Abstract
An inactivated Japanese encephalitis virus (JEV) vaccine, which induces neutralizing antibodies, has been used for many years in Japan. In the present study, the JEV prM-E protein gene was cloned, inserted at the P/M junction of measles AIK-C cDNA, and an infectious virus was recovered. The JEV E protein was expressed in B95a cells infected with the recombinant virus. Cotton rats were inoculated with recombinant virus. Measles PA antibodies were detected three weeks after immunization. Neutralizing antibodies against JEV developed one week after inoculation, and EIA antibodies were detected three weeks after immunization. The measles AIK-C-based recombinant virus simultaneously induced measles and JEV immune responses, and may be a candidate for infant vaccines. Therefore, the present strategy of recombinant viruses based on a measles vaccine vector would be applicable to the platform for vaccine development.
Introduction
Japanese encephalitis virus (JEV) is a member of the Flaviviridae family that is transmitted through the bites of Culex tritaeniorhynchus infected with JEV, and is a potential cause of acute encephalitis in humans. Swine are amplifier animals of JEV, while humans are infected as the terminal host through mosquito bites [1]. Acute encephalitis develops in one out of approximately 250 infected cases after a 2-week incubation period, and is characterized by a high fever and non-specific illness, followed by convulsion, headache, confusion, and paralytic illness. JE is still a life-threating disease with severe sequelae [2]. A total of 67,900 clinical cases have been estimated to occur annually, with approximately 20,000 deaths being reported in high risk countries in South East Asia [3]. Approximately 2,000 deaths caused by JEV infection were reported in Japan 1950, however, this number was reduced by the introduction of an inactivated JEV vaccine for children <15 years of age. Several cases of acute encephalitis caused by JEV infection have recently been reported annually, mostly in the elderly, in Japan [4]. Although the incidence of clinical encephalitis is rare, an estimated 3 billion individuals are at risk of JE in high risk countries. No specific therapeutic agent is currently available and prophylactic vaccines are the only effective method to control JE [1].
In Japan, severe cases of acute disseminated encephalomyelitis (ADEM) have been reported following JEV vaccines. The government decided to discontinue mouse brain-derived vaccines because of the potential risk of allergic neurological illnesses. Although mouse brain-derived inactivated vaccines were initially safe in clinical settings, the WHO announced it would be better to replace them with Vero cell culture-based JEV vaccines [5]. In 2010, Vero cell-derived vaccines were introduced in Japan for domestic use [6, 7]. The Vero cell-derived alum adjuvanted vaccine (SA 14-14-2 strain) is now used in most Asian countries, and induces neutralizing antibodies, similar to mouse brain-derived JEV vaccines [8].
Cell-based inactivated vaccines are now widely used in high risk areas and as traveler vaccines, and another vaccine formulation is a live attenuated vaccine. The live attenuated JEV vaccine, based on JEV strain SA 14-14-2 strain, was developed in China using conventional establishment in primary hamster kidney cells and additional passages in embryogenic chick cells, and is now widely used in China and other countries [9]. Through recent development of DNA engineering, chimeric Flavivirus vaccines based on yellow fever vaccine 17D, a well-known ChimeriVax platform, have been applied to Japanese encephalitis, Dengue, and West Nile viruses [10, 11]. The genome region of JEV prM-E in the 17D strain was replaced with that of the JEV SA 14-14-2 strain [10]. This chimeric JEV vaccine (IMOJEV) was shown to induce sufficient immunogenicity, similar to Vero-cell derived inactivated vaccines, in clinical trials, with the most attractive benefit being its efficacy after a single dose immunization [12].
Recombinant measles virus AIK-C vaccine strain expressing the fusion (F), glycoprotein (G), nucleoprotein (NP), and membrane (M)-2 proteins of respiratory syncytial virus (RSV) were constructed in our laboratory. The recombinant AIK-C stain expressing the RSV F or G protein induced neutralizing antibodies, while those expressing the F, NP, or M-2 protein induced cell-mediated immunity, thereby providing protective immunity [13, 14]. Following this line of AIK-C measles vaccine vector, a recombinant measles virus expressing the JEV antigen was investigated, using cotton rats because they are only small experimental animals susceptive to measles virus as shown in previous reports [13, 14]. The prM protein is cleaved to the M protein during virus maturation, to create a structural protein, while the E protein is a major protective envelop antigen that induces neutralizing (NT) and hemagglutination inhibition (HI) antibodies, and the non-structure (NS)-1 protein is thought to be related to replication activity and JEV maturation [15, 16, 17]. The prM-E region is considered to be related to protective antigens and, in the present study, was inserted at the P/M junction of AIK-C cDNA. The characteristics of infectious virus were the investigated for the JEV vaccine candidate.
