Abstract
Background
The Institute of Medicine (IOM) report, “Unequal Treatment,” which defines disparities as racially based, indicates that disparities in cancer diagnosis and treatment are less clear. While a number of studies have acknowledged cancer disparities, they have limitations of retrospective nature, small sample sizes, inability to control for covariates, and measurement errors.
Objective
The purpose of this study was to examine disparities as predictors of survival among newly diagnosed head and neck cancer patients recruited from 3 hospitals in Michigan, USA, while controlling for a number of covariates (health behaviors, medical comorbidities, and treatment modality).
Methods
Longitudinal data were collected from newly diagnosed head and neck cancer patients (N = 634). The independent variables were median household income, education, race, age, sex, and marital status. The outcome variables were overall, cancer-specific, and disease-free survival censored at 5 years. Kaplan-Meier curves and univariate and multivariate Cox proportional hazards models were performed to examine demographic disparities in relation to survival.
Results
Five-year overall, cancer-specific, and disease-free survival were 65.4% (407/622), 76.4% (487/622), and 67.0% (427/622), respectively. Lower income (HR, 1.5; 95% CI, 1.1–2.0 for overall survival; HR, 1.4; 95% CI, 1.0–1.9 for cancer-specific survival), high school education or less (HR, 1.4; 95% CI, 1.1–1.9 for overall survival; HR, 1.4; 95% CI, 1.1–1.9 for cancer-specific survival), and older age in decades (HR, 1.4; 95% CI, 1.2–1.7 for overall survival; HR, 1.2; 95% CI, 1.1–1.4 for cancer-specific survival) decreased both overall and disease-free survival rates. A high school education or less (HR, 1.4; 95% CI, 1.0–2.1) and advanced age (HR, 1.3; 95% CI, 1.1–1.6) were significant independent predictors of poor cancer-specific survival.
Conclusion
Low income, low education, and advanced age predicted poor survival while controlling for a number of covariates (health behaviors, medical comorbidities, and treatment modality). Recommendations from the Institute of Medicine’s Report to reduce disparities need to be implemented in treating head and neck cancer patients.
Introduction
Cancer disparities are endemic in the United States healthcare system and in many other industrialized nations. Disparities may be characterized by socioeconomic status (SES), including income, education, ethnicity/race, age, sex, and marital status, as well as other factors such as insurance, disability, geographic location, or sexual orientation.[1–5] The US Department of Health and Human Services’ Healthy People 2020 initiative has identified the elimination of health disparities as one of its overarching goals.[6] However, the Institute of Medicine (IOM) report, “Unequal Treatment,” which defines disparities as racially based, indicates that disparities in cancer diagnosis and treatment are less clear than disparities in other diagnoses such as cardiac care.[7] Nonetheless, a number of studies have been conducted that have begun to address disparities among cancer patients.
Several studies have noted racial differences in both survival and/or recurrence among head and neck cancer patients.[3, 5, 8–19] Other studies indicate that minorities tend to present at a later cancer stage at diagnosis and less likely to have insurance, which suggests that equal access to care may eliminate racial disparities.[10, 12, 15, 20–22] Some research including head and neck cancer patients,[9, 12, 23] demonstrates that when controlling for SES and behavioral factors (e.g., smoking), racial disparities are diminished or no longer present.[9, 12, 21, 23–25]
Among cancer patients, age has been shown to predict survival in most studies, which can partially be explained by comorbidities and/or differential treatment given to older persons,[5, 13, 26–28] yet other studies have shown no age differences in survival in head and neck cancer patients.[10, 29] While women with head and neck cancer have been demonstrated to live longer than men,[5, 17–19, 30] other studies have indicated that sex was not predictive of survival or recurrence among head and neck cancer patients.[5, 8, 31–33] A relationship between marital status and survival and recurrence has also been reported among cancer patients.[17, 18, 31, 34, 35]
Limitations on the studies of disparities among head and neck cancer patients include their retrospective nature,[8, 10, 17] inability to control for covariates,[14, 19, 23, 32, 36] measurement errors (such as using postal address as a proxy for SES[5, 10] or using a county-level variables as a proxy of SES[17]), and small sample sizes.[31] Determining the nature and extent of disparities is important in identifying interventions to reduce disparities. Using data from a large longitudinal study, the specific aim of this study was to examine disparities as predictors of 5-year overall, cancer-specific, and disease-free survival among newly diagnosed head and neck cancer patients.
