Abstract
The unicellular green algae Chlamydomonas reinhardtii has long been studied for its unique fermentation pathways and has been evaluated as a candidate organism for biofuel production. Fermentation in C. reinhardtii is facilitated by a network of three predominant pathways producing four major byproducts: formate, ethanol, acetate and hydrogen. Previous microarray studies identified many genes as being highly up-regulated during anaerobiosis. For example, hybrid cluster protein 4 (HCP4) was found to be one of the most highly up-regulated genes under anoxic conditions. Hybrid cluster proteins have long been studied for their unique spectroscopic properties, yet their biological functions remain largely unclear. To probe its role during anaerobiosis, HCP4 was silenced using artificial microRNAs (ami-hcp4) followed by extensive phenotypic analyses of cells grown under anoxic conditions. Both the expression of key fermentative enzymes and their respective metabolites were significantly altered in ami-hcp4, with nitrogen uptake from the media also being significantly different than wild-type cells. The results strongly suggest a role for HCP4 in regulating key fermentative and nitrogen utilization pathways.
Introduction
Chlamydomonas reinhardtii is a predominantly soil dwelling microalgae found globally [1] that has long been used as a model system for studying photosynthesis, nutrient deprivation, flagellar function, and H2 production [2]. Interest in C. reinhardtii as model organism for biofuel production has been renewed in recent years due to: 1) the discovery of its ability to perform anaerobiosis in the light, 2) its rapid growth rates compared to terrestrial plants, and 3) development of ‘omics’ based approaches to elucidating metabolic pathways, including the development of genetic manipulation techniques, which can be used for the optimization of metabolic processes [3,4]. The generation of stable mutants in C. reinhardtii has traditionally been achieved by random genomic integration [4]. This approach is cumbersome and requires the screening of thousands of mutants using suitable phenotypic criteria or extensive DNA analysis. Recently, tools have been developed that enable targeted gene disruption through the use of artificial microRNAs (amiRNAs) [5,6] and CRISPR/Cas9 technologies [7].
Fermentation pathways in C. reinhardtii
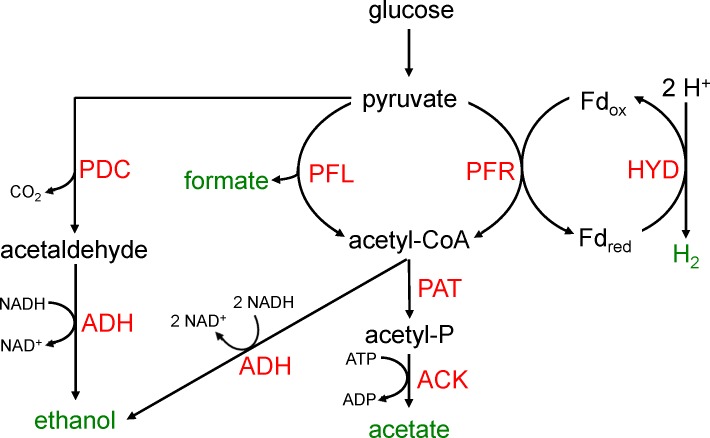
It is apparent that C. reinhardtii has evolved a diverse set of metabolic pathways to deal with the periods of anoxia it experiences in nature. Anoxia can be induced in the laboratory by placing sealed cultures in the dark, sparging oxygen from cultures (e.g. bubbling N2), or by placing cells in sulfur-free media and growing them in light (S is required for photosynthetic O2 evolution). Under the latter conditions, O2 uptake via respiration overcomes O2 production via photosynthesis leading to anoxia. As illustrated in Fig 1, fermentation in C. reinhardtii follows glycolysis by the breakdown of pyruvate. Six fermentation products are observed during darkness-induced fermentation: H2, CO2, acetate, ethanol, formate, and glycerol [8,9]. However, the main products of darkness-induced fermentation (anaerobiosis) are formate, acetate, and ethanol in a 2:1:1 ratio, with H2 and CO2 given off as minor byproducts [9,10]. A fermentation ratio of 2:2:1 of the respective metabolites has also been reported [2,8], though this discrepancy could be due to different culture conditions and strains of algae used in the studies [1].
Fig 1. Major fermentation pathways of Chlamydomonas reinhardtii.
Following glycolysis, pyruvate is further broken down to acetyl-CoA by pyruvate formate lyase (PFL) and pyruvate ferredoxin oxidoreductase (PFR). Acetaldehyde is formed by pyruvate decarboxylase (PDC) from pyruvate. Hydrogenase (HYD) oxidizes reduced ferredoxin to form H2 gas. Ethanol is formed from acetaldehyde and acetyl-CoA via Alcohol dehydrogenase (ADH). Acetate is formed from acetyl-CoA via phosphoacetyl transferase (PAT) and acetate kinase (ACK). Lactate is formed via aactate dehydrognenase (LDH). Modified from [2].
C. reinhardtii is unique among eukaryotes in that it contains four enzymes used in pyruvate fermentation, including: pyruvate formate lyase (PFL), pyruvate ferredoxin oxidoreductase (PFR), lactate dehydrogenase (LDH), and pyruvate decarboxylase (PDC).
In addition to the four fermentative enzymes mentioned above, C. reinhardtii also expresses [FeFe] hydrogenases (HYD) and associated maturation proteins, which are rarely found in eukaryotes [1,2]. Hydrogen production in green algae was first demonstrated in 1942 by Hans Gaffron [11] and is facilitated in C. reinhardtii by reversible [FeFe] hydrogenases, of which there are two isoforms (HYDA1 and HYDA2) bound to the photosynthetic apparatus by ferredoxin [11]. H2 production takes place in strictly anaerobic environments, as HYD transcription and enzyme stability is severely compromised in the presence of oxygen (≈3% O2) [12]. Algal hydrogenases show high similarity to hydrogenases found in strict anaerobes, fungi and protists [13], but is directly reduced by ferredoxin, unlike other hydrogenases that rely on putative electron relays comprised of FeS clusters, either [2Fe2S] or [4Fe-4S] [13]. HYD expression is induced 100-fold upon darkness-induced anaerobiosis, though starchless mutants show attenuated hydrogenase expression suggesting other transcriptional regulators other than O2 [2,14,15]. This is likely due to the fact that dark fermentative H2 production pathway is directly coupled to starch catabolism [8,9]. In this pathway the oxidation of pyruvate is directly coupled to the reduction of ferredoxin by PFR while producing acetyl-CoA and CO2, with HYD then oxidizing the reduced ferredoxin forming H2. However, H2 is a very minor fermentation product during darkness-induced anaerobiosis [9,10].
Hybrid cluster protein 4
The low H2 output observed during darkness induced anaerobiosis leads to questions regarding the presence of limiting steps or pathways competing with HYD for reduced ferredoxin. For example, hybrid cluster protein 4 (HCP4, Cre09.g391650) displays a rapid and large increase in expression during darkness-induced anaerobiosis [2]. There are four HCP family members encoded by the C. reinhardtii genome, but only HCP4 appears to be highly upregulated during anoxia [2]. Similar to HYD, HCP4 is an iron sulfur protein containing two subunits, a [4Fe-4S]2+/1+ or [2Fe-2S]2+/1+ and [4Fe-2S-2O], the so-called “hybrid cluster” [16]. The binding motif of [4Fe-4S] and [2Fe-2S] observed in HCP4 shows unique spacing of conserved cysteines making it similar to HCPs found in strict anaerobes [2]. Although this family of proteins has been studied extensively on a structural basis, its physiological role is not fully understood. E. coli HCP was found to be induced by hydrogen peroxide and is believed to play a role in oxidative stress defense [16]. E. coli HCP also shows up-regulation upon addition of nitrate or nitrite to the media, and purified HCP displays hydroxylamine reductase activity, reducing hydroxylamine to ammonia [17]. Further, hydroxylamine production was shown to require ferredoxin in Clostridium pasteurianum [18]. Due to these findings, it has been proposed that HCP4 in C. reinhardtii could oxidize reduced ferredoxin, thereby directly competing with HYD for electrons and thus lowering potential H2 yield [2].
