Abstract
M cells in the follicle-associated epithelium (FAE) of Peyer’s patches (PPs) serve as a main portal for external antigens and function as a sentinel in mucosal immune responses. The scarcity of these cells has hampered identification of M cell-specific molecules. Recent efforts have begun to provide insight into antigen transcytosis and differentiation of M cells; however, the molecular mechanisms underlying these processes are not fully elucidated. Small non-coding RNAs including microRNA (miRNA) have been reported to regulate gene expression and control various biological processes such as cellular differentiation and function. To evaluate the expression of miRNAs in FAE, including M cells, we previously performed microarray analysis comparing intestinal villous epithelium (VE) and PP FAE. Here we confirmed FAE specific miRNA expression levels by quantitative PCR. To gain insight into miRNA function, we generated mice with intestinal epithelial cell-specific deletion of Dicer1 (DicerΔIEC) and analyzed intestinal phenotypes, including M-cell differentiation, morphology and function. DicerΔIEC mice had a marked decrease in M cells compared to control floxed Dicer mice, suggesting an essential role of miRNAs in maturation of these cells. Furthermore, transmission electron microscopic analysis revealed that depletion of miRNA caused the loss of endosomal structures in M cells. In addition, antigen uptake by M cells was impaired in DicerΔIEC mice. These results suggest that miRNAs play a significant role in M cell differentiation and help secure mucosal immune homeostasis.
Introduction
The gastrointestinal tract is the site for digestion and absorption of nutrients, but at the same time it is exposed to foreign antigens including enormous numbers of commensal microorganisms as wells as pathogens. To protect from these foreign antigens, the gastrointestinal tract is equipped with a specialized gut-associated lymphoid tissue (GALT) as well as a variety of non-immunologic barriers, including gastric acid, pancreatic juice, bile, glycocalyx, a mucus layer, intercellular junctional complexes (e.g., tight junctions and adherens junctions), and rapid cell turnover [1, 2]. The mucosal surface is also protected by secretory antibody, especially immunoglobulin A, as well as antimicrobial peptides secreted from Paneth cells and enterocytes. In addition, GALT serves as the leading edge of an immunological barrier. GALT, comprised of Peyer’s patches (PPs), isolated lymphoid follicles, appendix and colonic patches is the main inductive site for mucosal immune responses [3]. Luminal surfaces of PPs are covered by the follicle-associated epithelium (FAE), which contains relatively limited numbers of goblet cells and enteroendocrine cells but harbors a unique subset of epithelial cells, membranous or microfold cells (M cells) [4]. Unlike the villus epithelium (VE), the FAE is specially designed to promote contact with luminal antigens to induce mucosal immune responses. For example, there are limited numbers of goblet cells in FAE and a thinner mucus layer compared to the VE region [4]. It has also been reported that FAE enterocytes lack polymeric Ig receptors for the local transport and secretion of secretory IgA [5]. In addition, antimicrobial peptide-producing Paneth cells are not present in the FAE crypts [6]. These features provide easier access to FAE by luminal particulate antigens such as bacteria and viruses. By contrast, the VE consists primarily of enterocytes, with scattered goblet cells and occasional enteroendocrine cells. The main function of the VE is the digestion and absorption of nutrients. Thus, the cellular composition and function of FAE and VE are quite different; however, the mechanisms that differentially regulate FAE and VE differentiation remain unknown.
M cells are specialized epithelial cells located in the FAE [7] that continuously sample and transport luminal antigens to the underlying GALT. The antigens are then captured by immature dendritic cells (DCs) residing in the subepithelial dome region beneath the FAE. The antigen-primed DCs undergo maturation and migrate to the T-cell area of GALT to present antigens to T cells, leading to activation of antigen-specific B cells and ultimately the production of IgA antibodies by lamina propria plasma cells [3]. Accumulated studies have begun to provide insight into antigen transcytosis and differentiation of M cells [8–12]; however, the molecular mechanisms underlying these processes are not fully elucidated.
MicroRNAs (miRNA) are ~19–25 nucleotide non-coding RNA molecules that regulate gene expression via repression of target mRNA. Binding of miRNAs to the 3' untranslated region of target mRNAs leads to translation inhibition or mRNA degradation [13]. A substantial number of studies have shown that miRNAs regulate many biological processes including cell or tissue development timing, differentiation and growth control [14]. The miRNAs are transcribed by RNA polymerase II as primary transcripts that are later processed by the RNase III-type endonuclease called Dicer into mature miRNAs. However, the complete loss of Dicer leads to embryonic lethality in mice [15], making its many functions difficult to study. To elucidate the importance of miRNAs in a particular tissue development or cell differentiation process, many groups have therefore used tissue or cell type-specific recombination approaches to deplete the Dicer1 gene[16–19]. Expression profiles and functions of miRNAs in intestinal epithelium have been examined in jejunal and colonic mucosa [20], but those in FAE remain unknown. We generated intestinal epithelium-specific Dicer1 deletion mice and investigated the role of miRNA in this tissue, focusing on the FAE. Here we report that miRNAs in FAE contribute to M-cell differentiation and that loss of miRNAs leads to impaired antigen transcytosis function via depletion of endosomes.
