Abstract
Background
Olanzapine (OLZ) treatment is associated with a high risk of weight gain, and may cause abnormalities in glycolipid metabolism. Therefore, the underlying mechanism of OLZ-related weight gain is needed to clarify but not yet been adequately determined. In recent years, adipocytokines such as leptin, adiponectin, and tumor necrosis factor (TNF)-α, which play important roles in energy homeostasis, have been suggested as biomarkers of weight gain. Here, we determined if baseline plasma concentrations of leptin, adiponectin, and TNF-α predict weight gain following OLZ treatment.
Methods
We recruited 31 schizophrenia outpatients (12 men and 19 women, 28.8 ± 10.2 years old) that were unmedicated or on another antipsychotic monotherapy medication. Baseline body mass index (BMI) and plasma levels of leptin, adiponectin, and TNF-α were obtained. All patients started or were switched to OLZ monotherapy for a maximum of 1 year. BMI was also obtained at the endpoint.
Results
Mean BMI change following OLZ treatment was 2.1 ± 2.7 kg/m2. BMI change from baseline to endpoint negatively-correlated with baseline leptin levels in female patients (r = −0.514, P = 0.024), but not male patients. Baseline adiponectin or TNF-α levels were not correlated with BMI change.
Conclusion
Baseline plasma leptin can have an effect on subsequent weight gain following OLZ treatment in female patients with schizophrenia.
Introduction
Pharmacological treatment options for schizophrenic disorder have increased considerably with the arrival of second-generation antipsychotics (SGAs). Among SGAs, olanzapine (OLZ) is effective and well-tolerated, and therefore frequently administered [1], although it is also most frequently associated with the side-effect of weight gain [2–3]. Weight gain can lead to glycolipid metabolic disorders and decreased treatment adherence [4], therefore predicting and preventing weight gain is desirable. However, individual variations in weight gain due to OLZ are large [5]. The mechanisms of variations are partly explained by the activity at histamine H1, serotonin 5-HT2C, 5-HT2A, muscarinic M3, adrenergic receptors [6], orexigenic/anorexigenic neuropeptides and their receptors [7], but have not yet been adequately explained, prediction and prevention are difficult.
In recent years, adipocytokines secreted by adipose cells, namely, leptin, adiponectin, and tumor necrosis factor (TNF)-α, have gained attention in the medical field, and are known to be involved in weight change through roles in food intake, energy consumption, and glycolipid metabolism [8]. Leptin increases in proportion to the amount of fat [9]. States of energy excess are associated with hyperleptinemia, but the hypothalamus is resistant or tolerant to the effects of increased leptin (dashed line). Energy deficiency results in hypoleptinemia. As a result, a complex neural circuit comprising orexigenic and anorexigenic signals is activated to increase food intake. Furthermore, for the same body fat, women have greater leptin concentrations than men, as a result of differences in sex hormones. Adiponectin, an antiatherogenic and antiinflammatory protein, enhances insulin sensitivity and is inversely correlated with obesity and visceral fat [10]. In addition, adiponectin leads to central nervous system-induced weight loss [11]. TNF-α, a cytokine widely known as a biological defense mechanism that acts via inflammation, increases lipolysis, inhibits adipogenesis, and decreases weight due to infection. TNF-α is significantly observed in adipose cells of obese individuals, and triggers insulin resistance [12]. Additionally, there are reports suggesting correlation between activation of the TNF-α system by OLZ and weight gain [13].
Based on these reports, we previously focused on the relationship between metabolic disorders due to SGA, and adipocytokines. By comparing a patient group using SGAs (including OLZ) with a healthy control group, we found lower adiponectin in blood serum in the SGA user group, and in contrast, higher leptin that correlated with weight [14]. Furthermore, switching from OLZ to aripiprazole treatment led to increased adiponectin and decreased TNF-α in serum [15], suggesting that serum adipocytokine levels may be related to OLZ-induced weight gain. Moreover, although the study sample size was small, baseline leptin concentration was shown to affect change in body mass index (BMI) after OLZ treatment [16]. Here, we examine the hypothesis that baseline serum concentrations of leptin, adiponectin, and TNF-α are related to post-OLZ use weight change.