Nitrogen metabolism in C. reinhardtii
In C. reinhardtii, nitrate and ammonium are the predominant sources for nitrogen assimilation [19,20]. Nitrate is converted into nitrite in the cytoplasm by the enzyme nitrate reductase (NR) and is further transported into the chloroplast by NAR1 nitrite transporters [19,20]. In the chloroplast nitrite is converted to ammonium via the ferredoxin-dependent nitrite reductase (NiR) and is incorporated into L-glutamate via the glutamine synthetase/glutamate synthase cycle (GS/GOGAT) [19,20]. Light/dark cycles have been found to regulate NR activity with nitrate and nitrite uptake being highest in the light and lowest in the dark [21].
Purpose of this study
Cumulatively, the extraordinary collection of fermentation pathways makes C. reinhardtii especially well adapted to anaerobiosis, and is able to produce multiple biofuels such as triacylglycerols, ethanol and H2 [22]. These fermentation products, along with the ability of C. reinhardtii to grow quickly to very high biomass densities in environments that will not compete with food stocks, makes it an excellent candidate for the development of biofuels. Indeed, C. reinhardtii and other microalgae have been the focus of global research into biofuel production for decades [23]. Despite this effort, the pathways involved in the diverse fermentation metabolism of C. reinhardtii are not fully understood. A further understanding of the interactions between the various fermentation pathways active in anaerobic C. reinhardtii will yield more precise targets in the effort to engineer better microalgae strains for biofuel production.
It is also clear that C. reinhardtii has as a diverse set of enzymes and pathways that are utilized in the nitrogen uptake and assimilation. Somewhat surprisingly the uptake of nitrogen has not been widely studied during anaerobic conditions. Ferredoxin plays a large role in nitrogen metabolism at the NiR, Fd-GOGAT, and possibly the NR stages, and it is known that darkness inhibited electron flow around PSI and PSII will slow nitrate, nitrite and ammonia uptake [24]. However the interplay between anaerobic energy production and anaerobic nitrogen metabolism has not been elucidated. HCP4 may be associated with anaerobic nitrogen metabolism based on the high upregulation of HCP4 during anaerobiosis and the hydroxylamine reductase activity observed in related proteins, as well as its hypothesized ability to oxidize reduced ferredoxin.
The purpose of this study was to use C. reinhardtii as a model system to investigate the role of HCP4 on the interactions of various fermentation pathways in darkness-induced anaerobiosis. A further understanding of regulatory networks coordinating metabolic flux in C. reinhardtii is paramount in developing informed metabolic engineering strategies to boost biofuel production.
Materials and Methods
Algal strains and standard growth conditions
Chlamydomonas reinhardtii type cc425 arg2 cw15 sr-u-2-60 mt+ (referred to as cc425 or wild-type hereafter) was used as the wild-type background for all studies. All cultures were grown mixotrophically in Tris Acetate Phosphate (TAP) media [1] under 12 hr day / 12 hr night cycles with shaking at 120 rpm. During growth, wild-type strains were supplemented with 100 μg/ml arginine. Cultures were illuminated with a photosynthetic photon flux of 150 μmol m-2 s-1 and temperature of 23°C.
Generation of amiRNA vectors
The vector pChlami2 was obtained from the Chlamydomonas resource center (University of Minnesota) and prepared according to Molnar et al. [5]. amiRNA inserts were generated for HCP4 using WMD (web based microRNA designer) version 3 (http://wmd3.weigelworld.org/cgi-bin/webapp.cgi?page=Home;project=stdwmd). A schematic of HCP4 gene structure and the location of amiRNA targeting are indicated in Fig 2A. HCP4 (XM_001694402) mRNA sequence was used to generate an appropriate amiRNA insert. Ninety nucleotide long oligonucleotides (S1 Table) were synthesized by Integrated DNA Technologies (IDT, Coralville, IA). Inserts were resuspended to a final concentration of 100 μM. To anneal the insert oligos, 10 μl of forward and reverse insert oligos were mixed with 20 μl 2X annealing buffer (20mM Tris pH 8.0, 2mM EDTA, 100mM NaCl). The mixture was boiled for five minutes and gradually cooled overnight. The double stranded insert was purified using Qiagen PCR clean up kit (Qiagen, Venlo, Netherlands). The insert was phosphorylated with using Promega T4 Polynucleotide Kinase (Promega Corp., Madison, WI, USA). The vector pChlami2 was digested with SpeI and dephosphorylated with calf intestinal alkaline phosphatase (CAIP). The dephosphorylated vectors were purified using Qiagen PCR clean up kit. The phosphorylated insert was then cloned into the vectors using Promega T4 DNA ligase (Promega Corp., Madison, WI, USA). Mach1 E. coli were transformed by electroporation with the vectors and plated on 150 μg/ml ampicillin LB agar plates.
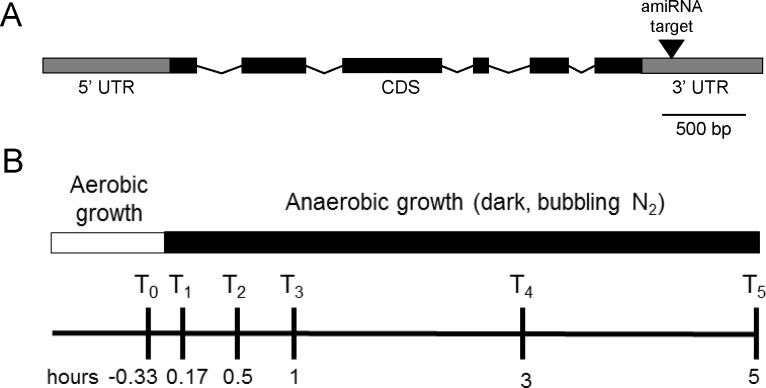
Fig 2. Experimental design for silencing HCP4 and induction of anaerobiosis.
(A) Schematic of HCP4 gene structure (Cre09.g391650), which was targeted in the 3’ UTR for degradation using a pChlamiRNA 2 vector (Molnar et al. 2009). (B) Schematic of time points of subsamples taken from anaerobic cultures. Cultures grown aerobically in TAP media were pelleted, resuspended in 28mM HEPES, pH 7.5 buffer (represented by far left of white box), and then allowed to acclimate for 50 minutes in the light with shaking prior to induction of anaerobiosis. Samples were taken from anaerobic cultures at 0.33 hours before anaerobic induction and then at 0.17, 0.5, 1, 3, and 5 hours post-anaerobiosis.
Individual E. coli colonies were selected and grown in LB broth at 37°C and DNA was extracted via Qiagen miniprep kit (Qiagen, Venlo, Netherlands). PCR reactions were performed to locate transformed colonies containing the vector and insert in the correct orientation using primers AmiRNAprecfor (5’-GGTGTTGGGTCGGTGTTTTTG-3’) and Spacerrev (5’-TAGCGCTGATCACCACCACCC-3’) were used with Promega GoTaq Green Master Mix according to the manufacturer’s instruction (Promega Corp., Madison, WI, USA). Candidate constructs containing the insert in the correct orientation were sequenced at the University of Michigan Sequencing Core (Ann Arbor, Michigan).
