Abstract
BACKGROUND
Some recent studies reported human epidermal growth factor receptor (HER-2/neu) as a marker that can be used in immunological studies of colorectal carcinoma for predicting the prognosis and the treatment. Therefore, we aimed to investigate the frequency of HER-2 expression in patients with colorectal cancer, and explore the relationship between clinicopathological prognostic factors and its expression based on immunohistochemical analysis.
METHODS
This study included 50 patients with a histologically proven diagnosis of colorectal carcinoma who received surgery at Imam Khomeini Hospital affiliated to Mazandaran University of Medical Sciences. First, HER-2/neu protein expressions were detected by immunohistochemistry and then the data extracted from recorded files.
RESULTS
The median age of the patients was 60.2±13.9 years (range: 25-93 years). There was no significant relationship between size of tumor, age, sex, lymph node metastases, distant metastasis, differentiation, and stage of the disease with positive expression of HER-2 in this study.
CONCLUSION
No significant relationship between expression of HER-2 and clinicopathological prognostic factors was found in our study. Further comprehensive and prospective trial with standard method to evaluate the role of HER-2 expression among patients with colorectal cancer is needed.
Keywords: Her-2/neu, Colorectal cancer, Northern of Iran, Immunohistochemistry
INTRODUCTION
Colorectal cancer (CRC) is one of the major causes of morbidity and mortality in worldwide.1 According to the annual report of the Iranian National Cancer Registry, CRC is considerably prevalent among Iranian under 50 years population. It is the fourth most common cancer after skin, breast, and gastric cancers.2 Although, surgery is an efficient way to remove the primary tumor but many patients (up to 35%) develop distant metastasis during or after the primary surgery.3
Despite advances in surgical techniques, and adjuvant chemotherapy, a considerable proportion of patients with CRC respond poorly to treatments. Therefore, researchers attempt to identify molecular biomarkers as potential therapeutic targets or a way of predicting therapeutic response and outcome in such patients.4
Several antibodies have been developed for treatment of cancers. One of the most known is trastuzumab (anti-HER-2/neu), which showed successful results for breast cancer.5-6 Using trastuzumab as an adjuvant therapy after surgery leads to significant improvement in survival of patients. Trastuzumab has been approved by USA FDA as an effective treatment, for patients with metastatic breast cancer.7 Although this effectiveness is higher when the expression of HER-2 is high.
HER-2 gene amplification and protein overexpression are correlated with the pathogenesis and progression of several human cancers.8 Activation of HER-2/neu, activates the pathways that play a primary role in cell proliferation and cell differentiation. Recent studies reported that deregulated HER-2 expression/activity is pivotal in oncogenic transformation, tumorigenesis, and metastasis.9 Previous studies showed overexpression of HER-2 in wide range of 0-84% among patients with CRC.8-10
Integrated trastuzumab and chemotherapy regimen enhanced the response rate in other cancer types such as breast cancer. Especially in those with the intestinal type of cancer.11-12 However in patients with CRC, contrast results have been reported in term of investigating the relationship between HER-2/neu expression and some of clinicopathological characteristics.13-14 Researchers recommended further study in this area to recognize and introduce the related factors.13-14
Therefore, we aimed to explore the expression of HER-2/neu and its correlation with clinicopathological parameters among patients with CRC during 2003-2013 in Imam Khomeini Hospital affiliated to Mazandaran University of Medical Science
MATERIALS AND METHODS
Sampling
In this retrospective study, 50 consecutive patients with CRC who referred to the educational hospital of Mazandaran University of Medical Sciences during 2003-2013, were included. All included patients underwent colectomy without history of chemotherapy or radiotherapy before surgery.
The patients’ information and clinical data such as age, sex, differentiation, histological types of the tumor, stage, size of tumor, metastases, involvement of regional lymph nodes, duration from diagnosis to death, survival, and recurrence were retrieved from clinical and pathological databases recorded in the patients’ files.
Procedure of immunohistochemistry
All specimens were fixed in 10% formalin and embedded in paraffin sections with 4-micron thickness. The specimens were prepared from each paraffin block and one of them was stained by Haematoxyl for histological typing and grading of the lesions.