Materials and Methods
Study Design
This was a prospective observational longitudinal study of patients enrolled in the University of Michigan Head and Neck Cancer Specialized Program of Research Excellence (SPORE). The independent variables were income, education, ethnicity/race, age, sex, and marital status. Covariates were smoking,[5, 37] problem drinking,[5, 38] body mass index (BMI),[39, 40] depression,[4, 41] cancer site,[2, 5, 9, 12] cancer stage,[5, 17, 42] comorbidities,[4, 5] and treatment.[5, 42] The outcome variables were overall, cancer-specific, and disease-free survival censored at 5 years post-diagnosis or April 1, 2009, whichever came first. Human subjects approval was received from the Medical School Institutional Review Board (IRBMED) at the University of Michigan, the VA Ann Arbor Healthcare System, and Henry Ford Hospital. Recruitment was conducted from January 2003 to November 2008.
Study Population
Newly diagnosed patients with squamous cell carcinoma of the head and neck were recruited to participate in this study. To help ensure a diverse patient population of minorities and those of lower socioeconomic status, in addition to recruiting patients from the University of Michigan, patients were also recruited from the Veterans Affairs (VA) Ann Arbor Healthcare System, and Henry Ford Hospital in Detroit. Exclusion criteria were those: 1) less than 18 years of age; 2) pregnant; 3) non-English speaking; 4) mentally unstable; 5) with non-upper aerodigestive tract cancers (such as thyroid or skin cancer); 6) with a historical diagnosis and treatment for head and neck cancer; or 7) in stage 0 at diagnosis. Out of 1185 patients approached, 934 consented to participate, yielding a response rate of 79%. Of those consented, 796 met all eligibility requirements for this analysis. Survival curves and additional analyses included only subjects with no missing survey data, leaving a sample size of 622 (78% of eligible patients). Those with missing data were significantly more likely to be black, older age, unmarried, current smokers, and have more comorbidities, but did not have problem drinking and not receive radiation or chemotherapy (Table 1).
Table 1. Comparison between those included and those excluded in the analysis.
| Univariate models | |||
|---|---|---|---|
| Parameter | Included | Excluded | p value |
| (n = 622) | (n = 174) | ||
| Ethnicity/race | < .001 | ||
| White | 548 (79.8) | 139 (20.2) | |
| Black | 41 (60.3) | 27 (39.7) | |
| Hispanic, other (Native American) | 33 (89.2) | 4 (10.8) | |
| Age (in decades) | 58.4 (10.8) | 61.6 (12.5) | .003 |
| Marital status | .037 | ||
| Married | 374 (81.3) | 86 (18.7) | |
| Separated/Widowed/Divorced | 248 (75.2) | 82 (24.8) | |
| Smoking statusb | .009 | ||
| Current smoker | 155 (71.4) | 62 (28.6) | |
| Former smoker | 358 (81.7) | 80 (18.3 | |
| Never | 109 (80.2) | 27 (19.8) | |
| Problem drinkingc | .004 | ||
| Yes | 160 (88.9) | 20 (11.1) | |
| No | 462 (79.3) | 121 (20.7) | |
| ACE-27 comorbidity score | .008 | ||
| None | 179 (82.5) | 38 (17.5) | |
| Mild | 237 (81.4) | 54 (18.6) | |
| Moderate | 138 (75.8) | 44 (24.2) | |
| Severe | 68 (67.3) | 33 (32.7) | |
| Radiation treatment | .008 | ||
| Received | 541 (82.3) | 116 (17.7) | |
| Not Received | 81 (71.7) | 32 (28.3) | |
| Chemotherapy treatment | .001 | ||
| Received | 427 (84.2) | 80 (15.8) | |
| Not Received | 195 (74.4) | 67 (25.6) | |
Median income, education, sex, BMI, depression, cancer site, stage, surgery, and hospital site were not significantly different between those included in the analysis and those excluded in the analysis.
Procedure
Research assistants recruited patients to the study in the waiting rooms of otolaryngology clinics. Written informed consent was obtained, and patients completed written surveys on demographics and health behaviors. A medical record review was conducted for each study participant. Subjects enrolled in the study were then resurveyed every 3 months for 2 years, and yearly thereafter.
Independent Variables
Median household income for the census tract of each subject was found using American Fact Finder data for the 2000 US Census from the www.census.gov website. Low income was defined as the lowest quartile of incomes (<$35,000). Standard questions on demographics were collected from the patient surveys, including education, ethnicity/race, age, sex, and marital status. Ethnicity/race was measured using two separate questions about Hispanic/Latino origin and race. Due to sample size limitations, ethnicity/race was classified as white, black, or Hispanic/other (e.g., Native American).