C. reinhardtii transformation
C. reinhardtii strain cc425 was stably transformed with the vector pChlami2 containing the HCP4 amiRNA insert using a modified Kindle’s glass bead method [25]. Wild-type cc425 cells were re-suspended to a density of 1x106 cells/ml. 300 μl of cells, 100 μl 20% polyethylene glycol (PEG), 2 μg HCP4 amiRNA vector, and 300 μg glass beads (0.5mm diameter) were added to a 1.5 ml microcentrifuge tube and vortexed on high for 30 seconds. 150 μl of cells were plated on a 1.5% TAP agar plates and incubated. Multiple independent transformants growing on TAP were selected and the sequence verified at the University of Michigan sequencing core (Ann Arbor, Michigan).
Induction of anaerobic conditions
Liquid cultures were initiated by inoculating 500 ml of TAP with wild-type and HCP4-silenced cells (hereafter termed ami-hcp4). Cultures were grown for four days under 12 hour light-dark cycles, illuminated with a photosynthetic photon flux of 150 μmol m-2 s-1 and temperature of 23°C. Following incubation cells were counted via hemocytometer and 160x106 cells (both wild-type and ami-hcp4) were pelleted and washed with 25 ml HEPES buffer (28mM, pH 7.5). Pellets were then resuspended in 40ml HEPES buffer (28mM, pH 7.5) to a final concentration of 4x106 cells/ml in a 50ml conical tube and incubated in the light for 1 hour. Following incubation, cell vitality was assayed by noting swimming cells in each culture. The tubes were wrapped in foil, with Parafilm loosely applied to the tops. N2 gas was bubbled through the cultures and light excluded by placing a foil covered box over the cultures. Dissolved oxygen was assayed using a Clark-type electrode following 10 minutes of N2 bubbling to ensure anoxic conditions were achieved. As illustrated in Fig 2B, subsamples were collected from cultures that were resuspended in 28 mM HEPES, pH 7.5 buffer at T0: following 40 minutes incubation in light under aerobic conditions, which was equivalent to 20 minutes before (-0.33 hours) induction of anaerobiosis, T1: 10 minutes after the initiation of anaerobiosis (following 10 minutes N2 bubbling in dark, 0.17 hours), T2: 0.5 hours post-anaerobiosis, T3: 1 hour post-anaerobiosis, T4: 3 hours post-anaerobiosis, T5: 5 hours post-anaerobiosis. Cell viability and motility were verified at each experimental time point.
Gene expression analyses
Trizol (Invitrogen, San Diego, California) was used to extract RNA from the frozen pellets. Pellets were resuspended in 1ml Trizol by pipetting and samples were then incubated at room temp for 5 minutes; 200 μl of chloroform was then added and tubes shaken by hand for 15 seconds then incubated at room temp for 2 minutes. Samples were spun at 11,000 g for two minutes at 4°C. The upper aqueous phase was placed in a new tube, 0.5 ml of isopropyl alcohol was added and the tube was incubated at room temperature for 10 minutes. Samples were then centrifuged at 11,000 g for 10 minutes at 4°C. The supernatant was removed and replaced with 1ml of 75% ethanol and mixed gently by hand. The mixture was centrifuged at 7,000 g for five minutes at 4°C. The supernatant was removed and let air dry for five minutes. The RNA pellet was resuspended in 40 μl RNase-free H2O and incubated at 55°C for 10 minutes. RNA was quantified and integrity was verified by running 500 μg of RNA in a 2% agarose gel.
Reverse transcription was carried out using a Qiagen Quantitect Reverse Transcription kit and 500 μg RNA according to manufacturer’s instructions (Qiagen, Venlo, Netherlands). Real time PCR was performed using Rotor-gene SYBR Green PCR Kit (Qiagen, Venlo, Netherlands) and Corbett Research RG-3000 thermocycler (Qiagen, Venlo, Netherlands). One μl of cDNA was used for each reaction. Previously published primers were used to amplify 100–200 nucleotide regions of the following genes; Rack1, beta-tubulin, PDC, HYD, PFL, PFR, and HCP4 (S2 Table) [2]. Cycling parameters contained a melting step at 95°C for 10 minutes followed by 65 cycles of a 95°C (10 sec) melting step followed by a 60°C (15 sec) annealing/elongation step. Data were acquired on the FAM/Sybrgreen channel during the annealing/elongation step. A 10 minute step at 72°C ended the cycle. Relative expression was calculated using the comparative Ct Method (Applied Biosystems). Rack1 and beta-tubulin were used as constitutively expressed controls.
Organic metabolite assays
The metabolites formate, ethanol and acetate were measured in the media using kits (formate; 10979732035, acetate; 10148261035, ethanol; 10176290035) from Boehringer Mannheim / r-biopharm, Darmstadt, Germany. These enzymatic assays measure sample dependent production of NADH. NADH was measured at 340nm for the ethanol and acetate assays using a Beckman DU 650 spectrophotometer (Beckman Coulter, Brea, CA). NADH was measured in the formate assay using a Nanodrop ND-1000 spectrophotometer (ThermoScientific, Waltham, MA). Supernatant fractions from each time point were used as samples and HEPES buffer used as the blank. Manufacturer instructions were followed with slight modifications. The ethanol reaction volume was reduced to 525 μl and 100 μl sample volume was used in each reaction. The formate reaction volume was reduced to 61 μl and 40 μl sample volume was used. The Acetate reaction volume was reduced to 537 μl using a sample volume of 100 μl. Results are reported as the average of quadruplicate samples for formate and ethanol and triplicate samples of acetate at each time point.
Nitrogen uptake assays
Uptake of nitrate or ammonium was measured over a 24 hour period. Liquid cultures were initiated by spiking 500ml of TAP liquid with equal amounts of wild-type and ami-hcp4 cells. Cultures were grown for four days under 12 hour light dark cycles, illuminated with a photosynthetic photon flux of 150 μmol m-2 s-1 and temperature of 23°C. Following incubation the cells density was measured using a hemocytometer and 160x106 cells were pelleted and washed with ammonium solution (12mM Na-acetate, 28mM HEPES, 10mM NH4Cl) or nitrate solution (12mM Na-acetate, 28mM HEPES, 10mM KNO3). Cells were then resuspended in 40 ml of their respective ammonia or nitrate solutions at 4x106 cells/ml. Cells were bubbled with N2 in the dark for ten minutes then capped and placed in the dark. Two ml subsamples were taken from the ammonium uptake experiment every two hours for 12 hours and then every 4 hours until 24 hours had elapsed. After being taken, samples were immediately centrifuged and separated into pellet and supernatant fractions. RNA was extracted from the four hour time point of the ammonia and nitrate uptake samples and silencing of HCP4 was confirmed as described earlier.
Ammonium and nitrate remaining in solution were assayed using kits (ammonium; 11112732035, nitrate; 10905658035) from Boehringer Mannheim / r-biopharm, Darmstadt, Germany. Manufacturer’s directions were followed with slight modifications. Reaction volume for the ammonia and nitrate assays were reduced to 503.3 μl, and 508.33μl respectively. NADH was measured spectroscopically using a Nanodrop 2000c spectrophotometer (Thermo scientific, Waltham, MA).
Results
Previous studied identified HCP4 as one of the most highly upregulated genes during anaerobiosis [2]. To examine the role of HCP4 in C. reinhardtii fermentation and nitrogen utilization, a construct encoding an amiRNA targeting the 3’ UTR of HCP4 (Fig 2A) was generated and used to stably transform cc425 (wild-type) C. reinhardtii.