Methods
Subjects
In total, 31 outpatients aged 18–60 years and diagnosed with schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision (DSM-IV-TR) at the Department of Psychiatry of Niigata University Medical and Dental Hospital were recruited. Participants were required to have been untreated with antipsychotics over 4 weeks or receiving the same daily dose of single SGA treatment of aripiprazole, risperidone, blonanserin, or perospirone for at least 4 weeks. Participants were excluded if they met the following criteria: diabetes, dyslipidemia, endocrine disease, concurrent treatment with other antipsychotic agents, or concurrent treatment with any drugs other than benzodiazepines. The study was performed with approval from the Gene Ethics Committee of Niigata University Graduate School of Medical and Dental Sciences. All patients and/or their families were given thorough explanations prior to obtaining written consent.
Methods for Drug Administration and Evaluation
At baseline, scores on the Brief Psychiatric Rating Scale (BPRS) were evaluated, and fasting blood samples were drawn after an overnight fast of at least 8 h to examine leptin, adiponectin, TNF-α and fasting blood glucose. Plasma samples were centrifuged at 4°C and stored at −80°C. Serum analyses were performed by standard methods (SRL, Inc., Japan). Participants’ BMI (weight in kilograms divided by the square of their height in meters) was calculated. Following baseline assessments, for individuals who were receiving antipsychotic medications without OLZ, the dosage was reduced to a 400-mg/day chlorpromazine equivalent over 2 weeks and then discontinued within 4weeks. In parallel, OLZ treatment was started at an initial dose of 5 mg/day and OLZ dose was adjusted every 2 to 4 weeks in the range of 20mg/day on the basis of clinical judgments. BMI and BPRS were calculated at the endpoint, which was defined as either the point after 1 year from baseline or at OLZ discontinuation for any reason.
Statistical Analysis
BMI change was defined as the amount of subsequent BMI change from baseline to endpoint. The Shapiro–Wilk test was used to assess whether the data were normally distributed. Because almost all the data without smoker’s number were normally distributed, a Pearson-correlation analysis was performed between BMI change and following variables; age, baseline BMI, duration of illness, olanzapine-treatment period, daily olanzapine dose, fasting blood glucose at baseline, baseline BPRS, and baseline concentration of leptin, adiponectin, TNF-α. Spearman’s rank correlation test was performed between BMI change and smoker’s number. Using the variables significantly correlated with BMI change, a stepwise multiple regression analysis was performed to evaluate the effects of independent variables. Statistical significance threshold was defined as P < 0.05. SPSS-21.0 (IBM Japan, Ltd., Tokyo, Japan) was used for statistical calculations.
Results
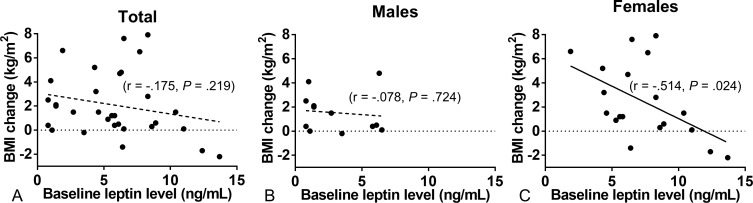
Clinical characteristics and baseline data of patients are shown in Table 1. At baseline, leptin and adiponectin levels in females were higher than in males, while TNF-α levels were higher in males than females. There was no significant difference between males and females in age, baseline BMI, duration of illness, treatment period of olanzapine, daily olanzapine dose, fasting blood glucose at baseline, baseline BPRS, and number of smokers. Nineteen patients were successfully treated with OLZ over 1 year. Twelve patients discontinued OLZ before 1 year because of insufficient or adverse effect. In either case, BMI and BPRS were calculated at the endpoint in all 31 patients. Changes in BMI and BPRS from baseline to endpoint are shown in Table 2. Mean BMI change following OLZ treatment was 2.1 ± 2.7 kg/m2, and did not correlate with any variables in any patients. However, in female patients, BMI change was negatively-correlated with baseline leptin levels (r = −.514, P = .024) (Fig 1) and olanzapine-treatment period (r = −.520, P = .027), but not correlated with other variables. Moreover, baseline leptin levels were not correlated with olanzapine-treatment period in in female patients. In male patients, BMI change was not correlated with any variables. We conducted a stepwise multiple regression analysis in female patients to evaluate the effects of baseline leptin levels and olanzapine-treatment period on BMI change. Higher baseline leptin level was found to contribute to lower BMI gain (adjusted R2 = .275, β = -.584, P = .046).