For immunohistochemistry, sections of the selected block were placed on slides of Saylyn S3003 and were incubated in 60ºc for 1 hour in room temperature. In order to clean paraffin, Gzyll solutions, absolute ethanol, and ethanol 96° (2 times for 5 minutes, each time in one solution) were used in three steps, then were washed by the running water. After drying, the slides were transferred to 1% hydrogen peroxide mixture (to eliminate internal peroxidase) and methanol followed to the target solution after 10 min. Henceforth they were placed in an autoclave for 13 min up to boiling. Then the tissue was removed after 15 min and left to reach to room temperature. The tissues washed with running water and wash buffer ( Dako pen), placed in a moist chamber and incubated the samples at envision for 60 minutes using diagnostic kits, anti c-erb 2 ( anti HER-2/neu ) biogenexe. Then, two times washed with wash buffer. DAB solution was poured on glass slides and if a brown color change appeared after 1-2 min, they were placed again in wash buffer for 2 minutes. Afterward, the washed slides with distilled water, were stained with hematoxylin Meyer, rinsed in distilled water and fixed in Gzyll. Finally, the slides were mounted with Entellan. The positive control was a case of invasive duct carcinoma of the breast, in which strong HER2/neu expression had been detected in the membrane of malignant epithelial cells. For the negative control, the primary antibody did not shed. The procedures monitored by two expert pathologists.
Scoring
Scoring was done on the basis of the system used to evaluate Hercep-Test:
0 (Negative) No immunoreactivity or immunoreactivity in <10% of tumor cells
1+ (Negative) Faint weak immunoreactivity in >10% of tumor cells but only a portion of the membrane is positive (incomplete)
2+ (Weak positive) Weak to moderate complete membrane immunoreactivity in >10% of tumor cells
3+ (Positive) Moderate to strong complete immunoreactivity in >10% of tumor cells.[13]
Ethical statement
All data were extracted at the Imam Khomeini Hospital. The retrospective study was performed using the stored samples after the pathological diagnosis, and all of the samples were anonymous before the study. The Mazandaran University of Medical Science approved the study.
Statistic
For statistical data processing, the SPSS software version 20 was used and the data were analyzed through descriptive statistics (frequencies, percentages, medians). To examine the relationship between expression of HER-2 and clinicopathological characteristics, chi-square test was used.
RESULTS
Out of the total 50 patients, 27(54%) were men and 23(46%) were women. Mean age of the patients was 60.2 ± 13.9 years. Most of the cases aged between 5th to 7th decades of their lives (68%). The age range was between 25-93 years.
The presentation of lesions were; 16 (32%) in colon, 14 (28%) in cecum, 9 (18%) in sigmoid, 6 (12%) in rectosigmoid, and 5 (10%) in rectum, respectively. The tumor size ranged from 1cm to 10 cm. The size of 3 cm was the most frequent (22%).
In regard to the degree of differentiation, most of the cases (31[62%]) were well-differentiated, 14 (28%) were moderately differentiated, and only 5 (10%) were poor differentiated. 19 (38%) cases were involved with metastatic nodal deposits and 13 (26%) had distance metastasis (tables 1 and 2).
Table 1 . Characteristics of patients .
| Characters | Frequency | Percentage | p value | |
| Age | 50> | 16 | 32% | 0.692 |
| 50< | 34 | 68% | ||
| Gender | Male | 27 | 54% | 0.908 |
| Female | 23 | 46% | ||
| Treatment | Surgery | 8 | 16% | 0.446 |
| Surgery & chemotherapy | 33 | 66% | ||
| Surgery & radiotherapy | 9 | 18% | ||
| Differentiation | Well differentiated | 31 | 62% | 0.232 |
| Moderate differentiated | 14 | 28% | ||
| Poor differentiated | 5 | 10% | ||
| Location | Colon | 16 | 30% | 0.464 |
| Sigmoid | 9 | 10% | ||
| Rectum | 5 | 12% | ||
| Rectosigmoid | 6 | 26% | ||
| Cecum | 14 | 28% | ||
| Tumor size | Median | 4.6cm | 0.557 | |
| Range | 1-10cm | |||
| Depth of tumor | T1 | 9 | 18% | 0.941 |
| T2 | 7 | 14% | ||
| T3 | 28 | 56% | ||
| T4 | 6 | 12% | ||
| Stage | I | 6 | 12% | 0.868 |
| IIA | 21 | 42% | ||
| IIB | 3 | 6% | ||
| IIIA | 6 | 12% | ||
| IIIB | 13 | 26% | ||
| IIIC | 1 | 2% | ||
| Histological | Adenocarcinoma | 46 | 92% | 0.523 |
| Mucoid adenocarcinoma | 4 | 8% |
Table 2 . Metastasis and recurrence of tumor .