Gene expression profiling
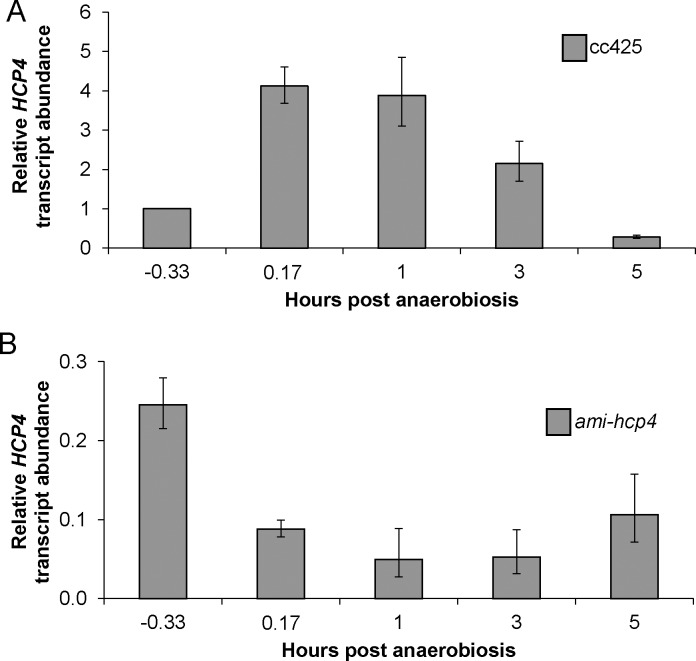
Wild-type cc425 and ami-hcp4 cells were grown in TAP media, pelleted, washed, and resuspended in 28mM HEPES buffer and subjected to five hours of anoxic conditions through growth in the dark while being bubbled with a constant stream of N2 gas. No gross phenotypic differences relative to wild-type were noted in ami-hcp4 cell number, viability, or motility during either aerobic or anaerobic growth (in either TAP media or HEPES buffer). Subsamples were taken at -0.33, 0.17, 0.5, 1, 3, and 5 hours after induction of anaerobic conditions as outlined in Fig 2B. To confirm that HCP4 expression was upregulated in wild-type cells grown under the selected anaerobic conditions, and that HCP4 was indeed silenced in transgenic mutants (ami-hcp4), mRNA was extracted and evaluated by quantitative RT-PCR. Indeed, under our experimental conditions, HCP4 transcription was significantly elevated within 10 minutes of induction of anaerobic conditions (Fig 3A), and remained elevated for at least 3 hours. However, HCP4 expression was ~3-fold less than wild-type cells after 5 hours of growth under anaerobic conditions.
Fig 3. Analysis of HCP4 transcripts under experimental conditions.
(A) Induction of HCP4 transcription in wild-type cells under darkness-induced anaerobic conditions. All post-anaerobiosis time points were significantly different than pre-anaerobiosis (data presented relative -0.33 hours in wild-type CC425, p<0.05). (B) The effect of amiRNA vector targeting HCP4 on transcript levels under the same anaerobic conditions as wild-type cells. HCP4 mRNA levels were strongly reduced in ami-hcp4 relative to wild-type throughout the course of anaerobiosis. mRNA was extracted at the indicated time points and cDNA levels measured by qPCR in wild-type and ami-hcp4 (data is presented as expression of HCP4 in ami-hcp4 relative to wild-type at each time point). n = 2 biological replicates with 3 technical replicates for both A and B.
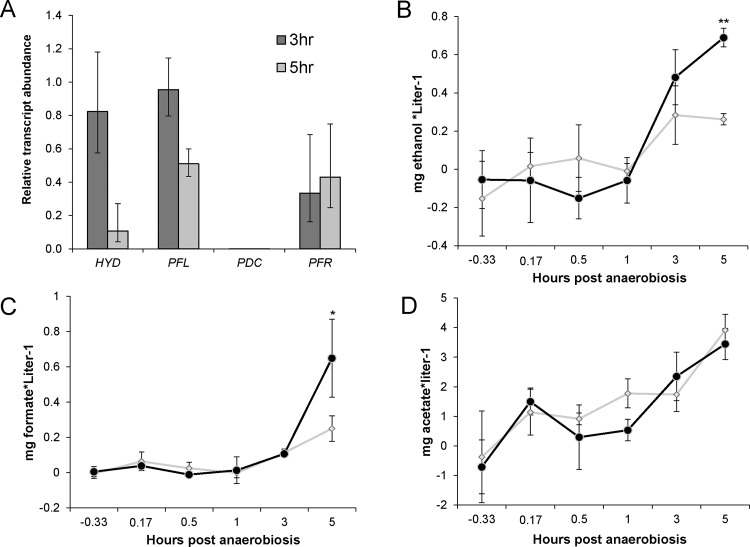
Quantitative RT-PCR analyses also demonstrated HCP4 transcript levels were significantly reduced in ami-hcp4 at all time points (Fig 3B). Silencing of HCP4 in ami-hcp4 ranged from 0.24 relative transcript abundance (4-fold knockdown) at 0.33 hours pre-anaerobiosis to 0.05 relative transcript abundance (20-fold knockdown) at 1 hour post-anaerobiosis. Once silencing of HCP4 was confirmed, the expression of other genes central to fermentation pathways, including HYD, PFL, PDC, and PFR, were investigated to elucidate the effects of silencing HCP4 (Fig 4A). Relative transcript abundance was measured using the same cDNA synthesized from 3 and 5 hours post-anaerobiosis described above. At three hours post-anaerobiosis HYD displayed a slight decrease in expression, but this change was not statistically significant. At five hours, however, HYD showed a 10-fold decrease in expression relative to wild-type. Similarly, PFL was not significantly downregulated at 3 hours post-anaerobiosis, but at 5 hours showed a significant 2-fold decrease in expression. PFR expression was decreased at both the 3 and 5 hour time points, displaying 0.33 and 0.44 relative transcript abundance, or a 3-fold and 2.3-fold decrease in expression, respectively. Surprisingly, PDC levels showed negligible expression in ami-hcp4 cells at both the three and five hour time points, which was confirmed in multiple independent transformants.
Fig 4. The effect of silencing HCP4 on fermentation pathways.
(A) Expression of key fermentation transcripts in ami-hcp4 relative to wild-type CC425 at both 3 and 5 hours post-anaerobiosis; n = 3 biological replicates with triplicate technical replicates. (B) Ethanol, (C) formate, and (D) acetate accumulation during darkness-induced anaerobiosis in wild-type cc425 (gray diamonds) and hcp4 (black circles). Cultures were resuspended in 28 mM HEPES, pH 7.5 at -1 hour post anaerobiosis and constantly bubbled with N2 in the dark starting at 0 hours post anaerobiosis. **p <0.01; * p<0.05; n = 4 biological replicates for ethanol and formate, n = 3 biological replicates for acetate.
Metabolite production during anaerobiosis
The downregulation of keys genes for several major fermentation pathways in ami-hcp4 suggested that the metabolites from these pathways may also be reduced. The metabolites ethanol, formate and acetate were measured using enzymatic assays at all time points taken during the course of anaerobiosis. Production of ethanol was not significantly different between wild-type and ami-hcp4 lines until 5 hours post-anaerobiosis (Fig 4B). At 3 hours post-anaerobiosis wild-type and ami-hcp4 showed measureable excreted ethanol at mean concentrations of 0.28 and 0.48 mg*ml-1 respectively, yet these values were not statistically significant from each other. At 5 hours, wild-type and ami-hcp4 production significantly diverge as ami-hcp4 displayed an average 2.6-fold increase in excreted ethanol. Significant difference was calculated at five hours by Student’s unpaired t-test with equal variance (p = 0.0002).