Covariates
Covariates were determined based on the current literature and clinical judgement and were controlled by constructing multivariate Cox proportional hazard models. Smoking status was characterized as current, former, or never smoking tobacco products (including cigarettes, cigars, and pipe tobacco) at diagnosis. The previously validated 10-item instrument, Alcohol Use Disorders Identification Test (AUDIT),[43] was used to measure alcohol use; the scores ranged from 0 to 40 with a score of 8 or more indicating problem drinking.[44] BMI (weight in kilograms divided by the square of height in meters) was calculated based on self-reported height (without shoes) and weight. Depressive symptoms were measured using the 5-item Geriatric Depression Scale–Short Form (GDS-SF), with a score of 4 or more indicating probable depression.[45] Cancer sites were classified into four groups: a) oral cavity, b) oropharynx, c) larynx, and d) other (hypopharynx, nasopharynx, sinus, and others). Cancer stage (I–IV) and TNM classification were measured using the American Joint Committee on Cancer (AJCC) staging classification system.[46, 47] Comorbidities were measured using the Adult Comorbidity Evaluation-27 (ACE-27) and classified as none, mild, moderate, or severe.[48, 49] Type of curative treatment received (surgery, radiation, and/or chemotherapy [yes/no]) was recorded by yearly chart audit or patient self-report when treated at an outside facility.
Outcome Variable
The three main outcome variables were overall, cancer-specific, and disease-free survival. Survival was defined by the number of days from the date of initial diagnosis until the date of death from either all-cause (overall survival), cancer-related causes (cancer-specific survival), or the date of first recurrence (disease-free survival). Patients were contacted every 3 months to keep track of survival (dead or alive) and recurrence status (recurrence or recurrence-free) for the first 2 years after diagnosis and then yearly thereafter. If patients were lost to follow-up, the Social Security Administration Death Master File (DMF) was used to determine if and when they had died. Patients lost to follow-up and not found on the DMF were assumed alive. Subjects who were alive or free of recurrence at 5 years post-diagnosis were censored on April 1, 2009.
Statistical Analysis
Means and frequency distributions were examined for all variables. Associations between independent variables were conducted using chi-square for categorical variables, t-tests, and analysis of variance for continuous variables. All independent variables and covariates were treated as categorical variables except age and BMI. Kaplan-Meier plots and the log-rank test were used to compare the independent variables and survival. Univariate and multivariate Cox proportional hazards models were used to study the relationship between the independent variables, covariates, and dependent variables. Since hospital site was significantly correlated with income, education, race, and marital status, it was removed from the multivariate models. TNM classification was not included in the multivariate models because there were so few M1 (1.1%, n = 7) and TN classification were highly collinear with cancer stage; the Variance Inflation Factor (VIF) for stage increased from 3.8 to 7.0 when TN classification added to the model. The VIF values ranged from 1.0 through 3.7 indicating no significant multicollinearity among variables in the multivariate models.[50] Values for p < .05 were reported.
Results
Description of the Sample
The patient characteristics are described in Table 2 (N = 622). The median household income was $43,996. There were nearly equal numbers of patients who had attended some college or more as those with a high school education or less. The mean age was 58 years. Most of the respondents were non-Hispanic whites (n = 548, 88.1%). Just over three-quarters were male (n = 491, 78.9%), and more than half were married (n = 374, 60.1%).
Table 2. Pretreatment patient characteristics of newly diagnosed head and neck cancer patients.
(N = 622).