| Characters | Frequency | Percentage | p value | |
| LN metastasis | Absent | 31 | 62% | 0.465 |
| Present | 19 | 38% | ||
|
Distant metastasis |
No | 27 | 54% | 0.797 |
| Yes | 13 | 26% | ||
| No response | 10 | 20% | ||
| Recurrence | Absent | 30 | 60% | 0.663 |
| Present | 10 | 20% | ||
| No response | 10 | 20% | ||
| Vascular | Absent | 29 | 58% | 0.726 |
| Present | 21 | 42% | ||
| Lymphatic | Absent | 47 | 94% | 0.803 |
| Present | 3 | 6% |
In this study of immunohistochemical expression of HER-2/neu, scores (0 and +1) were considered negative and scores (+2 and +3) were considered positive. According to the findings, most of the studied cases (30/50 [60%]) were categorized as negative (figure 1).
Fig. 1 .
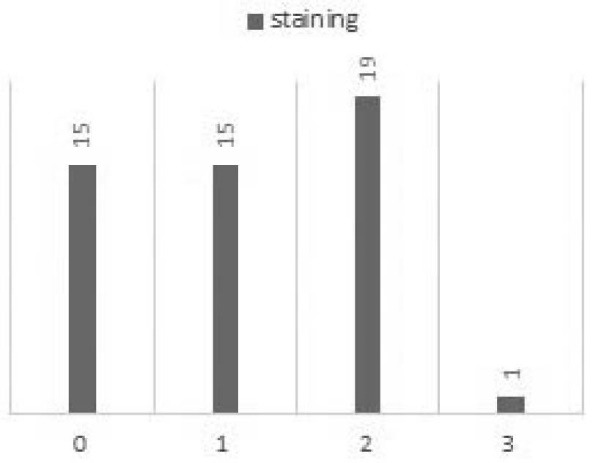
Scores of expression of HER-2/neu
Figure 1 shows 30% of samples presented +1, 30% were zero, 38% of cases presented +2, and only 2% was +3. Therefore 40% of samples considered as positive (38% (+2) and 2% (+3)).
There was no significant relationship between expression of HER and age (p=0.692), differentiation (p=0.232), stage (p=0.868), histological types (p=0.523), depth of tumor (p=0.941), size of tumor (p=0.557), treatment type (p=0.446), and sex (p=0.908).
There was no significant relationship between HER-2 expression and distant metastases (p =0.731), lymph node metastases (p=0.465), recurrence (p=0.663), vascular invasion (p=0.726) and lymphatic invasion (p=0.803). The figure -2- shows positive and negative expression of samples. In positive expression the samples colure changed (figure 2).
Fig. 2 .
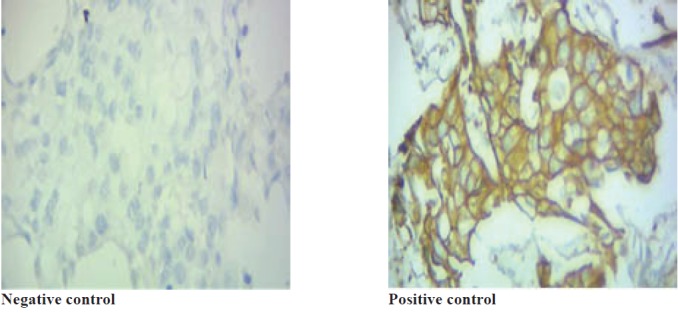
Positive and Negative control
DISCUSSION
Our study showed that 40% of the samples presented positive HER-2 expression and there was no significant relationship between size of tumor, age, sex, location, differentiation stage, metastatic lymph nodes, and distant metastasis and HER-2 positivity. Similar to our findings other studies14-17 also reported that there was no correlation between HER-2 positivity and clinicopathological factors. Kruszewski, also found that staining intensity did not provide any prognostic information.18 However, this result is different from Gill’s findings who reported relationship between age and metastatic lymph nodes and positivity of HER-2. This difference can be explained by different sample size and intensity of expression in their study, which showed that of the 40 cases, 65% (30% = +3 and 12.5% = +2) presented expression of HER-2/neu, while in our study out of the 50 cases 40% (2%= +3 and 38% = +3) expressed HER-2.19
Our results also revealed 40% (2%= +3 and 38% = +3) expression for HER-2, which is similar to findings of Rossi and colleagues,20 who reported 39% expression in patients with CRC. However, Essapen and co-workers,21 reported 87% expression of HER-2 in CRC cases, which is much higher than our results. Seo,4 believed that when accepted staining and scoring techniques were used, the rate of membranous and cytoplasmic overexpression for HER-2 can be approximately 5% and 30%, respectively. Blok and colleagues attributed the discrepancies among studies to several factors including type of primary antibody used, different scoring systems for HER-2 protein expression, variability in technical approach, sample size, racial differences, and heterogeneity of study population.10-22