Production of formate was not detectable until 3 hours post-anaerobiosis (Fig 4C). At 3 hours levels of formate excreted were not significantly different between wild-type and ami-hcp4 which were 0.11 and 0.10 mg formate*liter-1 respectively. However, at 5 hours post-anaerobiosis production of formate significantly diverged between wild-type and ami-hcp4, with wild-type accumulating 0.25 mg formate*liter-1 and ami-hcp4 accumulating 0.65 mg formate*liter-1. Thus ami-hcp4 had a ~2.6-fold increase in formate accumulation at 5 hours post-anaerobiosis relative to wild-type. Significant difference was calculated at 5 hours by Student’s unpaired t-test with equal variance (p = 0.045).
Acetate accumulation during anaerobiosis was not significantly different at any time point between wild-type and ami-hcp4 (Fig 4D). Both cultures showed acetate accumulation beginning at 0.17 hours post-anaerobiosis. Acetate accumulation progressively increased in both cultures until 5 hours post-anaerobiosis when wild-type and ami-hcp4 accumulated an average of 3.9 and 3.4 mg acetate*liter-1 respectively.
As reported in Table 1, at 3 hours post-anaerobiosis wild-type accumulated the metabolites formate, acetate and ethanol at a 1:12:2.5 ratio. At the same time point ami-hcp4 accumulated these metabolites at a 1:16:4.2 ratio. At 5 hours post-anaerobiosis wild-type accumulated the metabolites formate, acetate and ethanol at a ratio of 1:12:1, whereas ami-hcp4 accumulated these metabolites at a ratio of 1:4:1.
Table 1. Mean metabolite totals from 3 and 5 hours after induction of anaerobiosis.
| Strain | Formate (mg) | Acetate (mg) | Ethanol (mg) | Molar ratio (formate:acetate:ethanol) | |
|---|---|---|---|---|---|
| 3 hour | cc425 | 0.11 ± 0.02 | 1.74 ± 0.56 | 0.28 ± 0.15 | 1:12:2.5 |
| ami-hcp4 | 0.11 ± 0.01 | 2.35 ± 0.82 | 0.48 ± 0.14 | 1:16:4.2 | |
| 5 hour | cc425 | 0.25 ± 0.07 | 3.91 ± 0.53 | 0.26 ± 0.03 | 1:12:1 |
| ami-hcp4 | 0.65 ± 0.22* | 3.45 ± 0.53 | 0.69 ± 0.05** | 1:4:1 |
*p<0.05
**p<0.01 for ami-hcp4 relative to cc425 (wild-type) at the respective time points.
Nitrogen uptake assays
The expression of hybrid cluster proteins is up-regulated in E. coli cultures upon the addition of nitrate or nitrite [26]. Similarly, the only known biochemical function of any HCP from any species is that of hydroxylamine reductase in E.coli [17]. These findings suggest HCP4 may be involved in nitrogen metabolism in C. reinhardtii. Thus, nitrate and ammonium uptake were assayed in wild-type and ami-hcp4 strains under anaerobic conditions.
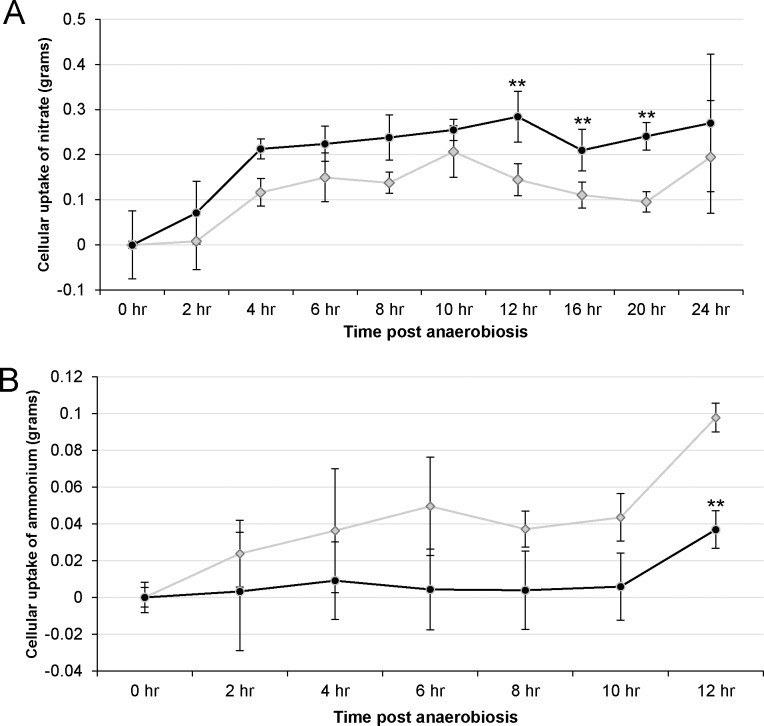
Nitrate uptake in wild-type and ami-hcp4 were similar to one another, but ami-hcp4 had a consistently higher uptake of nitrate, with three out of ten time points (12, 16 and 20 hours post-anaerobiosis) being statistically different than wild-type (Fig 5A). Four hours post-anaerobiosis marked the first notable divergence in nitrate uptake with wild-type and ami-hcp4 cells having uptaken an average of 0.12 and 0.22 grams of nitrate respectively. Twelve hours post-anaerobiosis marked the greatest divergence in nitrate uptake with wild-type and ami-hcp4 having uptaken a mean of 0.14 and 0.28 grams of nitrate respectively. The trend in this data shows that cc425 nitrate uptake peaked at 10 hours post-anaerobiosis while ami-hcp4 peaked at 12 hours.
Fig 5. Nitrate and ammonium uptake during anaerobiosis.
Nitrate and ammonium uptake were measured for 24 and 12 hours during anaerobiosis, respectively. Wild-type cc425 (gray diamonds) and hcp4 (black circles). Cultures were grown in 10 mM HEPES, 10 mM ammonium, and 12 mM acetate solution. Cells were bubbled with N2 for 10 minutes and kept sealed in the dark. Subsamples were taken every two hours and nitrate and ammonium remaining in solution measured. ** t-test, p <0.01, n = 3 biological replicates for A and B.
Ammonium uptake was measured for only 12 hours under anaerobic conditions (Fig 5B) due to low viability of the cells in the later stages of the experimental conditions used. Wild-type and ami-hcp4 ammonium uptake diverged at 8, 10, and 12 hour time points. At 8 hours post-anaerobiosis mean ammonium uptake in wild-type and ami-hcp4 were 0.03 and 0.003 grams respectively. At 12 hours post-anaerobiosis ammonium uptake in wild-type and ami-hcp4 were 0.1 and 0.06 grams of ammonium respectively. The general trend in Fig 5B shows relatively constant ammonium uptake in wild-type cells, but ammonium uptake in ami-hcp4 did not take place until 12 hours post-anaerobiosis. A significant difference in ammonium uptake was calculated at 12 hours post-anaerobiosis by Student’s unpaired t-test with equal variance (p = 0.012).
Discussion
C. reinhardtii has a uniquely diverse set of metabolic pathways that enable it to cope with a multitude of environmental situations including extended periods of anoxia. Recent metabolic and genetic studies have uncovered genes, pathways, and proteins that are up-regulated or activated during anaerobiosis [2,27,28]. The multiple pathways facilitating the continuation of glycolysis during anaerobiosis make C. reinhardtii fermentation one of the most diverse in the plant world and have been shown to be plastic in nature [29]. Being one of the most highly up-regulated genes during anaerobiosis in C. reinhardtii, HCP4 proves to be an interesting candidate for study as its physiological role in organisms has not been precisely determined, yet is activated during O2 deprivation in many prokaryotes. This study demonstrates that silencing of HCP4 impacts multiple fermentation pathways, including changes in gene transcription, as well as metabolite flux and nitrogen uptake.