Materials and Methods
2.1. Virus and cells
JEV Beijing-1 and AIK-C measles vaccine seed stocked strains (Kitasato Daiichi Sankyo Vaccine: Kitamoto, Saitama prefecture, Japan) were used for this work. Vero CCL-81 cells purchased from ATCC were maintained in VP-SFM supplemented with 4 mM L-glutamine. B95a cells were maintained in RPMI-1640 medium supplemented with 10% FBS. 293T cells were maintained in MEM supplemented with 5% FBS, and B95a cells in RPMI 1640 supplemented with10% FCS. BHK cells were maintained in MEM supplemented with 5% FBS for an analysis of the development of NT antibodies against JEV. All media were supplemented with 10,000 IU/ml penicillin and 10,000 μg/ml streptomycin, and irradiated FBS was used. All cell lines were maintained within 50–100 passages and validated in the quality control units of Kitasato Daiichi-Sankyo Vaccine.
2.2. Construction of cDNA expressing the JEV prM-E antigen
Construction strategy is shown in Fig 1. The AIK-C cloning vector for the foreign genome was previously constructed from the Sac II (genome position 2040 of AIK-C) to Pac I (genome position 7238 of AIK-C) sites, and the GGCGCG sequence was added upstream of CC of genome position 3433, introducing a new Asc I restriction site. Restriction sites R1 linked with Nco I and R2 linked with Not I sequences were subsequently added [13, 14]. The JEV prM-E gene was cloned from nt position 447 to 2447 of the JEV genome, which had been amplified by PCR using linker primers having Nco I and Not I sites from the plasmid reported by Hashimoto et al. [18]. The JEV prM-E gene was then inserted at the P/M junction of the cDNA of the AIK-C strain and the full-length cDNA, pMVAIK/JEVprM-E, was constructed.
Fig 1. Construction strategy of recombinant cDNA.
The PrM-E region was amplified from the Beijin strain of Japanese encephalitis virus and cloned into the P/M junction of pMVAIK, using Nco I and Not I restriction enzyme sites.
2.3. Recovery of infectious virus
293T cells were infected with the non-replicative recombinant vaccinia virus, MVA T7 Pol, expressing T7 RNA polymerase, and pMVAIK/JEVprM-E was transfected together with TransIT-LT1 Reagent (Mirus Bio Corporation, US) and helper plasmids encoding the Nucleo (N), Phospho (P), and Large (L) proteins of AIK-C. After being incubated for 3 h, the medium was replaced with fresh MEM supplied with 5% FBS. 293T cells were detached after a 2-day culture, and the co-cultured with B95a cells [13, 14].
2.4. Virus growth
In order to examine the viral growth, B95a cells were infected with MVAIK/JEVprM-E (m.o.i. = 0.02), and plates were incubated at temperatures of 33, 35, 37, and 39°C. Culture fluids were obtained on days 1, 3, 5, and 7 and infective titers were determined. Infective titers were expressed as TCID50/ml.
2.5. Expression of the E protein of JEV
B95a cells were infected with MVAIK/JEVprM-E, and cells were fixed with ethanol. Monoclonal antibodies against the JEV E and measles N proteins were used, and cells were stained using anti-mouse polyclonal antibodies conjugated with FITC or rodamine.
Vero cells were infected with MVAIK/JEVprM-E, and culture supernatants, infected cells, and cell lysates were immunoprecipitated using a polyclonal antibody against JEV raised in rabbits. Proteins were electrophoresed, transferred, and stained using monoclonal antibodies.
2.6. Immunogenicity in cotton rats
Seven week-old female cotton rats were purchased from Harlan Laboratories, USA, and were infected with recombinant virus MVAIK/JEVprM-E through intra-muscular route and serum samples were obtained to examine the development of antibodies against measles virus and JEV. Measles PA antibodies were assayed using a PA antibody kit (Fuji revio, Tokyo). EIA and neutralizing antibodies against JEV were examined using the Beijing strain, as previously reported [19, 20]. The study design was approved by the Committee of Animal Research of Kitasato Institute for Life Sciences (Approval Number; 13–003).