The distribution of the location of our CRC samples (left colon 2% and right colon 30%) is consistent with epidemiological reports from Safaee in Iran.2 Results of this study revealed that age range of patients were from 25 to 93 years with mean age of 60.2 ±13.9 years. This finding is similar to findings of a recent survey on national cancer registry data of Iran, which reported overall mean age at diagnosis time as 58.9 ± 15.4 years.2
Moreover, 68% of cases included in our study were above 50 years. This finding was close to those reported by office for national statistics,16 that 86% of cases were detected in people who were 60 years or older. In current study, 46% were women and 54% were men. Difference of sex distribution was not considerable in this study, which is similar to other Iranian surveys such as Safaee’s.2
In conclusion, we found 40 % of the CRC cases in Mazandaran, Iran, were positive for HER-2 and there was not statistical relationship between positivity of HER-2 and age, sex, lymph node metastasis, distant metastasis, size of tumor, and stage of disease. This may have implications for selecting CRC cases that may benefit from anti-HER-2/neu therapy. Further studies in larger scales are needed to confirm our results.
Regarding limitations such as small study populations, different design methods, an insufficient follow-up period, low concentrations of HER-2, lack of a standard scoring method, and lack of experience, there is need for more comprehensive studies with larger samples targeting with standard design and methods.
The treatment of CRC is very costly therefore considering the sensitivity of determining HER-2 status is substantial for prescribing Herceptin. In addition, our findings are helpful in selection of patients for IHC test to prevent extra tests. The authors recommend researchers to apply fluorescence in situ hybridization (FISH) test to benchmarking the overexpression of HER-2 marker with amplification gene in case of +1 and +2 scores. Comparing different scoring methods in term of HER-2 overexpression is also suggested.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Torabizadeh Z, Nosrati A, Tahvildari S. Human Epidermal Growth Factor Receptor Expression in Colorectal Cancer and Its Relationship with Clinicopathological Characteristics. Middle East J Dig Dis 2015;8:24-30. DOI: 10.15171/mejdd.2016.03
References
- 1.Fatima A, Haggar Haggar, M M, Robin P. Boushey Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin Colon Rectal Surg. 2009;22:191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safaee A, Seyed- Reza F, Sara A, Mohsen V, Bijan M, Reza Zali Z. Four years Incidence Rate of Colorectal Cancer in Iran: A Survey of National Cancer Registry Data - Implications for Screening. Asian Pac J Cancer Prev. 2012;13:2695–8. doi: 10.7314/APJCP.2012.13.6.2695. [DOI] [PubMed] [Google Scholar]
- 3.Mojarad EN, Kuppen PH. HER2 and immunotherapy using monoclonal antibodies in colorectal cancer. Immunotherapy. 2013;5:1267–9. doi: 10.2217/imt.13.131. [DOI] [PubMed] [Google Scholar]
- 4.Seo A, Kwak Y, Kim D, Kang S, Choe G, Kim W. et al. HER2 Status in Colorectal Cancer: Its Clinical Significance and the Relationship between HER2 Gene Amplification and Expression. PLoS One. 2014;9:e98528. doi: 10.1371/journal.pone.0098528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosrati A, Naghshvar F, Torabizadeh Z, Salehi F. Correlastion of colorectal cancer stem cell marker D24 expression with clinicopathologic features and survival of patient with colorectal cancer. Govaresh. 2014;19:86–94. [Google Scholar]