Materials and Methods
Animals
BALB/cA and C57BL/6 mice were purchased from CLEA Japan. Dicer1 flox mice (DicerF/F) were purchased from The Jackson laboratory and were backcrossed onto the C57BL/6 background. To generate intestinal epithelial cell-specific Dicer1 knockout (DicerΔIEC) mice, we crossed Dicer1 flox mice with villin-cre transgenic mice. DicerΔIEC and control DicerF/F littermates were maintained under specific pathogen-free conditions. Mice are sacrificed by cervical dislocation. Animal experiments were approved by the Animal Research Committees of RIKEN and Yokohama City University [(Permit Numbers Kei 24–005 (RIKEN) and T11-001 (Yokohama City University)].
Preparation FAE and VE for quantitative PCR
PPs were harvested from mice using curved scissors and flushed out the luminal contents with Hank’s balanced salt solution (HBSS). PPs were soaked in HBSS containing 30 mM EDTA for 20 min at 4°C. Epithelial cell sheets were peeled off from PPs, and FAE and VE were dissected by using 26G needles under stereomicroscopic monitoring ([21]) to obtain epithelium sheets of FAE and VE ([21, 22]). To count and calculate follicle numbers and surface area, PPs incubated with 3% acetic acid for 15 min at room temperature were analyzed using a SZX16 stereoscopic microscope (Olympus). Follicle surface area was calculated using DP2-BSW (Olympus).
Quantitative PCR of miRNA
Total RNA including the small RNA fraction was extracted from murine FAE and VE using a mirVana kit (Ambion) and was reverse transcribed with a Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems). Quantitative PCR was performed to quantify miRNA expression levels using the TaqMan Universal PCR Master Mix II w/ UNG (Applied Biosystems) and the Thermal Cycler Dice Real Time System (TAKARA). Values were normalized relative to the small nucleolar RNA Sno202. Specific primer sets were purchased from Applied Biosystems.
Quantitative PCR of mRNA
Total RNA was extracted from murine FAE and VE using a mirVana kit (Ambion) and was reverse-transcribed using ReverTra Ace-α (TOYOBO). Quantitative PCR was performed to quantify mRNA expression levels using the SYBR Premix Ex Taq and the Thermal Cycler Dice Real Time System (TAKARA). Values were normalized to Gapdh. Specific primer pairs for each gene are listed in Table 1.
Table 1. Primer sequence of q-PCR.
| Gene Symbol | Sequence |
|---|---|
| Ccl9 | Forward 5’-TACTGCCCTCTCCTTCCTCA-3’ |
| Reverse 5’-TTGAAAGCCCATGTGAAACA-3' | |
| GAPDH | Forward 5’-TGTGTCCGTCGTGGATCTGA-3’ |
| Reverse 5’-TTGCTGTTGAAGTCGCAGGAG-3' | |
| Gp2 | Forward 5’-GATACTGCACAGACCCCTCCA-3’ |
| Reverse 5’-GCAGTTCCGGTCATTGAGGTA-3' | |
| Marcksl1 | Forward 5’-TTTTGCCCTCCTGTGGATTCT-3’ |
| Reverse 5’-CCACTAGGCACAGCACAAGAGA-3' | |
| SpiB | Forward 5’-AGCGCATGACGTATCAGAAGC-3’ |
| Reverse 5’-GGAATCCTATACACGGCACAGG-3' | |
| Dicer1 | Forward 5’-TGCCCTTGTCAATAACACCA-3’ |
| Reverse 5’-GCTCAGAGTCCATTCCTTGC-3' | |
| Mybl2 | Forward 5’-GGATGAAGATGGGAAGCTGA-3’ |
| Reverse 5’-TGAGCAGGCTGTTACCCTCT-3' |
Hematoxylin and Eosin (H&E) stain
Paraffin embedded tissue sections were deparaffinized and rehydrated. Then, sections were stained with hematoxylin and eosin.
Whole mount immunostaining
PPs were excised from the small intestine, fixed with Cytofix/Cytoperm (BD Biosciences) for 1 hour at 4°C and then incubated with 10 μg/ml anti-CD16/32 monoclonal antibody (93; eBioscience) / 0.1% saponin / 0.2% BSA in phosphate buffered saline (PBS) to block non-specific Fc binding. The whole mount specimens were then stained overnight at 4°C with 1 μg/ml Alexa Fluor 488-conjugated anti-mouse GP2 (2F11C3; MBL) and 1 U/ml Alexa Fluor 555-conjugated Phalloidin (Molecular probes). The specimens were analyzed with a DM-IRE2 confocal laser scanning microscope and Leica confocal software (Leica Microsystems). To count M cells number, FAE sheet were peeled off from PPs as describe above. Then, FAE sheets were fixed with Cytofix/Cytoperm (BD Biosciences) for 1 hour at 4°C and incubated with 10 μg/ml anti-CD16/32 monoclonal antibody (93; eBioscience) / 0.1% saponin / 0.2% BSA in phosphate buffered saline (PBS) to block non-specific Fc binding. The whole mount specimens were then stained overnight at 4°C with 1 μg/ml Alexa Fluor 488-conjugated anti-mouse GP2 (2F11C3; MBL). The specimens were analyzed with a BX51 fluorescence microscope (Olympus). M cell count and FAE area measurement were examined using ImageJ software.