HCP4 transcription in ami-hcp4 was decreased consistently during anaerobiosis. The -0.33 hour time point showed a 4-fold knockdown, while one hour post-anaerobiosis a 20-fold knockdown was achieved. This increase in silencing level across time points in ami-hcp4 compared to wild-type was likely due to the rapid induction of HCP4 upon anaerobiosis as shown in Fig 3A. Knockdown of HCP4 also led to dramatic differences in the expression of key genes for different fermentation pathways (Fig 4A). HYD and PFL transcripts showed no significant change in expression after 3 hours of anaerobiosis, but were dramatically reduced after 5 hours of anaerobiosis. PFR, which links pyruvate metabolism to ferredoxin, was shown to be consistently lower at 3 and 5 hours post-anaerobiosis. PDC expression, however, was also dramatically reduced at all time points. PDC transcript levels were also measured at earlier time points and in multiple independent transformants and found to be similarly silenced. Taken together, a general repression in gene expression for fermentation pathways was noted. These findings strongly suggest HCP4 is either directly or indirectly involved in regulating cellular responses to anonxia.
Metabolite assays also showed a significant difference between wild-type and ami-hcp4. As shown in Fig 4, the accumulation of ethanol and formate in the media began at 3 hours post-anaerobiosis. Significant differences in ethanol and formate accumulation between wild-type and ami-hcp4 appeared at 5 hours post-anaerobiosis (Fig 4B and 4C). At 3 hours post-anaerobiosis ami-hcp4 displayed a ratio of ethanol-to-formate production of ~4:1, although neither metabolite displayed significantly different accumulation between ami-hcp4 and wild-type at this time point. This ~4:1 ratio of ethanol to formate production perhaps indicates that acetyl-CoA is being produced by both PFL and PFR and is being converted to acetate as well as ethanol. It is possible that enough PFR activity is still present even with the reduced transcript levels witnessed at this time point which contributes acetyl-CoA that is favorably converted to acetate.
Interestingly, both formate and ethanol accumulation are roughly 2.6-fold higher in ami-hcp4 than wild-type at five hours post-anaerobiosis, displaying a 1:1 ratio of production. Similarly, ethanol and formate production in wild-type occur at a roughly 2:1 ratio at three hours post-anaerobiosis and a ratio of 1:1 at five hours post-anaerobiosis. PFL facilitates the breakdown of pyruvate into formate and acetyl-CoA in a 1:1 ratio. If we assume that the down-regulation of PFR shown in ami-hcp4 effectively blocks the breakdown of pyruvate by this pathway, and the more than anticipated acetate accumulation during fermentation in the media is due to excretion of acetate accumulated during mixotrophic growth, then these data suggest that the ethanol produced in C. reinhardtii at 5 hours post-anaerobiosis is facilitated exclusively by PFL in both wild-type and ami-hcp4. Conversely, at 3 hours post-anaerobiosis the ratio of ethanol-to-formate suggest that PFR is actively converting pyruvate into acetyl-CoA while reducing ferredoxin or acetyl-CoA produced by PFL is being converted to acetate. This shift in metabolic flux happens precisely when ethanol and formate start to accumulate in the media. It seems possible that the accumulation of metabolites may act as a regulator during anaerobiosis, possibly being detected directly, or by secondary signaling molecules such as pH or oxidative stress. Taken together, a general model of the response of fermentation pathway gene expression and metabolic flux is presented in Fig 6. At 5 hours post-anaerobiosis there is a general decrease in fermentation gene transcription, albeit with a hypothesized increase in electron flux through the PFL and ADH pathways.
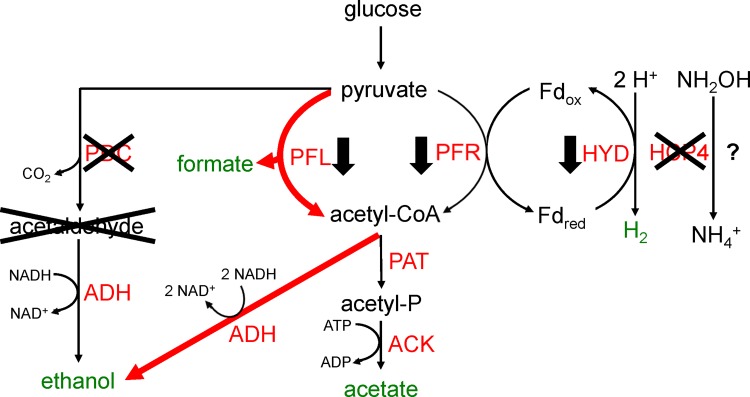
Fig 6. Summary of altered fermentation pathways upon HCP4 silencing.
PDC, PFL, PFR, and HYD gene transcripts were significantly reduced in ami-hcp4 relative to wild-type at five hours post-anaerobiosis. It is proposed that at five hours post anaerobiosis greater electron flux through PFL and ADH (red lines) leads to increased formate and ethanol production in hcp4. It is also possible that HCP4 may be competing with HYD for electrons from reduced ferredoxin in wild-type cells. A putative function for HCP4 as a hydroxylamine reductase is indicated. The effect of silencing HCP4 on H2 production is unknown.
One of the most startling findings of this study was the drastic down-regulation of PDC transcription. PDC is a cytoplasmic protein that is an integral component in yeast alcoholic fermentation. It has been shown that PDC does not participate highly in C. reinhardtii fermentation, but its activity and gene expression are both increased during anaerobiosis [2,29]. In yeast, PDC shows optimal activity in cells grown at a pH 6.0, but a decrease of only 7% in activity was shown when resuspended at a pH of 7.4, which is close to the pH of this experiment (7.5) [30]. PDC in maize is induced upon anaerobiosis only when cellular pH dropped from 7.4 to 6.8 [31]. Expression data in C. reinhardtii shows PDC up-regulation takes place in media at pH 7.0 and pH 7.3 [2,32]. Thus extracellular pH does not appear to similarly regulate PDC expression in C. reinhardtii. It is possible however that intracellular pH levels drop during anaerobiosis causing PDC to be activated. It is possible that an intracellular drop in pH is inhibited in ami-hcp4, thereby inhibiting PDC transcription. A potential mechanism of HCP4 in controlling intracellular pH is currently unknown.
Acetate accumulation in wild-type and ami-hcp4 cultures were not significantly different at any time point, and levels in both cell types increased steadily throughout the course of the experiment (Fig 4D). Acetate levels were significantly higher than ethanol and formate, which conflicts with previously published data stating the ratio of formate:acetate:ethanol should be roughly 2:1:1 or 2:2:1 [2,8,9]. While the data presented here offer a much different ratio of metabolic byproducts, differences in experimental procedures may account for this. In the Gfeller et al. [9] and Ohta et al. [8] studies, cells were grown autotrophically using CO2 as a carbon source, while also using different strains (F-60 and MGA 161, respectively). In this study however, acetate was used as a carbon source during mixotrophic growth. Thus when washed and resuspended in HEPES buffer before anaerobiosis, the cells possibly contained high internal stores of acetate (or starch leading to acetate production) which may have been excreted during anaerobiosis. Less clear are the differences in metabolite accumulation observed in this study and that from Mus et al. [2], which used the same strain and similar growth conditions. The only notable difference in growth conditions was that the cells in the current study were resuspended in 28 mM HEPES, pH 7.5 prior to induction of anoxia, whereas Mus et al. used 50 mM potassium phosphate (pH 7.0) supplemented with 3 mM MgCl2. It would be interesting to repeat the current study using alternative buffers during anoxic growth to determine how the culture conditions impact metabolite evolution.