During experimental procedures, vaccination, and blood sampling, animals were anesthetized with 3% isoflurane with oxygen. After the research, animals were deeply anesthetized additionally with pentobarbital into peritoneal cavity and necropsied. In the protocol of experimental research, clinical signs to determine when to euthanize the animals were loss of appetite and loss of activities. No animal died during the course of the experiments until they were euthanized.
Results
3.1. Growth of recombinant MVAIK/JEVprM-E virus in B95a cells
B95a cells were infected with MVAIK/JEVprM-E with moi = 0.02 at different temperatures. Culture supernatants were harvested and the results of infectivity are shown in Fig 2. The virus grew well at 33°C three days after the inoculation, with a peak being on day 7 of the infection. Virus growth was reduced at 35°C and absent at 37°C and 39°C. The recombinant MVAIK/JEVprM-E strain maintained the ts characteristics, as demonstrated in the original measles AIK-C [21].
Fig 2. Virus growth in Vero cells at different temperatures of 33, 35, 37, and 39°C.
Virus infectivity was assayed as TCID50 in Vero cells and each bar represents the mean ± 1.0 SD.
3.2. Expression of the JEV protein
B95a cells were infected with MVAIK/JEVprM-E and fixed. The results are shown in Fig 3. Cells were stained with monoclonal antibodies against the JEV E and measles N proteins. The JEV E protein was expressed at the same location as the expression of measles N protein.
Fig 3. Expression of JEV E and measles N proteins.
Monoclonal antibodies against the JEV E and measles N proteins were used, and visualized by anti-mouse antibodies conjugated with FITC or rhodamine.
The infected B95a cell lysate, culture supernatant, and cell culture were immuno- precipitated using polyclonal antibodies against JEV and subjected to Western blotting. They were stained with a monoclonal antibody against the JEV E protein, and the results are shown in Fig 4A. Three major bands were from 50–75 KD. The molecular size of the JEV E protein was estimated to be approximately 50K, similar to previously findings reported in expression systems [16, 22].
Fig 4. Protein expression of JEV of culture fluid and purified measles virus particles.
Vero cells were infected with a recombinant virus, and cell lysates, culture supernatants, and cell culture fluids were then immunoprecipitated using a polyclonal antibody against JEV. They were subjected to Western blotting (4A). Recombinant virus particles were purified through sucrose discontinuous density gradients. Fractions 1, 2, and 3 were collected and subjected the Western blotting. Polyclonal antibodies against measles virus (left panel) and monoclonal antibodies against the JEV E protein were used (4B).
Vero cells were infected with MVAIK/JEVprM-E and the culture supernatant was collected. It was ultra-centrifuged through 60%, 45%, and 30% sucrose discontinuous gradients. Three fractions were collected and Western blotting was performed using polyclonal antibodies against measles virus and JEV, as shown in Fig 4B. Measles virus was detected in fraction 2. JEV proteins were detected in fraction 1 (supernatant). Fraction 3 contained residual measles particles, but the JEV E protein was not detected in fraction 2 or 3. This result suggested that the JEV E protein was not incorporated into the particles.
3.3. Immune responses of the recombinant virus in cotton rats
Three cotton rats were immunized with MVAIK/JEVprM-E and re-immunized eight weeks after the first dose. The development of PA antibodies to measles virus is shown in Fig 5. PA antibodies developed three weeks after immunization and peaked at five weeks after immunization. No booster response was noted after re-immunization.
Fig 5. Antibody response against measles virus.
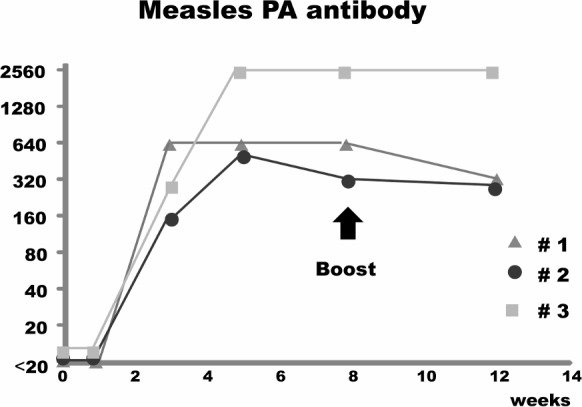
Three cotton rats were immunized with a recombinant virus and reimmunized eight weeks after the first immunization. Serum samples were obtained after 1, 3, 5, 8, and 12 weeks. Measles antibodies were examined using a PA antibody kit.
