal phenazine‐1‐carboxamide from Pseudomonas sp. against dimorphic fungi Benjaminiella poitrasii and human pathogen Candida albicans. J Appl Microbiol. 2015; 118 (1): 39–48. 10.1111/jam.12675 [DOI] [PubMed] [Google Scholar]
- 24.Cheng J, Park TS, Chio LC, Fischl AS, Ye XS. Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Mol Cell Biol.2003; 23: 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousavi SA, Robson GD. Oxidative and amphotericin B-mediated cell death in the opportunistic pathogen Aspergillus fumigatus is associated with an apoptotic-like phenotype. Microbiol. 2004; 150: 1937–1945. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Dickman MB. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc Natl Acad Sci USA. 2005; 102: 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leiter E, Szappanos H, Oberparleiter C, Kaiserer L, Csernoch L, Pusztahelyi T, et al. Antifungal protein PAF severely affects the integrity of the plasma membrane of Aspergillus nidulans and induces an apoptosis like phenotype. Antimicrob Agents Chemother. 2005; 49:2445–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briard B, Bomme P, Lechner BE, Mislin GL, Lair V, Prévost MC, et al. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci Rep. 2015; 5:8220 10.1038/srep08220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moree WJ, Phelan VV, Wu CH, Bandeira N, Cornett DS, Duggan BM, et al. Interkingdom metabolic transformations captured by microbial imaging mass spectrometry. Proc Natl Acad Sci USA. 2012; 109(34):13811–13816. 10.1073/pnas.1206855109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, Newman DK. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol. 2012; 47: 738–745. 10.1165/rcmb.2012-0088OC [DOI] [PubMed] [Google Scholar]
- 31.Zheng H, Kim J, Liew M, Herrera O, Bok JW, Kelleher NL, et al. Redox metabolites signal polymicrobial biofilm development via the NapA oxidative stress cascade in Aspergillus. Curr Biol. 2015; 25:29–37. 10.1016/j.cub.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi D, Kondo K, Uehara N, Otokozawa S, Tsuji N, Yagihashi A, et al. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob Agents Chemother. 2002; 46:3113–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi GF, Zhu FY, Du P, Yang X, Qiu D, Yu Z,et al. Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides 2010; 31:1978–1986. 10.1016/j.peptides.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 34.Cotoras M, Castro P, Vivanco H, Melo R, Mendoza L. Farnesol induces apoptosis-like phenotype in the phytopathogenic fungus Botrytis cinerea. Mycologia 2013; 105:28–33. 10.3852/12-012 [DOI] [PubMed] [Google Scholar]
- 35.Machida K, Tanaka T, Yano Y, Otani S, Taniguchi M Farnesol-induced growth inhibition in Saccharomyces cerevisiae by a cell cycle mechanism. Microbiol. 1999; 145: 293–299. [DOI] [PubMed] [Google Scholar]
- 36.Benhar M, Dalyot I, Engelberg D, Levitzki A. Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol. 2001; 21:6913–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemmerlin A, Bach TJ. Farnesol-induced cell death and stimulation of 3-hydroxy-3-methylglutaryl-coenzyme a reductase activity in tobacco Bright Yellow-2 cells. Plant Physiol. 2000; 123: 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004; 54: 1212–1223. [DOI] [PubMed] [Google Scholar]
- 39.Sharon A, Finkelstein A, Shlezinger N, Hatam I. Fungal apoptosis: function, genes and gene function. FEMS Microbiol Rev. 2009; 33:833–854. 10.1111/j.1574-6976.2009.00180.x [DOI] [PubMed] [Google Scholar]
- 40.Baxter CG, Rautemaa R, Jones AM, Webb AK, Bull M, Mahenthiralingam E, et al. Intravenous antibiotics reduce the presence of Aspergillus in adult cystic fibrosis sputum. Thorax. 2013; 68(7):652–6577 10.1136/thoraxjnl-2012-202412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available in the paper.