HCP-family proteins have been implicated in nitrogen metabolism by studies in E.coli. HCP expression is up-regulated in E. coli upon addition of nitrate or nitrite [26], and purified HCP also has hydroxylamine reductase activity [17]. Hydroxylamine reductase catalyzes the reversible conversion of hydroxylamine to ammonia using ferredoxin as an electron donor [18]. HCP4 in C. reinhardtii shows moderate amino acid sequence similarity with the E.coli HCP (which has demonstrated hydroxylamine reductase activity), with 41.7% identity and 59.1% similarity (S1 Fig). Our study also shows a potential effect of HCP4 on nitrogen utilization during anaerobiosis. ami-hcp4 showed increased nitrate uptake, but decreased ammonia uptake compared to wild-type (Fig 5). It has been hypothesized that nitrite reductase requires ferredoxin to convert nitrite to ammonium [33]. The data presented here shows nitrate uptake being enhanced in ami-hcp4, which may indicate HCP4 is also competing with nitrite reductase for electrons from ferredoxin. It is interesting that significant changes in ammonium uptake were not present in ami-hcp4 until 12 hours post-anaerobiosis. This lag in ammonium uptake in ami-hcp4 may indicate that HCP4 has a primary role responsible for ammonium uptake in C. reinhardtii. Alternatively, it is certainly possible that the observed changes in N uptake in ami-hcp4 may be due to secondary effects of silencing HCP4. Clearly, future experiments examining hydroxylamine and nitrite reductase activities (as well as N utilization with more temporal resolution) in both mutant and wild-type cultures are warranted.
Taken together these data suggest a preliminary model of HCP4’s role in the cell during darkness-induced anaerobiosis. The sequence similarity of HCP4 in C. reinhardtii to HCP in E. coli, and the increase in nitrate uptake when HCP4 is silenced, suggest that HCP4 is oxidizing reduced ferredoxin. With this working hypothesis, the other data collected in this experiment can be analyzed and a preliminary model produced. The silencing of HCP4 may cause PFR to be downregulated due to the reduced electron flow out of ferredoxin. Whether or not HCP4 competition for reduced ferredoxin has an impact on H2 output is unknown. In this model HCP4 acts as a “release valve” for electrons from ferredoxin. A decreased reduction of pyruvate due to the depressed transcription of PFR may lead to a decrease of acetyl-CoA, which in turn would cause a decrease in NAD+ and ATP production through the PAT/ACK or ADH pathways (Fig 1 and Fig 6). This loss in NAD+ and/or ATP production would be compensated for by the accumulation of PFL transcripts and an increased production of formate and acetyl-CoA. The decrease in PFL transcripts at five hours post-anaerobiosis could possibly be explained by the increase in formate accumulation acting as a negative feedback to PFL gene transcription. PFL proteins present up to that point may still be active. This hypothetical model displays the functional compensation of fermentation pathways in C. reinhardtii similarly shown in other studies [29,34].
While it is clear that HCP4 silencing affects the transcript levels of central fermentation pathways, as well as metabolite production in C. reinhardtii, further work is needed to fully characterize HCP4’s role in anaerobic metabolism. Transcript abundance changes drastically upon silencing of HCP4, but previous studies have shown that PFL and HYD are regulated not only at the transcriptional level, but are also activated post-transcriptionally [29,34]. Further investigation of protein levels in wild-type and ami-hcp4 would further guide understanding of the plasticity of these fermentation pathways.
Unfortunately, H2 gas evolution was not measured in this study due to technical difficulties. Thus the results presented here provide an incomplete picture of metabolic flux during anoxia. Quantifying H2 production in C. reinhardtii in ami-hcp4 and wild-type would further support or reject the hypothesis that HCP4 competes with HYD for electrons from ferredoxin, as well as the extent to which silencing HCP4 has in increasing the viability of H2 production via C. reinhardtii at a commercial level. In vitro experiments with isolated ferredoxin and HCP4 could be used examine their potential interactions, such as seen in Wolfe et al. 2002 [17]. Following these experiments, a more global examination of metabolites (including quantification of H2 production using mass-spec and total starch breakdown) would generate the data needed to pinpoint HCP4’s position in C. reinhardtii fermentation, as well as the potential HCP4 mutants have for increasing the production of biofuels. It is also imperative to address potential functional redundancy between HCP4 and the other three HCPs encoded by the C. reinhardtii genome, which all share extensive sequence similarity (S1 Fig).
The drastic downregulation of PDC was an unexpected and interesting finding. Although PDC and hydrogenase seem to have similar expression patterns (being upregulated during oxidative stress and anaerobiosis) their direct link is unclear. These results strongly suggest HCP4 somehow regulates the expression of the PDC. PDC expression was shown in other organisms to be dependent on intracellular pH. In this experiment cultures were heavily buffered at pH 7.5. It would be interesting to see whether intracellular or extracellular pH changes occur upon silencing of HCP4 in a less heavily buffered media.
While HCP4 is most highly upregulated during anaerobiosis, it is also up-regulated during sulfur deprivation-induced anaerobiosis, which creates much more H2 gas than darkness-induced anaerobiosis [35]. If the hypothesis that HCP4 oxidizes reduced ferredoxin is supported, then the increase in H2 output could be drastically increased in these cultures. Therefore investigation of ami-hcp4 mutants must be examined in these conditions in order to determine HCP4’s potential impact on the economic feasibility of H2 production in C. reinhardtii.