EIA and neutralizing (NT) antibodies were examined using the Beijing strain, and the results are shown in Fig 6. EIA antibodies developed three weeks after and NT antibodies one week after immunization. No booster response was demonstrated. NT antibodies against JEV were induced three weeks after immunization. Enhanced immune response was noted in two out of three rats but was not significant; one developed a high titer of 10 1.65 six weeks after immunization without antibody response.
Fig 6. Antibody responses of EIA and NT antibodies against JEV.
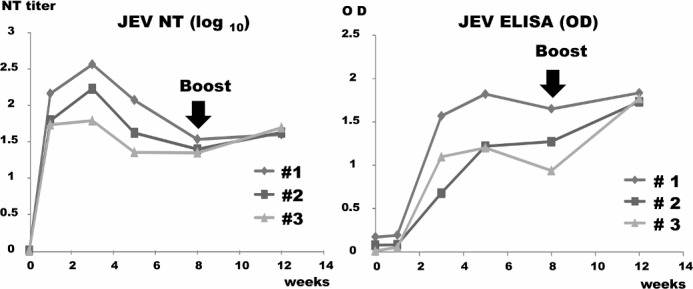
Three cotton rats were immunized with a recombinant MVAIK/JEVprM-E virus and reimmunized eight weeks after the first immunization. Serum samples were obtained after 1, 3, 5, 8, and 12 weeks. EIA and NT antibodies against JEV were measured.
Discussion
JEV is still prevalent in South-East Asia and South Asia. The number of reported cases markedly decreased to less than 10 patients in one year recently in Japan [23]. Antibodies and CTL are both considered to protect against JEV infection based on the findings of animal experiments using the artificial transfer of sera and CTL. Among several JEV proteins, the E protein is regarded as the protective antigen target [16, 17]. Therefore, an inactivated JEV vaccine was developed and recommended for routine immunization. Mouse brain-based vaccines were initially used, but cell-culture based vaccines were subsequently developed [1, 6, 7, 8]. Vero cell-based JEV-inactivated vaccines were shown to induce cross-reactive protective antibodies against Murray Valley encephalitis virus and West Nile virus, with a delta inulin-based adjuvant (Advax) [24]. The addition of an adjuvant resulted in the induction of a broad range of antibodies.
Live attenuated SA14-14-2 was developed by a mutant virus with the deletion of the 3’ non-coding region and has been used mainly in China, inducing long-lasting effective neutralizing antibodies [25]. Besides inactivated and live attenuated JEV vaccines, recombinant yellow fever vaccine-based vectored vaccine is available. The 17D204 strain is a live attenuated yellow fever vaccine with sufficient immunogenicity and safety and has been used for a long time. Yellow fever virus is a family of Flaviridae family, like JEV, and the prM-E protein region was replaced by that of the SA14-14-2 live-attenuated JEV vaccine strain [12]. The 17D yellow fever vector has been applied to Dengue vaccines [26]. A live attenuated vaccine has been used as the back-bone for the development of recombinant vaccines, and the JEV prM-E gene was inserted into vaccinia virus cDNA [27].
In the present study, a live attenuated measles AIK-C strain was used as a virus vector. The AIK-C strain was developed in our institute, established through small plaque cloning at 32.5°C [28]. It showed the unique characteristics of extremely low or no virus growth at 39°C, and a low incidence of febrile illness after immunization [29]. The molecular basis for this attenuation was investigated: Pro at the 439 amino acid position of the P protein was responsible for the ts phenotype, and Leu at the 278 position of the F protein was important for small plaque formation [21, 30]. The AIK-C strain has been used as a recommended vaccine without any serious adverse events in Japan since 1977, and is also used for young infants [31, 32, 33]. An AIK-C-based chimera recombinant virus expressing the preM-E protein of Japanese encephalitis virus is theoretically safe and immunogenic. In the present study, JEV NT antibodies developed one week after immunization, more promptly than JEV EIA or measles PA antibodies. The prM-E protein may have been produced in infected cells and released. The same findings were obtained when cotton rats were immunized with recombinant measles AIK-C strains expressing respiratory syncytial virus [13].
The target age of the recommended vaccine for measles is one year of age and just before entering a primary school, while that for the JEV vaccine is more than 6 months of age, preferably at three years of age. Two doses of primary immunization are recommended, a booster one year after the primary immunization, and additional vaccination at 10 years of age using inactivated JEV vaccine [34]. The target generations for vaccination of measles and JEV vaccines are similar and the recombinant measles vaccine expressing JEV prM-E has the clinical benefit of inducing both humoral and cellular immune responses of the live vectored vaccine and simplifying the immunization schedule, thereby reducing the number of immunizations.