Scanning electron microscopy
PPs were excised and fixed for 2.5 hours with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH7.4). The tissues were completely dehydrated in a graded ethanol series. Specimens were coated with a gold layer using a sputter coater MSP-1S (Shinku Device) and observed by SEM (VE-7800, KEYENCE).
Transmission electron microscopy
PPs and VE were excised and fixed for 48 hours with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH7.4). The tissues were cut into 1–2 mm pieces, immersed for an additional 4 hours in the same fixative, postfixed for 1.5 hours with 1% OsO4 dissolved in distilled water, dehydrated in a graded series of ethanol, and embedded in Epon. Ultrathin sections were cut on an ultramicrotome and stained with uranyl acetate and lead citrate for observation under an electron microscope (H7100, Hitachi).
Evaluation of bead uptake
8- to 10-week-old DiecrF/F or DicerΔIEC mice (four mice per group) were inoculated by gavage with 1 x 1011 FluoSpheres (Invitrogen). After 4 hours, PPs were dissected and incubated at 25°C in sterile PBS. Prepared frozen sections were examined using a BX51 fluorescence microscope (Olympus) and beads in PP follicles were counted manually.
Evaluation of oral infection
8- to 10-week-old DiecrF/F or DicerΔIEC mice (five mice per group) were inoculated intragastrically by gavage with 0.2 ml of 0.1 M sodium bicarbonate to neutralize gastric acid. Mice were then inoculated intragastrically by gavage with 1 x 108 CFU of Yersinia enterocolitica (ATCC27729). After 24 hours, three PPs from the ileal end were dissected and incubated at 25°C in sterile PBS containing 20 μg/ml gentamicin for 30 minutes, homogenized in sterile PBS and plated on Yersinia Selective Agar base (OXID) with Yersinia selective supplement (OXID) to determine CFU.
Statistics
Statistical analysis was performed with the Mann-Whitney U test. Differences were considered as significant at P < 0.05.
Results
Different miRNA expression profiles in FAE and VE
To examine the miRNA expression profile in FAE, we have previously dissected FAE and VE surrounding the FAE of PPs [11, 22] and compared their miRNA expression profiles by microarray analysis. We identified 43 miRNAs up-regulated at least two-fold in FAE compared with VE and 9 miRNAs down-regulated in FAE by at least two-fold [21]. The expression levels of the 43 miRNAs upregulated in FAE were further examined in C57BL/6 and BALB/cA mice by quantitative PCR (q-PCR). Only 5 miRNAs were commonly up-regulated at least two-fold in FAE compared with VE in both strains (Fig 1 and data not shown). We therefore reasoned that these five miRNAs are likely involved in FAE-specific translational regulation.
Fig 1. miRNA expression profiles in intestinal epithelium.
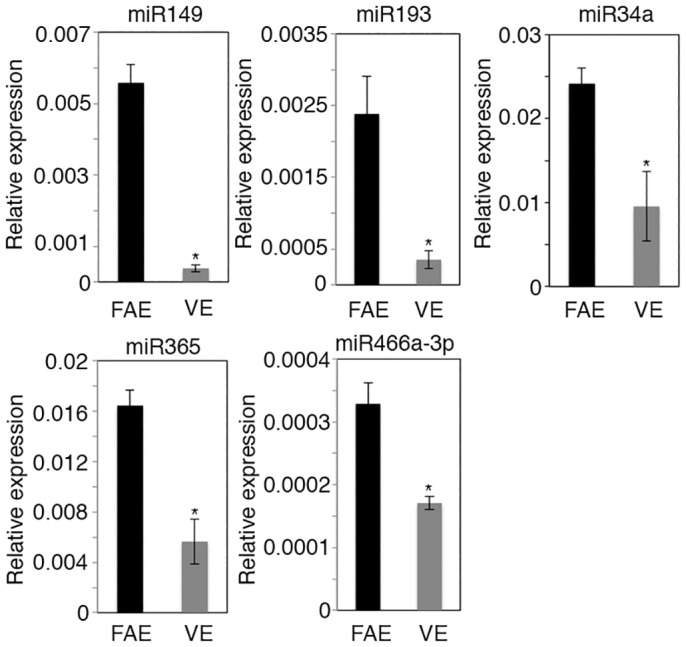
Q-PCR analysis was performed for miRNA expression in FAE and VE. The relative levels of each miRNA relative to the small nucleolar RNA Sno202 are shown. Values are mean ± SE of three samples from different mice. *P<0.05.