Conclusions
This study demonstrates C. reinhardtii fermentative metabolism is not only phylogenetically diverse and functionally plastic, but that reverse genetic techniques can be applied to increase biofuel production. For example, down-regulating HCP4 resulted in a significant increase in ethanol production. The increased ethanol, and potentially increased H2 production rates, in this mutant may have sizeable benefits to mass production of biofuels in C. reinhardtii. Its potential role in nitrogen metabolism may also be an important avenue of future investigation.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Abbreviations
- PFL
pyruvate formate lyase
- PDC
pyruvate decarboxylase
- PFR
pyruvate ferredoxin oxidoreductase
- LDH
lactate dehydrogenase
- HYD
hydrogenase
- ADH
alcohol dehydrogenase
- PAT1/PAT2
phosphoacetyl transferase
- ACK1/ACK2
acetate kinase
- NR
nitrate reductase
- NiR
nitrite reductase
- GS/GOGAT
glutamine sythetase/glutamate synthase cycle
- GOGAT
glutamine oxoglutarate amidotransferase
- TAP
Tris acetate phosphate media
- amiRNA
artificial microRNA
- ami-hcp4
transgenic C. reinhardtii containing amiRNA targeting HCP4
- HCP
hybrid cluster protein
- HCP4
hybrid cluster protein 4
- CAIP
calf intestinal alkaline phosphatase
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the University of Minnesota Grant-in-Aid of Research, Artistry & Scholarship Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Harris EH (1989) The Chlamydomonas sourcebook: a comprehensive guide to biology and laboratory use San Diego: Academic Press; xiv, 780 p. p. [DOI] [PubMed] [Google Scholar]
- 2.Mus F, Dubini A, Seibert M, Posewitz MC, Grossman AR (2007) Anaerobic acclimation in Chlamydomonas reinhardtii: anoxic gene expression, hydrogenase induction, and metabolic pathways. Journal of Biological Chemistry 282: 25475–25486. [DOI] [PubMed] [Google Scholar]
- 3.Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiology 122: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beer LL, Boyd ES, Peters JW, Posewitz MC (2009) Engineering algae for biohydrogen and biofuel production. Current Opinion in Biotechnology 20: 264–271. 10.1016/j.copbio.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Molnar A, Bassett A, Thuenemann E, Schwach F, Karkare S, Ossowski S, et al. (2009) Highly specific gene silencing by artificial microRNAs in the unicellular alga Chlamydomonas reinhardtii. Plant Journal 58: 165–174. 10.1111/j.1365-313X.2008.03767.x [DOI] [PubMed] [Google Scholar]
- 6.Zhao T, Wang W, Bai X, Qi Y (2009) Gene silencing by artificial microRNAs in Chlamydomonas. Plant Journal 58: 157–164. 10.1111/j.1365-313X.2008.03758.x [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Brueggeman AJ, Horken KM, Plucinak TM, Weeks DP (2014) Successful transient expression of Cas9 and single guide RNA genes in Chlamydomonas reinhardtii. Eukaryot Cell 13: 1465–1469. 10.1128/EC.00213-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohta S, Miyamoto K, Miura Y (1987) Hydrogen evolution as a consumption mode of reducing equivalents in green algal fermentation. Plant Physiology 83: 1022–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gfeller RP, Gibbs M (1984) Fermentative metabolism of Chlamydomonas reinhardtii: I. Analysis of fermentative products from starch in dark and light. Plant Physiology 75: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreuzberg K (1984) Starch fermentation via a formate producing pathway in Chlamydomonas reinhardii, Chlorogonium elongatum and Chlorella fusca. Physiologia Plantarum 61: 87–94. [Google Scholar]
- 11.Happe T, Mosler B, Naber JD (1994) Induction, localization and metal content of hydrogenase in the green alga Chlamydomonas reinhardtii. European Journal of Biochemistry 222: 769–774. [DOI] [PubMed] [Google Scholar]
- 12.Ghirardi ML, Togasaki RK, Seibert M (1997) Oxygen sensitivity of algal H2- production. Applied Biochemistry and Biotechnology 63–65: 141–151. [DOI] [PubMed] [Google Scholar]
- 13.Ghirardi ML, Posewitz MC, Maness PC, Dubini A, Yu J, Seibert M (2007) Hydrogenases and hydrogen photoproduction in oxygenic photosynthetic organisms. Annual Review of Plant Biology 58: 71–91. [DOI] [PubMed] [Google Scholar]
- 14.Zabawinski C, Van Den Koornhuyse N, D'Hulst C, Schlichting R, Giersch C, Delrue B, et al. (2001) Starchless mutants of Chlamydomonas reinhardtii lack the small subunit of a heterotetrameric ADP-glucose pyrophosphorylase. Journal of Bacteriology 183: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posewitz MC, Smolinski SL, Kanakagiri S, Melis A, Seibert M, Ghirardi ML (2004) Hydrogen photoproduction is attenuated by disruption of an isoamylase gene in Chlamydomonas reinhardtii. Plant Cell 16: 2151–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida CC, Romao CV, Lindley PF, Teixeira M, Saraiva LM (2006) The role of the hybrid cluster protein in oxidative stress defense. Journal of Biological Chemistry 281: 32445–32450. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe MT, Heo J, Garavelli JS, Ludden PW (2002) Hydroxylamine reductase activity of the hybrid cluster protein from Escherichia coli. Journal of Bacteriology 184: 5898–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valentine RC, Jackson RL, Wolfe RS (1962) Role of ferredoxin in hydrogen metabolism of Micrococcus lactilyticus. Biochemical and Biophysical Research Communications 7: 453–456. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez E, Galvan A (2008) Nitrate assimilation in Chlamydomonas. Eukaryotic Cell 7: 555–559. 10.1128/EC.00431-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirasawa M, Tripathy J, Sommer F, Somasundaram R, Chung J-S, Nestander M, et al. (2010) Enzymatic properties of the ferredoxin-dependent nitrite reductase from Chlamydomonas reinhardtii; Evidence for hydroxylamine as a late intermediate in ammonia production. Photosynthesis Research 103: 67–77. 10.1007/s11120-009-9512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittag M, Waltenberger H (1997) In vitro mutagenesis of binding site elements for the clock-controlled proteins CCTR and Chlamy 1. Biological Chemistry 378: 1167–1170. [PubMed] [Google Scholar]
- 22.Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryotic Cell 9: 486–501. 10.1128/EC.00364-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melis A, Happe T (2004) Trails of green alga hydrogen research—from hans gaffron to new frontiers. Photosynthesis Research 80: 401–409. [DOI] [PubMed] [Google Scholar]
- 24.Thacker A, Syrett PJ (1972) The assimilation of nitrate and ammonium by Chlamydomonas reinhardi. New Phytologist 71: 423–433. [Google Scholar]
- 25.Kindle KL (1990) High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences of the United States of America 87: 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filenko NA, Browning DF, Cole JA (2005) Transcriptional regulation (prismane) protein of a hybrid cluster. Biochemical Society Transactions 33: 195–197. [DOI] [PubMed] [Google Scholar]
- 27.Grossman AR, Croft M, Gladyshev VN, Merchant SS, Posewitz MC, Prochnik S, et al. (2007) Novel metabolism in Chlamydomonas through the lens of genomics. Current Opinion in Plant Biology 10: 190–198. [DOI] [PubMed] [Google Scholar]
- 28.Terashima M, Specht M, Naumann B, Hippler M (2010) Characterizing the anaerobic response of Chlamydomonas reinhardtii by quantitative proteomics. Molecular & Cellular Proteomics 9: 1514–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philipps G, Krawietz D, Hemschemeier A, Happe T (2011) A pyruvate formate lyase-deficient Chlamydomonas reinhardtii strain provides evidence for a link between fermentation and hydrogen production in green algae. Plant Journal 66: 330–340. 10.1111/j.1365-313X.2011.04494.x [DOI] [PubMed] [Google Scholar]
- 30.MORRELL S, GREENWAY H, DAVIES DD (1990) Regulation of pyruvate decarboxylase in vitro and in vivo. Journal of Experimental Botany 41: 131–139. [Google Scholar]
- 31.Roberts JK, Wemmer D, Ray PM, Jardetzky O (1982) Regulation of cytoplasmic and vacuolar pH in maize root tips under different experimental conditions. Plant Physiology 69: 1344–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen AV, Thomas-Hall SR, Malnoe A, Timmins M, Mussgnug JH, Rupprecht J, et al. (2008) The transcriptome of photo-biological hydrogen production induced by sulphur deprivation in the green alga Chlamydomonas reinhardtii. Eukaryotic Cell: EC.00418-00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirasawa M, Knaff DB (1985) Interaction of ferredoxin-linked nitrite reductase with ferredoxin. Biochimica Et Biophysica Acta 830: 173–180. [DOI] [PubMed] [Google Scholar]
- 34.Dubini A, Mus F, Seibert M, Grossman AR, Posewitz MC (2009) Flexibility in anaerobic metabolism as revealed in a mutant of Chlamydomonas reinhardtii lacking hydrogenase activity. Journal of Biological Chemistry 284: 7201–7213. 10.1074/jbc.M803917200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morsy FM (2011) Acetate versus sulfur deprivation role in creating anaerobiosis in light for hydrogen production by Chlamydomonas reinhardtii and Spirulina platensis: two different organisms and two different mechanisms. Photochemistry and Photobiology 87: 137–142. 10.1111/j.1751-1097.2010.00823.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.