Data Availability
All relevant data are within the paper and available.
Funding Statement
TN had research funding from Daiichi Sankyo, and some parts of the study were supported by the fund. Daiichi Sankyo provided support in the form of salaries for authors AH, HT, and TK and provided JEV vaccine strain, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the author contributions section.
References
- 1.WHO (2015) Japanese encephalitis vaccines: WHO position paper–February 2015. Wkly Epidemiol Rec 90: 69–88. [PubMed] [Google Scholar]
- 2.Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, et al. (2011) Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ l 89: 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halstead S, Jacobson J, Dubischar-Kastner K (2013) Japanese encephalitis vaccines In Plotkin S, Orenstein W, Offit P, editors. Vaccines 6 th edition, Saunders Elesvier; pp312–351. [Google Scholar]
- 4.Konishi E, Shida M, Yamamoto S, Arai S, Tanaka-Taya K, Okabe N (2006) Natural infection with Japanese encephalitis virus among inhabitants of Japan: a nationwide survey of antibodies against nonstructural 1 protein. Vaccine 24: 3054–3056. [DOI] [PubMed] [Google Scholar]
- 5.WHO Meeting report (2007) WHO informal consultation on the scientific basis for production of inactivated Japanese encephalitis vaccines for human use, Geneva, Switzerland, 1–2 June 2006. Vaccine 25: 5233–5243. [DOI] [PubMed] [Google Scholar]
- 6.Sugawara K, Nishiyama K, Ishikawa Y, Abe M, Sonoda K, Komatsu K, et al. (2002) Development of Vero cell-derived inactivated Japanese encephalitis vaccine. Biologicals 30: 303–314. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki C, Okada K, Ozaki T, Hirose M, Iribe K, Yokote H, et al. (2014) Phase III clinical trials comparing the immunogenicity and safety of Vero cell-derived Japanese encephalitis vaccine ENCEVAC(R) with those of mouse brain-derived vaccine using the Beijing-1 strain. Clin Vaccine Immunol 21: 188–95. 10.1128/CVI.00377-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, et al. (2007) Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomized controlled trial. Lancet 370: 1847–1853. [DOI] [PubMed] [Google Scholar]
- 9.Ni H, Chang GJ, Xie H, Trent DW, Barrett AD (1995) Molecular basis of attenuation of neurovirulence of wild-type Japanese encephalitis virus strain SA14. J Gen Virol 76; 409–413. [DOI] [PubMed] [Google Scholar]
- 10.Chambers TJ, Nestorowicz A, Mason PW, Rice CM (1999) Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol 73: 3095–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J (2010) Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 28, 632–649. 10.1016/j.vaccine.2009.09.098 [DOI] [PubMed] [Google Scholar]
- 12.Appaiahgari MB, Vrati S, (2010) IMOJEV®: a Yellow fever virus-based novel Japanese encephalitis vaccine. Expert Rev Vaccines 9:1371–1384. 10.1586/erv.10.139 [DOI] [PubMed] [Google Scholar]
- 13.Sawada A, Komase K, Nakayama T. (2011) AIK-C measles vaccine expressing fusion protein respiratory syncytial virus induces protective antibodies in cotton rats. Vaccine 29: 1481–1490. 10.1016/j.vaccine.2010.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaji Y, Nakayama T (2014) Recombinant measles viruses expressing respiratory syncytial virus proteins induced virus-specific CTL responses in cotton rats. Vaccine 32: 4529–4536. 10.1016/j.vaccine.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 15.Kimura-Kuroda J, Yasui K (1988) Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J Immunol 141: 3606–3610. [PubMed] [Google Scholar]
- 16.Konishi E, Yamaoka M, Khin SW, Kurane I, Takada K, Mason PW (1999) The anamnestic neutralizing antibody response is critical for protection of mice from challenge following vaccination with a plasmid encoding the Japanese encephalitis virus premembrane and envelope genes. J Virol 73: 5527–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan CH, Chen HW, Huang HW, Tao MH (2001) Protective mechanisms induced by a Japanese encephalitis virus DNA vaccine: requirement for antibody but not CD8(+) cytotoxic T-cell responses. J Virol 75: 11457–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto T, Nomoto A, Watanabe K, Mori T, Takezawa T, Aizawa C, et al. (1988) Molecular cloning and complete nucleotide sequence of the genome of Japanese encephalitis virus Beijing-1 strain. Virus Genes 1: 305–317. [DOI] [PubMed] [Google Scholar]