miRNAs in FAE contribute to M-cell maturation
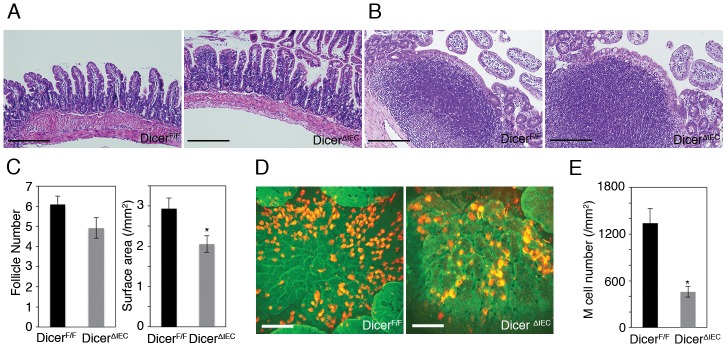
To elucidate the functions of miRNAs in intestinal epithelium, including FAE, we generated mice lacking mature miRNAs in intestinal epithelium by crossing floxed Dicer mice to villin-cre mice. Both DicerΔIEC FAE and VE displayed approximately 85–95% reduction of Dicer1 mRNA expression levels compared with DicerF/F (S1 Fig) DicerΔIEC mice showed decreased number of goblet cells and increased number of apoptotic cells in the crypt region. In addition, the number of Paneth and enteroendocrine cells was similar in both mice. These phenotypes are consistent with previous reports [20]. On the other hands, villous length and PPs structure in DicerΔIEC were similar to DicerF/F (Fig 2A and 2B). Total number of PPs and follicles remained unchanged in DicerΔIEC mice (Fig 2C, S2A and S2B Fig). By contrast, the surface area of FAE in DicerΔIEC was less than that of control floxed Dicer (DicerF/F) mice (Fig 2C, S2A and S2B Fig). In addition, whole mount immunostaining revealed that the density of M cells in FAE was decreased by more than half in DicerΔIEC compared to DicerF/F mice (Fig 2D and 2E, S3 Fig).
Fig 2. Total number of M cells in Peyer’s patches is decreased in DicerΔIEC mice.
(A) H&E staining of small intestines VE region of DicerΔIEC and DicerF/F. (B) H&E staining in PPs of DicerΔIEC and DicerF/F. (C) The total number of follicles and surface area in DicerΔIEC and DicerF/F. Data are means± SE (n = 3). *P < 0.05. (D) Whole mount immunostaining of PPs with anti-GP2 (red) and F-actin (green) analyzed using a confocal microscope. Scale bars: 100 μm (E) M cell number/mm2 in FAE of each mouse strain. Data are means and SE. *P < 0.05.
Recent studies suggests that maturing M cells can be classified into distinct differentiation stages based on the expression of M-cell markers (Fig 3A and [10, 23]), therefore, we next examined their expression by q-PCR. The mRNA levels of SpiB, Ccl9 and Gp2 were significantly decreased in DicerΔIEC FAE compared to DicerF/F; however, expression of the earliest known M-cell marker Marcksl1 was the same in both mice (Fig 3B). We also examined the morphology of M cells by electron microscopy. Scanning electron micrographs of FAE showed a decrease in the number of cells with the typical M-cell morphology, i.e. organized microvilli on the apical surface, in DicerΔIEC FAE (Fig 4A). To understand in more detail the M-cell morphology, we also performed transmission electron microscopic (TEM) analysis, confirming the loss of organized microvilli (Fig 4B). On the other hand, morphology of microvilli in DicerΔIEC VE was similar to that in DicerF/F (Fig 4B). These results are consistent with whole mount immunostaining. Taken together, these results indicate that miRNAs in FAE are important for M-cell maturation.
Fig 3. FAE miRNAs involved in M cell maturation.
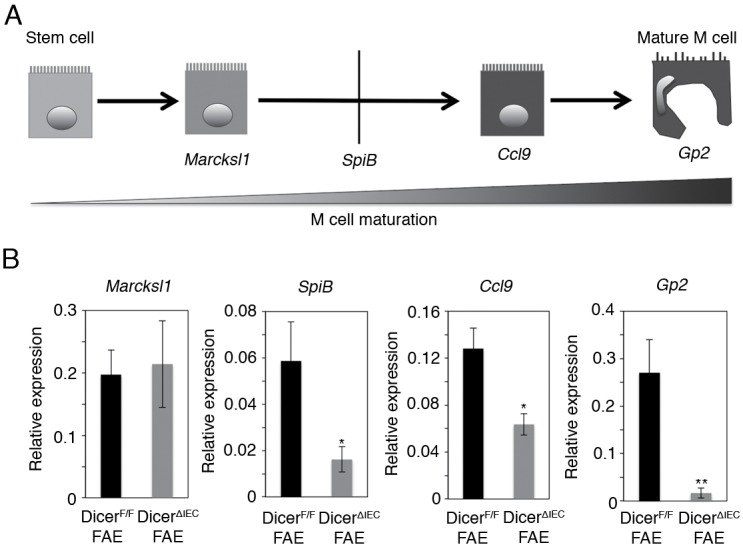
(A) Flowchart of M cell maturation. (B) Q-PCR analysis was performed for Marcksl1, SpiB, Ccl9 and Gp2 mRNA expression in DicerΔIEC FAE and DicerF/F FAE. The relative expression levels of each gene to Gapdh are shown. Values represent the mean ± SD of three samples from different mice. *P < 0.05 **P < 0.01.
Fig 4. Morphology of M cells in DicerΔIEC.