Table 1. Clinical characteristics and baseline data.
| Total (n = 31) | Male (n = 12) | Female (n = 19) | P | |
|---|---|---|---|---|
| Drug, n | ||||
| None | 19 | 9 | 10 | - |
| Risperidone | 8 | 3 | 5 | - |
| Aripiprazole | 2 | 0 | 2 | - |
| Blonanserin | 1 | 0 | 1 | - |
| Perospirone | 1 | 0 | 1 | - |
| Duration of illness, year* | 5.6 ± 5.9 | 6.4 ± 6.7 | 5.1 ± 5.5 | NS† |
| Age, year* | 28.8 ± 10.2 | 25.9 ± 8.7 | 30.7 ± 10.8 | NS† |
| Smoker, n (%) | 9 (29.0) | 4 (33.3) | 5 (26.3) | NS‡ |
| BPRS* | 32.6 ± 7.9 | 35.9 ± 6.2 | 30.0 ± 8.3 | NS† |
| BMI, kg/m2* | 20.7 ± 3.0 | 21.0 ± 3.6 | 20.5 ± 2.6 | NS† |
| Leptin, ng/mL* | 5.7 ± 3.5 | 3.1 ± 2.4 | 7.4 ± 3.0 | < 0.001† |
| Adiponectin, μg/mL* | 10.4 ± 4.8 | 7.5 ± 2.7 | 12.2 ± 5.0 | 0.02† |
| TNF-α, pg/mL* | 1.7 ± 0.5 | 2.0 ± 0.7 | 1.5 ± 0.2 | 0.049† |
*Data are expressed as mean ± standard deviation.
†Unpaired t-test was used to compare between males and females.
‡Chi-square test was used to compare between males and females.
Abbreviations: BPRS, Brief Psychiatric Rating Scale; BMI, body mass index; NS, not significant; TNF-α, tumor necrosis factor-α.
Table 2. BMI change following olanzapine treatment.
| Total (n = 31) | Male (n = 12) | Female (n = 19) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | P | Baseline | Endpoint | P | Baseline | Endpoint | P | |
| Olanzapine dose, mg/day* | 0.0 ± 0.0 | 12.4 ± 7.1 | - | 0.0 ± 0.0 | 13.8 ± 8.6 | - | 0.0 ± 0.0 | 11.6 ± 6.0 | - |
| Duration of olanzapine medication, weeks* | 0.0 ± 0.0 | 37.0 ± 27.5 | - | 0.0 ± 0.0 | 33.6 ± 33.5 | - | 0.0 ± 0.0 | 39.0 ± 24.0 | - |
| BPRS* | 32.6 ± 7.9 | 26.2 ± 7.3 | .004† | 35.9 ± 6.2 | 31.7 ± 6.2 | NS† | 30.0 ± 8.3 | 22.8 ± 5.7 | .031† |
| BMI, kg/m2* | 20.7 ± 3.0 | 22.8 ± 3.9 | < .001† | 21.0 ± 3.6 | 22.5 ± 3.9 | .008† | 20.5 ± 2.6 | 23.0 ± 3.9 | .003† |
*Data are expressed as mean ± standard deviation.
†Paired t-test was used.
Abbreviations: BPRS, Brief Psychiatric Rating Scale; BMI, body mass index; NS, not significant.