- 6.Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast. 2013;22:S152–5. doi: 10.1016/j.breast.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T. et al. PHARE trial investigators 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–8. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 8.Aprile G, De Maglio G, Menis J, Casagrande M, Tuniz F, Pisa EF. et al. HER-2 Expression in Brain Metastases from Colorectal Cancer and Corresponding Primary Tumors: A Case Cohort Series. Int J Mol Sci. 2013;14:2370–87. doi: 10.3390/ijms14022370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Bu X, Wu P, Wu P, Huang P. The ‘‘HER2–PI3K/Akt–FASN Axis’’ Regulated Malignant Phenotype of Colorectal Cancer Cells. Lipids. 2012;47:403–11. doi: 10.1007/s11745-011-3649-7. [DOI] [PubMed] [Google Scholar]
- 10.Blok EJ, Kuppen PJ, van Leeuwen JE, Sier CF. Cytoplasmic Overexpression of HER2: a Key Factor in Colorectal Cancer. Clin Med Insights Oncol. 2013;7:41–51. doi: 10.4137/CMO.S10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albarello L, Pecciarini L, Doglioni C. HER2 testing in gastric cancer. Adv Anat Pathol. 2011;18:53–9. doi: 10.1097/PAP.0b013e3182026d72. [DOI] [PubMed] [Google Scholar]
- 12.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 13.Elwy DA, Adelaziz AM, El-sheikh SA, Ebrahim Ebrahim, H H. Immunohistochemical Expression of HER2/neu in Colorectal Carcinoma. Med J Cairo Univ. 2012;80:467–77. [Google Scholar]
- 14.Azadeh S, Moghimi-Dehkordi B, Fatem SR. Colorectal cancer in Iran: an epidemiological study. Asian Pac J Cancer Prev. 2008;9:123–6. [PubMed] [Google Scholar]
- 15.Karaca H, Deniz K, Berk V, Inanc M, Ozkan M. Association of Human Epidermal Growth Factor Receptor-2Expression and Clinicopathological Findings in Patients with Colorectal Cancer. Asian Pac J Cancer Prev. 2012;13:6221–5. doi: 10.7314/APJCP.2012.13.12.6221. [DOI] [PubMed] [Google Scholar]
- 16.Al-Kuraya K1, Novotny H, Bavi P, Siraj AK, Uddin S, Ezzat A. et al. HER2, TOP2A, CCND1, EGFR and C-MYC oncogene amplification in colorectal cancer. J Clin Pathol. 2007;60:768–72. doi: 10.1136/jcp.2006.038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molaei M, Pejhan Pejhan, S S, Nayer BN, Moradi A, Ghiasi S, Zali MR. Human epidermal growth factor receptor-2 family in colorectal adenocarcinoma: correlation with survival and clinicopathological findings. Eur J Gastroenterol Hepatol. 2009;21:289–93. doi: 10.1097/MEG.0b013e32830b82ba. [DOI] [PubMed] [Google Scholar]
- 18.Gill MK, Manjari M, Jain K, Kaur T. Expression of Her-2/neu in Colon Carcinoma and Its Correlation with the Histological Grades and the Lymph Nodes Status. J Clin Diag Res. 2011;5:1564–8. [Google Scholar]
- 19.Kruszewski WJ, Rzepko R, Ciesielski M, Szefel J, Zieliński J, Szajewski M. et al. Expression of HER2 in colorectal cancer does not correlate with prognosis. Dis Markers. 2010;29:207–12. doi: 10.3233/DMA-2010-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi HA, Liu Q, Banner B, Hsieh CC, Savas L, Savarese D. The prognostic value of invariant chain (Ii) and Her-2/neu expression in curatively resected colorectal cancer. Cancer J. 2002;8:268–75. doi: 10.1097/00130404-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Essapen S, Thomas H, Green M, De Vries C, Cook MG, Topham C. et al. The expression and prognostic significance of HER-2 in colorectal cancer and its relationship with clinicopathological parameters. Int J Oncol. 2004;24:241–8. doi: 10.3892/ijo.24.2.241. [DOI] [PubMed] [Google Scholar]
- 22. Office for National Statistics. Cancer registration statistics England, 2012.2014.Available from: URL:http://www.ons.gov.uk/ons/rel/vsob1/cancer-statistics-registrations--england--series-mb1-/no--43--2012/stb-cancer-registrations-2012.html.