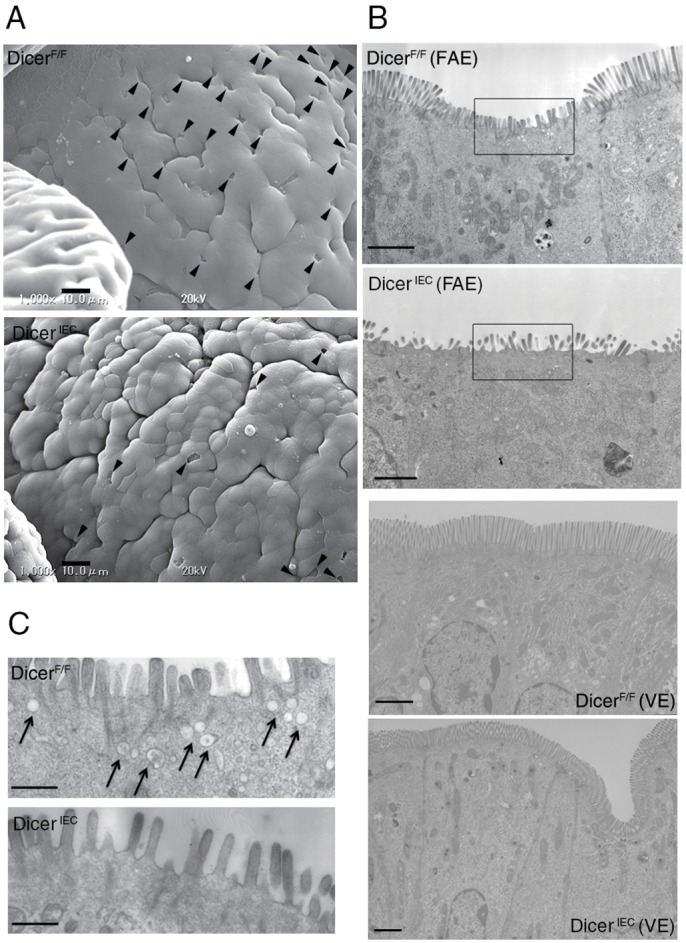
Electron micrographs of DicerF/F and DicerΔIEC PP. (A) Surface of FAE by scanning electron microscopy. Arrowheads indicate M cells. Scale bars: 10 μm (B) Transmission electron micrographs of an M cell in FAE and enterocyte in VE. Scale bars: 1.8 μm (C) High magnification image of (B). Arrow indicate the endosomes in M cell. Scale bars: 500 nm.
Impaired antigen uptake by DicerΔIEC M cells
Many endocytic/endosomal structures were observed by TEM in the DicerF/F M cells, whereas these structures were nearly absent in the DicerΔIEC M cells (Fig 4B and 4C), suggesting that endocytic activity is impaired in DicerΔIEC M cells.
To verify this point, we inoculated mice with fluorescent beads via the oral route. The beads were easily detected in the subepithelial dome region of DicerF/F PPs, whereas very few were observed in DicerΔIEC PPs (Fig 5A and 5B). We further investigated the impaired antigen uptake by M cells in the PPs of DicerΔIEC mice by examining the translocation of orally administered Yersinia enterocolitica and found that it was markedly reduced in DicerΔIEC compared to DicerF/F mice (Fig 5C). Taken together, our results indicate that FAE miRNA is important for both morphological and functional maturation of M cells.
Fig 5. Impaired antigen uptake by DicerΔIEC M cells.
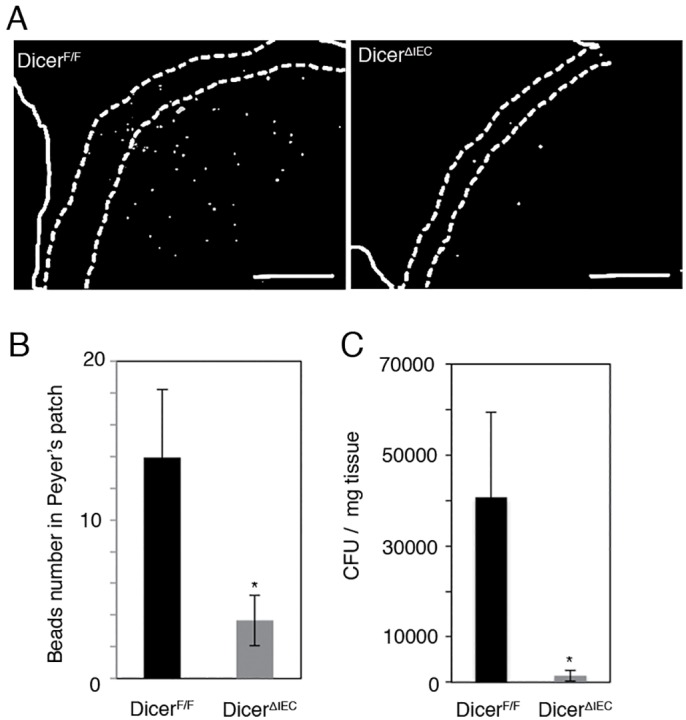
(A) DicerΔIEC and DicerF/F mice were inoculated by gavage with 1 x 1011 FluoSpheres. After 4 hours, frozen sections were prepared to examine translocated beads in PPs. Scale bars: 100 μm (B) Count data of beads taken up in PP each mouse strain. Data are expressed as the mean ± SE of four different samples for each group. **P < 0.01. (C) DicerΔIEC and DicerF/F mice were inoculated intragastrically by gavage with 1 x 108 CFU of Yersinia enterocolitica. After 24 hours, the bacterial translocation to Peyer’s patches was examined by plating PP homogenates. Data are expressed as the mean ± SE of five different mice/each group. *P < 0.05.