Fig 1. Relationship between BMI change following olanzapine treatment and baseline plasma leptin concentration.
(A) In all patients, BMI change did not correlate with baseline leptin levels. (B) In male patients, BMI change did not correlate with baseline leptin levels. (C) In female patients, BMI change negatively-correlated with baseline leptin levels (r = -0.514, P = 0.024).
Discussion
Our results show that body weight change in females may be associated with peripheral blood leptin levels prior to commencing OLZ treatment.
Wang et al. [16] used OLZ treatment for 14 days in nine (5 male and 4 female) patients with schizophrenia, and measured BMI along with blood leptin concentration prior to and following treatment. They observed a negative correlation between BMI change and leptin concentration prior to treatment, which corresponds with our finding in females. The mechanism by which body weight change is associated with blood leptin levels prior to OLZ treatment is not clear. Leptin acts on leptin receptors, causing strong suppression of food intake, and leading to accelerated energy consumption and maintained energy homeostasis [17]. It has been reported that OLZ administration results in decreased leptin receptors and increased leptin concentration [18]. It is possible that patients with low leptin concentrations may have higher sensitivity towards leptin receptors than patients with higher leptin concentrations. Therefore body weight maintenance and energy homeostasis may be more susceptible to the effect of declined leptin receptors due to OLZ administration, consequently leading to significant weight gain. In contrast, Monteleone et al. [19] treated 22 (13 male and 9 female) schizophrenic patients with clozapine, measuring BMI and blood leptin levels every 2 weeks for a maximum of 32 weeks, but found no relationship between leptin levels prior to treatment and body weight change. Our results do not correspond with their results; however, Monteleone et al. did not examine males and females separately, so the details are unknown.
We found that BMI change from an average OLZ administration period of 37 weeks was 2.1 kg/m2. The OLZ administration period varies greatly in past reports (6 weeks to approximately 2 years), with BMI increment due to OLZ treatment ranging from 0.91 to 4.9 kg/m2 [20–23], consistent with our results. Taking gender differences into consideration, we found that BMI change was greater in females: 1.5 kg/m2 in males and 2.5 kg/m2 in females (Table 2). Although it is known that OLZ causes weight gain in both males and females, BMI increment is greater in females compared with males [21, 23–24]. In rats, significant weight gain is only observed in female rats administered OLZ compared with controls [25–27]. An explanation for this has been proposed based on observed gender differences in the anorexigenic effect of leptin [28] and the number of leptin receptors expressed in the brain [29], which may affect the gender difference in body weight change. Furthermore, a gender difference in energy consumption [30–31] may also be involved. However, some clinical studies have observed no gender difference in BMI change [20, 32–33], and consistent conclusions in patients have not yet been shown.
We observed no relationship between weight gain and adiponectin or TNF-α levels prior to administration. Adiponectin increases insulin sensitivity and shows an inverse correlation with body weight and visceral fat [10]. TNF-α is an inflammatory cytokine that is known to cause insulin resistance [12]. To date, there have not been any reports indicating a relationship between adiponectin or TNF-α levels and subsequent body weight change, necessitating further studies in the future.
There are several limitations to our study. First, the sample size is small, therefore the result in male patients should be reanalyzed using larger samples. Second, medications prior to OLZ administration were not controlled, and an effect of the previous drug on weight may have remained even after commencing OLZ treatment. Third, the treatment period from commencing OLZ treatment to the endpoint varied. Further studies (e.g. on animals) are needed to reveal molecular mechanism of leptin-related olanzapine-induced weight gain.
Conclusions
In this study, it was suggested that the serum leptin may have effect on weight gain in female patients following treatment with olanzapine. If leptin contribute to clarify the mechanism of OLZ-related weight gain, weight gain may be predicted prior to treatment, additional interventions such as nutrition education may be used from an early period of treatment, thereby preventing weight gain.
Acknowledgments
We would like to express our gratitude to the study participants. We thank Hiroshi Kusano and Nanako Yamazaki for their excellent technical assistance.