- 19.Toriniwa H, Komiya T (2008) Long-term stability of Vero cell-derived inactivated Japanese encephalitis vaccine prepared using serum-free medium. Vaccine 26: 3680–3689. 10.1016/j.vaccine.2008.04.076 [DOI] [PubMed] [Google Scholar]
- 20.Lee T, Komiya T, Watanabe K, Aizawa C, Hashimoto H (1995) Immune response in mice infected with the attenuated Japanese encephalitis vaccine strain SA14-14-2. Arch Virol 39: 161–164. [PubMed] [Google Scholar]
- 21.Komase K, Nakayama T, Iijima M, Miki K, Kawanishi R, Uejima H (2006) The phosphoprotein of attenuated measles AIK-C vaccine strain contributes to its temperature-sensitive phenotype. Vaccine 24: 826–834. [DOI] [PubMed] [Google Scholar]
- 22.Xu X-G, Wang Z-S, Zhang Q, Li Z-C, Zhao H-N, Li W, et al. (2011) Baculovirus surface display of E envelope glycoprotein of Japanese encephalitis virus and its immunogenicity of the displayed proteins in mouse and swine models. Vaccine 29: 636–643. 10.1016/j.vaccine.2010.11.045 [DOI] [PubMed] [Google Scholar]
- 23.Konishi E1, Kitai Y, Nishimura K, Harada S (2012) Follow-Up Survey of Japanese Encephalitis Virus Infection in Kumamoto Prefecture, South-West Japan: Status during 2009–2011. Jpn J Infect Dis 65: 448–450. [PubMed] [Google Scholar]
- 24.Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, Komiya T, et al. (2010) An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol 91: 1407–1417. 10.1099/vir.0.019190-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohn YM, Tandan JB, Yoksan S, Ji M, Ohrr H (2008) A 5-year follow-up of antibody response in children vaccinated with single dose of live attenuated SA14-14-12 Japanese encephalitis vaccine: immunogenicity and anamnestic responses. Vaccine 26: 1638–1643. 10.1016/j.vaccine.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 26.Bray M, Lai CJ (1991) Construction of intertypic chimeric dengue viruses by substitution of structural protein genes. Proc. Natl. Acad. Sci. U. S. A. 88: 10342–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam JH, Wyatt LS, Chae SL, Cho HW, Park YK, Moss B (1999) Protection against lethal Japanese encephalitis virus infection of mice by immunization with the highly attenuated MVA strain of vaccinia virus expressing JEV prM and E genes. Vaccine 17: 261–268. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki K (1974) Studies on the modification of the live AIK measles vaccine. I. Adaptation of the further attenuated AIK measles virus (the strain of AIK-L33) to chick embryo cells. Kitasato Arch Exp Med 47: 1–12. [PubMed] [Google Scholar]
- 29.Makino S (1983) Development and characteristics of live AIK-C measles virus vaccine: a brief report. Rev Infect Dis 5: 504–505. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama T, Komase K, Uzuka R, Hoshi A, Okafuji T. (2001) Leucine at position 278 of the AIK-C measles virus vaccine strain fusion protein is responsible for reduced syncytium formation. J Gen Virol 82: 2143–2150. [DOI] [PubMed] [Google Scholar]
- 31.Tidjani O, Grunitsky B, Guérin N, Lévy-Bruhl D, Lecam N, Xuereff C, et al. (1989) Serological effects of Edmonston-Zagreb, Schwarz, and AIK-C measles vaccine strains given at ages 4–5 or 8–10 months. Lancet II (8676): 1357–1360. [DOI] [PubMed] [Google Scholar]
- 32.Bolotovski VM, Grabowsky M, Clements CJ, Albrecht P, Brenner ER, Zargaryantzs AI, et al. (1994) Immunization of 6 and 9 month old infants with AIK-C, Edmonston- Zagreb, Leningrad-16 and Schwarz strains of measles vaccine. Int J Epidemiol 23: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 33.Nkrumah FK, Osei-Kwasi M, Dunyo SK, Koram KA, Afari EA (1998) Comparison of AIK-C measles vaccine in infants at 6 months with Schwarz vaccine at 9 months: a randomized controlled trial in Ghana. Bull WHO 76: 353–359. [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama T (2013) Vaccine chronicle in Japan. J Infect Chemother 19: 787–798. 10.1007/s10156-013-0641-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and available.