Discussion
To ensure efficient antigen uptake, the cellular composition and function of FAE differs significantly from VE. Besides absorptive enterocytes, the VE is populated with scattered goblet cells and enteroendocrine cells, while the FAE consists of very few goblet and enteroendocrine cells and instead contains M cells [4]. We identified five miRNAs that are two-fold or more upregulated in FAE compared to VE in C57BL/6 (Fig 1) and BALB/cA mice (data not shown), thus it seemed likely that miRNAs may affect or regulate the cell distribution or function in FAE.
To test this possibility, we generated intestinal epithelium-specific Dicer-KO (DicerΔIEC) mice. A similar strategy has been taken by others, who have reported that miRNAs are involved in goblet-cell differentiation in the intestine [20], supporting the notion that this is a reliable approach to examine the role of miRNAs in intestinal epithelial cells. The total number of PPs as well as lymphoid follicles remained unchanged, but the size of the FAE in DicerΔIEC mice was smaller than in DicerF/F mice (Fig 2C). In addition, the total number of immune cells in PPs also decreased in DicerΔIEC mice (data not shown). Chemokines specifically expressed by the FAE are thought to be important for recruitment of immune cells to PPs [8, 24]. For example, CXCL16 expressed by the FAE plays a critical role in the recruitment and retention of T cells in the subepithelial dome [24], while CCL20 derived from FAE is important for migration of CCR6hiCD11cint B cells [8]. Furthermore, CCL20 is also a crucial chemokine for M-cell differentiation and/or maintenance, since mice lacking CCR6, the sole receptor for CCL20, had a reduction in M cells [25, 26]. Of note, Ccl20 and Cxcl16 mRNA was decreased in DicerΔIEC FAE compared to DicerF/F (data not shown). Collectively, the phenotype of DicerΔIEC PPs described in this study may at least partly reflect the dysregulated expression of these chemokines by intestinal epithelial miRNAs.
DicerΔIEC mice had a prominent decrease in the number of mature M cells (Fig 2D and 2E, S3 Fig). The M-cell reduction was confirmed by electron microscopy (Fig 4A). Concomitantly, one of the main functions of M cells, mucosal antigen transcytosis, was impaired in DicerΔIEC mice PP, as measured by uptake of beads and by bacterial translocation (Fig 5A, 5B and 5C). In addition, the small number of M cells remaining in DicerΔIEC FAE lack endocytic/endosomal structures normally observed (Fig 4B and 4C). These results indicate that FAE miRNAs are important for differentiation and/or maturation of functional M cells.
In this study, we identified five miRNAs that were up-regulated at least two-fold in FAE compared with VE in both C57BL/6 and BALB/cA mice (Fig 1). Among them, two miRNAs, miR34a and miR365, might be involved in M-cell maturation. The miR34a is a member of the miR-34 family, which is conserved from C. elegans to mammals. Recent studies have shown that miR-34a regulates the notch signaling pathway [27], for example, miR-34a represses Delta-like 1 (Dll1) [28] and miR34a down-regulation leads to increased Notch1 and Jag1 expression [29]. We showed in this study that the expression of M-cell marker genes such as SpiB, Ccl9 and Gp2 was much less in DicerΔIEC mice than in DicerF/F mice (Fig 3B). The Ets family transcription factor Spi-B plays a critical role in M-cell differentiation [10]. DicerΔIEC mice showed a phenotype similar to the Spib-knockout mice [10], which raises the possibility that miRNAs regulate Spib expression. It has been reported that Dll1-induced Notch1 signaling induces down-regulation of Spi-B in plasmacytoid DCs [30]. Taken together with our results, it seems possible that miR34a also regulates Dll1, Jag1 and Notch1 in FAE and this might, in turn, promote Spi-B expression required for M-cell maturation. Because miR34a was highly expressed in FAE among miRNAs examined, we tried to generate miR34a null mice for the examination of M cells development. However, miR34a heterozygous crossing could not give rise to any miRNA34a null mice, The result may indicate that miRNA34a plays a crucial role in murine embryonic development, and precludes the possibility for us to use these mice to study the role miR34a in M-cell/FAE development.
During colonic epithelium maturation, miR365 has been reported to regulate Myb-related protein B (MYBL2) expression, which is important in both cell cycle regulation and differentiation in various biological processes [31]. Reduction of MYBL2 expression is important for acquisition of full differentiation of colonic epithelial cell lines [32]. However Mybl2 mRNA expression was similar expression levels between DicerΔIEC FAE and DicerF/F (S4 Fig). Therefore, the Mybl2 expression level controlled by miR365 may not involve in M-cell maturation. Functions of the other three miRNAs, miR149, miR193 and miR466a-3p, have not been previously examined in the intestinal epithelium in vitro or in vivo. Generation of individual or multiple miRNA null or individual miRNA conditional knockout mice will help to further understand the mechanisms of M-cell maturational regulation by these miRNAs.