Data Availability
The data are restricted by the Gene Ethics Committee of Niigata University Graduate School of Medical and Dental Sciences. The contact e-mail address of the data administrator is the following: tsune@med.niigata-u.ac.jp.
Funding Statement
This study was funded by a Grant-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Research (#23591670) (https://www.jsps.go.jp/j-grantsinaid/) to YS, and Mitsubishi Tanabe Pharma Research Foundation and Health and Labour Sciences Research Grants (Research on Psychiatric and Neurological Diseases and Mental Health, H17-kokoro-002)(https://www.smrf.or.jp/) to T. Someya. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, et al. (2003) The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull. 29(1):15–31. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 353(12):1209–23. [DOI] [PubMed] [Google Scholar]
- 3.Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. (2010) Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 123(2–3):225–233. 10.1016/j.schres.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddad PM, Sharma SG. (2007) Adverse effects of atypical antipsychotics: differential risk and clinical implications. CNS Drugs. 21(11):911–936. [DOI] [PubMed] [Google Scholar]
- 5.Lipkovich I, Jacobson JG, Hardy TA, Hoffmann VP. (2008) Early evaluation of patient risk for substantial weight gain during olanzapine treatment for schizophrenia, schizophreniform, or schizoaffective disorder. BMC Psychiatry. 8:78 10.1186/1471-244X-8-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roerig JL, Steffen KJ, Mitchell JE. (2011) Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 25(12):1035–1059. 10.2165/11596300-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 7.Rojczyk E, Pałasz A, Wiaderkiewicz R. (2015) Effect of short and long-term treatment with antipsychotics on orexigenic/anorexigenic neuropeptides expression in the rat hypothalamus. Neuropeptides. 51:31–42. 10.1016/j.npep.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 8.Cao H. (2014) Adipocytokines in obesity and metabolic disease. J Endocrinol. 220(2):T47–59. 10.1530/JOE-13-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. (2011) Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 301(4):E567–584. 10.1152/ajpendo.00315.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 86(5):1930–1935. [DOI] [PubMed] [Google Scholar]
- 11.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. (2004) Adiponectin acts in the brain to decrease body weight. Nat Med. 10(5):524–529. [DOI] [PubMed] [Google Scholar]
- 12.Pollmächer T, Hinze-Selch D, Mullington J. (1996) Effects of clozapine on plasma cytokine and soluble cytokine receptor levels. J Clin Psychopharmacol. 16(5):403–409. [DOI] [PubMed] [Google Scholar]
- 13.Kluge M, Schuld A, Schacht A, Himmerich H, Dalal MA, Wehmeier PM, et al. (2009) Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology. 34(1):118–128. 10.1016/j.psyneuen.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 14.Sugai T, Suzuki Y, Fukui N, Ono S, Watanabe J, Tsuneyama N, et al. (2012) Dysregulation of adipocytokines related to second-generation antipsychotics in normal fasting glucose patients with schizophrenia. J Clin Psychopharmacol. 32(3):390–393. 10.1097/JCP.0b013e3182524393 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Sugai T, Ono S, Sawamura K, Fukui N, Watanabe J, et al. (2011) Changes in the metabolic parameters and QTc interval after switching from olanzapine to aripiprazole in Japanese patients with stable schizophrenia. J Clin Psychopharmacol. 31(4):526–528. 10.1097/JCP.0b013e318221e80d [DOI] [PubMed] [Google Scholar]
- 16.Wang HC, Chen PS, Lee IH, Yang YK, Yeh TL, Lu RB. (2006) Rapid leptin elevation after initiation of olanzapine? Neuropsychobiology. 54(3):182–185. [DOI] [PubMed] [Google Scholar]
- 17.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 269(5223):543–546. [DOI] [PubMed] [Google Scholar]