In conclusion, our data suggest that intestinal epithelial miRNAs are critical for the morphological and functional maturation of M cells. The appropriate regulation of gene expression by intestinal epithelial miRNAs should contribute to the cell fate decision and/or maintenance of cellular differentiation and functions in mucosal immune homeostasis.
Supporting Information
Q-PCR analysis was performed for Dicer1 mRNA expression in FAE and VE in DicerΔIEC and DicerF/F. The relative expression levels of each gene to Gapdh are shown. Values represent the mean ± SD of three samples from different mice. *P < 0.05.
(TIF)
Stereomicroscopic images of DicerF/F PPs (A) and DicerΔIEC PPs (B) after citric acid fixation. Asterisk showed individual follicle. Solid line in (A) showed representative area of calculated follicle surface. Scale bars: 500 μm.
(TIF)
Whole mount immunostaining of isolated epithelial sheet with anti-GP2 (Green) analyzed using BX51 fluorescence microscope (Olympus). Solid line showed FAE region. Scale bars: 200 μm.
(TIF)
Q-PCR analysis was performed for Mybl2 mRNA expression in DicerΔIEC FAE and DicerF/F FAE. The relative expression levels of each gene to Gapdh are shown. Values represent the mean ± SD of three samples from different mice.
(TIF)
Acknowledgments
We would like to thank P. D. Burrows for critical review and English editing of the manuscript and Y. Yamada for secretarial assistance.
Abbreviations
- GALT
gut-associated lymphoid tissue
- PPs
Peyer’s patches
- FAE
Follicle associated epithelium
- M cells
microfold cells
- VE
villus epithelium
- DC
dendritic cell
- miRNA
micro RNA
- PBS
Phosphate buffered saline
- Dll1
delta-like 1
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was supported by the following: From The Japan Society for the Promotion of Science, Grant-in-Aid for Research Activity Start-up (G.N.) (Grant number: 22890238), Grants-in-Aid for Young Scientists (B) (G.N.) (Grant number: 24790485), Grants-in-Aid for Young Scientists (A) (K.H.) (Grant number: 22689017), Grants-in-Aid for Scientific Research (A) (H.O.) (Grant number: 24249029); From The Ministry of Education, Culture, Sports, Science and Technology of Japan, AMED-CREST, AMED, Grant-in-Aid For Scientific Research on Priority Areas (K.H. and H.O.) (Grant number: 19041072); From The Japan Agency for Medical Research and Development, Advanced Research and Development Programs for Medical Innovation (H.O.) (Grant number: 15gm0710009h0002); Sasakawa Scientific Research Grant from the Japan Science Society (G.N.) (Grant number:22-453); The Sumitomo Foundation (K.H.) (Grant number:100737); The Uehara Memorial Foundation (K.H.) (Grant number:201120109); and Takeda Science Foundation (H.O.) (http://www.takeda-sci.or.jp/).
References
- 1.Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clinical microbiology reviews. 2006;19(2):315–37. 10.1128/CMR.19.2.315-337.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annual review of physiology. 2009;71:241–60. 10.1146/annurev.physiol.010908.163145 . [DOI] [PubMed] [Google Scholar]
- 3.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nature immunology. 2001;2(11):1004–9. 10.1038/ni1101-1004 . [DOI] [PubMed] [Google Scholar]
- 4.Owen RL. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer's patches—a personal and historical perspective. Seminars in immunology. 1999;11(3):157–63. 10.1006/smim.1999.0171 . [DOI] [PubMed] [Google Scholar]
- 5.Pappo J, Owen RL. Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology. 1988;95(5):1173–7. . [DOI] [PubMed] [Google Scholar]
- 6.Giannasca PJ, Giannasca KT, Falk P, Gordon JI, Neutra MR. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. The American journal of physiology. 1994;267(6 Pt 1):G1108–21. . [DOI] [PubMed] [Google Scholar]
- 7.Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS immunology and medical microbiology. 2008;52(1):2–12. 10.1111/j.1574-695X.2007.00359.x . [DOI] [PubMed] [Google Scholar]
- 8.Ebisawa M, Hase K, Takahashi D, Kitamura H, Knoop KA, Williams IR, et al. CCR6hiCD11c(int) B cells promote M-cell differentiation in Peyer's patch. International immunology. 2011;23(4):261–9. 10.1093/intimm/dxq478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462(7270):226–30. 10.1038/nature08529 . [DOI] [PubMed] [Google Scholar]
- 10.Kanaya T, Hase K, Takahashi D, Fukuda S, Hoshino K, Sasaki I, et al. The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nature immunology. 2012;13(8):729–36. 10.1038/ni.2352 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakato G, Fukuda S, Hase K, Goitsuka R, Cooper MD, Ohno H. New approach for m-cell-specific molecules screening by comprehensive transcriptome analysis. DNA research: an international journal for rapid publication of reports on genes and genomes. 2009;16(4):227–35. 10.1093/dnares/dsp013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakato G, Hase K, Suzuki M, Kimura M, Ato M, Hanazato M, et al. Cutting Edge: Brucella abortus exploits a cellular prion protein on intestinal M cells as an invasive receptor. Journal of immunology. 2012;189(4):1540–4. 10.4049/jimmunol.1103332 . [DOI] [PubMed] [Google Scholar]
- 13.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–55. 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell stem cell. 2010;7(1):36–41. 10.1016/j.stem.2010.06.012 . [DOI] [PubMed] [Google Scholar]
- 15.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nature genetics. 2003;35(3):215–7. 10.1038/ng1253 . [DOI] [PubMed] [Google Scholar]
- 16.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2111–6. 10.1073/pnas.0710228105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, et al. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Human molecular genetics. 2010;19(20):3959–69. 10.1093/hmg/ddq311 . [DOI] [PubMed] [Google Scholar]
- 18.Sadegh MK, Ekman M, Rippe C, Uvelius B, Sward K, Albinsson S. Deletion of Dicer in smooth muscle affects voiding pattern and reduces detrusor contractility and neuroeffector transmission. PloS one. 2012;7(4):e35882 10.1371/journal.pone.0035882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liston A, Lu LF, O'Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. The Journal of experimental medicine. 2008;205(9):1993–2004. 10.1084/jem.20081062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, et al. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139(5):1654–64, 64 e1 10.1053/j.gastro.2010.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakato G, Hase K, Ohno H. Distinct microRNA expression profiles in follicle-associated epithelium and villous epithelium. Genomics data. 2015;5:388–90. 10.1016/j.gdata.2015.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hase K, Ohshima S, Kawano K, Hashimoto N, Matsumoto K, Saito H, et al. Distinct gene expression profiles characterize cellular phenotypes of follicle-associated epithelium and M cells. DNA research: an international journal for rapid publication of reports on genes and genomes. 2005;12(2):127–37. 10.1093/dnares/12.2.127 . [DOI] [PubMed] [Google Scholar]
- 23.Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. Journal of immunology. 2009;183(9):5738–47. 10.4049/jimmunol.0901563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hase K, Murakami T, Takatsu H, Shimaoka T, Iimura M, Hamura K, et al. The membrane-bound chemokine CXCL16 expressed on follicle-associated epithelium and M cells mediates lympho-epithelial interaction in GALT. Journal of immunology. 2006;176(1):43–51. . [DOI] [PubMed] [Google Scholar]
- 25.Westphal S, Lugering A, von Wedel J, von Eiff C, Maaser C, Spahn T, et al. Resistance of chemokine receptor 6-deficient mice to Yersinia enterocolitica infection: evidence of defective M-cell formation in vivo. The American journal of pathology. 2008;172(3):671–80. 10.2353/ajpath.2008.070393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lugering A, Floer M, Westphal S, Maaser C, Spahn TW, Schmidt MA, et al. Absence of CCR6 inhibits CD4+ regulatory T-cell development and M-cell formation inside Peyer's patches. The American journal of pathology. 2005;166(6):1647–54. 10.1016/S0002-9440(10)62475-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. Journal of biochemical and molecular toxicology. 2012;26(2):79–86. 10.1002/jbt.20412 . [DOI] [PubMed] [Google Scholar]
- 28.de Antonellis P, Medaglia C, Cusanelli E, Andolfo I, Liguori L, De Vita G, et al. MiR-34a targeting of Notch ligand delta-like 1 impairs CD15+/CD133+ tumor-propagating cells and supports neural differentiation in medulloblastoma. PloS one. 2011;6(9):e24584 10.1371/journal.pone.0024584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L, et al. Hypoxia-induced down-regulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells. PloS one. 2012;7(2):e30771 10.1371/journal.pone.0030771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dontje W, Schotte R, Cupedo T, Nagasawa M, Scheeren F, Gimeno R, et al. Delta-like1-induced Notch1 signaling regulates the human plasmacytoid dendritic cell versus T-cell lineage decision through control of GATA-3 and Spi-B. Blood. 2006;107(6):2446–52. 10.1182/blood-2005-05-2090 . [DOI] [PubMed] [Google Scholar]
- 31.Sala A, Watson R. B-Myb protein in cellular proliferation, transcription control, and cancer: latest developments. Journal of cellular physiology. 1999;179(3):245–50. . [DOI] [PubMed] [Google Scholar]
- 32.Papetti M, Augenlicht LH. Mybl2, downregulated during colon epithelial cell maturation, is suppressed by miR-365. American journal of physiology Gastrointestinal and liver physiology. 2011;301(3):G508–18. 10.1152/ajpgi.00066.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Q-PCR analysis was performed for Dicer1 mRNA expression in FAE and VE in DicerΔIEC and DicerF/F. The relative expression levels of each gene to Gapdh are shown. Values represent the mean ± SD of three samples from different mice. *P < 0.05.
(TIF)
Stereomicroscopic images of DicerF/F PPs (A) and DicerΔIEC PPs (B) after citric acid fixation. Asterisk showed individual follicle. Solid line in (A) showed representative area of calculated follicle surface. Scale bars: 500 μm.
(TIF)
Whole mount immunostaining of isolated epithelial sheet with anti-GP2 (Green) analyzed using BX51 fluorescence microscope (Olympus). Solid line showed FAE region. Scale bars: 200 μm.
(TIF)
Q-PCR analysis was performed for Mybl2 mRNA expression in DicerΔIEC FAE and DicerF/F FAE. The relative expression levels of each gene to Gapdh are shown. Values represent the mean ± SD of three samples from different mice.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.