- 18.Ebenbichler C, Laimer M, Kranebitter M, Lechleitner M, Patsch JR, Baumgartner S, et al. (2005) The soluble leptin receptor in olanzapine-induced weight gain: results from a prospective study. Schizophr Res. 75(1):143–146. [DOI] [PubMed] [Google Scholar]
- 19.Monteleone P, Fabrazzo M, Tortorella A, La Pia S, Maj M. (2002) Pronounced early increase in circulating leptin predicts a lower weight gain during clozapine treatment. J Clin Psychopharmacol. 22(4):424–426. [DOI] [PubMed] [Google Scholar]
- 20.Wu RR, Zhao JP, Zhai JG, Guo XF, Guo WB. (2007) Sex difference in effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. J Clin Psychopharmacol. 27(4):374–379. [DOI] [PubMed] [Google Scholar]
- 21.Saddichha S, Manjunatha N, Ameen S, Akhtar S. (2007) Effect of olanzapine, risperidone, and haloperidol treatment on weight and body mass index in first-episode schizophrenia patients in India: a randomized, double-blind, controlled, prospective study. J Clin Psychiatry. 68(11):1793–1798. [DOI] [PubMed] [Google Scholar]
- 22.Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, Gebhardt N, Remschmidt H, Krieg JC, et al. (2009) Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. J Psychiatr Res. 43(6):620–626. 10.1016/j.jpsychires.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 23.Bobes J, Rejas J, Garcia-Garcia M, Rico-Villademoros F, García-Portilla MP, Fernández I, et al. (2003) Weight gain in patients with schizophrenia treated with risperidone, olanzapine, quetiapine or haloperidol: results of the EIRE study. Schizophr Res. 62(1–2):77–88. [DOI] [PubMed] [Google Scholar]
- 24.Andersen SW, Clemow DB, Corya SA. (2005) Long-term weight gain in patients treated with open-label olanzapine in combination with fluoxetine for major depressive disorder. J Clin Psychiatry. 66(11):1468–1476. [DOI] [PubMed] [Google Scholar]
- 25.Albaugh VL, Henry CR, Bello NT, Hajnal A, Lynch SL, Halle B, et al. (2006) Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity (Silver Spring). 14(1):36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi S, DiSilvio B, Unangst J, Fernstrom JD. (2007) Effect of chronic infusion of olanzapine and clozapine on food intake and body weight gain in male and female rats. Life Sci. 81(12):1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davey KJ, O'Mahony SM, Schellekens H, O'Sullivan O, Bienenstock J, Cotter PD, et al. (2012) Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl). 221(1):155–169. [DOI] [PubMed] [Google Scholar]
- 28.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. (2003) Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 52(3):682–687. [DOI] [PubMed] [Google Scholar]
- 29.Smith JT, Waddell BJ. (2003) Developmental changes in plasma leptin and hypothalamic leptin receptor expression in the rat: peripubertal changes and the emergence of sex differences. J Endocrinol. 176(3):313–319. [DOI] [PubMed] [Google Scholar]
- 30.Kirkby J, Metcalf BS, Jeffery AN, O'Riordan CF, Perkins J, Voss LD, et al. (2004) Sex differences in resting energy expenditure and their relation to insulin resistance in children (EarlyBird 13). Am J Clin Nutr. 80(2):430–435. [DOI] [PubMed] [Google Scholar]
- 31.Paul DR, Novotny JA, Rumpler WV. (2004) Effects of the interaction of sex and food intake on the relation between energy expenditure and body composition. Am J Clin Nutr. 79(3):385–389. [DOI] [PubMed] [Google Scholar]
- 32.Lee E, Leung CM, Wong E. (2004) Atypical antipsychotics and weight gain in Chinese patients: a comparison of olanzapine and risperidone. J Clin Psychiatry. 65(6):864–866. [DOI] [PubMed] [Google Scholar]
- 33.Deligiannidis KM, Rothschild AJ, Barton BA, Kroll-Desrosiers AR, Meyers BS, Flint AJ, et al. (2013) A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 74(10):1003–1009. 10.4088/JCP.13m08400 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are restricted by the Gene Ethics Committee of Niigata University Graduate School of Medical and Dental Sciences. The contact e-mail address of the data administrator is the following: tsune@med.niigata-u.ac.jp.