| Parameter | Mean (SD)/median | Range |
|---|---|---|
| Median follow-up time in days | 1,445.5 days | 19–1,826 days |
| Median household income | $43,996 | $11,232-$137,720 |
| Mean age in years | 58.4 years (10.8) | 21–92 years |
| Mean BMI | 26.8 (5.7) | 15.2–64.6 |
| No. of patients | Percentage | |
| Educational level | ||
| High school or less | 297 | 47.7 |
| Some college or more | 325 | 52.3 |
| Ethnicity/race | ||
| Non-Hispanic white | 548 | 88.1 |
| Black | 41 | 6.6 |
| Hispanic, other (Native American) | 33 | 5.3 |
| Sex | ||
| Male | 491 | 78.9 |
| Female | 131 | 21.1 |
| Marital status | ||
| Married | 374 | 60.1 |
| Not married | 248 | 39.9 |
| Smoking statusa | ||
| Current smoking | 155 | 24.9 |
| Former smoking | 358 | 57.6 |
| Never smoking | 109 | 17.5 |
| Problem drinkingb | 160 | 25.7 |
| Depressionc | 323 | 51.9 |
| Cancer site | ||
| Oral cavity | 136 | 21.9 |
| Oropharynx | 249 | 40.0 |
| Larynx | 140 | 22.5 |
| Hypopharynx | 34 | 5.5 |
| Nasopharynx | 8 | 1.3 |
| Sinus | 44 | 7.1 |
| Other | 5511 | 1.8 |
| Cancer stage | ||
| I | 65 | 10.5 |
| II | 60 | 9.7 |
| III | 93 | 15.0 |
| IV | 404 | 65.0 |
| TNM Classification | ||
| T Stage | ||
| TX | 46 | 7.4 |
| T1 | 121 | 19.5 |
| T2 | 158 | 25.4 |
| T3 | 111 | 17.8 |
| T4 | 186 | 29.9 |
| N Stage | ||
| NX | 1 | 0.2 |
| N0 | 213 | 34.2 |
| N1 | 82 | 13.2 |
| N2 | 269 | 43.3 |
| N3 | 57 | 9.2 |
| M Stage | ||
| MX | 29 | 4.7 |
| M0 | 586 | 94.2 |
| M1 | 7 | 1.1 |
| ACE-27 comorbidity | ||
| None | 179 | 28.8 |
| Mild | 237 | 38.1 |
| Moderate | 138 | 22.2 |
| Severe | 68 | 10.9 |
| Treatment | ||
| Radiation + Chemotherapy + Surgery | 167 | 26.9 |
| Radiation + Chemotherapy | 253 | 40.7 |
| Radiation + Surgery | 72 | 11.6 |
| Chemotherapy + Surgery | 2 | 0.3 |
| Radiation Only | 49 | 7.9 |
| Chemotherapy Only | 5 | 0.8 |
| Surgery Only | 68 | 10.9 |
| No Treatment | 6 | 1.0 |
| Hospital site | ||
| University of Michigan | 479 | 77.0 |
| Veterans Affairs Ann Arbor | 63 | 10.1 |
| Henry Ford Hospital | 80 | 12.9 |
a Includes cigarettes, cigars, and pipe tobacco.
b Alcohol Use Disorders Identification Test (AUDIT) ≥8.
c Geriatric Depressive Scale Short Form ≥4.
The majority of patients were current (n = 155, 24.9%) or former (n = 358, 57.5%) smokers. About one-quarter screened positive for problem drinking (n = 160, 25.7%), and half screened positive for significant depressive symptoms (n = 323, 51.9%). The mean BMI was 26.8 kg/m2 with more than half of the patients being either overweight (n = 233, 37.5%) or obese (n = 141, 22.7%). More than one-third of the sample had oropharyngeal cancer (n = 249, 40.0%), followed by laryngeal cancer (n = 140, 22.5%) and oral cavity cancer (n = 136, 21.9%). Almost two-thirds of the patients presented with stage IV disease (n = 404, 65.0%) and had none (n = 179, 28.8%) to mild (n = 237, 38.1%) comorbidities at diagnosis. The majority of patients received radiation (87.0%), chemotherapy (68.7%), and surgery (49.7%) and received a combination of treatments (e.g., radiation and chemotherapy, radiation and chemotherapy and surgery). The 5-year overall survival rate was 65.4% (n = 407/622), cancer-specific survival rate was 76.4% (n = 475/622), and disease-free survival rate was 67.0% (n = 417/622).
Associations Among Independent Variables
While low income and education were not associated with each other (p = .309), both were associated with black race (p < .0001 and p = .002, respectively), older age (p = .038 and p = .003, respectively), being unmarried (p = .002 and p = .0001, respectively), current smoking (p = .039 and p = .010, respectively), and cancer site (p = .010 and p < .0001, respectively), with higher education and higher income being more likely to have cancers of the oropharynx. In addition, lower educational levels were associated with being female (p = .024), problem drinking (p = .002), and higher levels of comorbidities (p = .001). Blacks were more likely to be unmarried (p = .018), have low BMI (p = .008), and not be receiving chemotherapy (p = .002).
Older patients tended not to have problem drinking (p = .0003), but have low BMI (p = .015), less depression (p = .004), and more comorbidities (p < .0001). While older persons had more cancers of the larynx, younger persons had more cancers of the oral cavity and oropharynx (p = .0003). Younger persons were more likely to receive surgery (p = .008) and chemotherapy (p = .0008). Female patients tended to be unmarried (p = .0002) and have, cancers of the oral cavity (p < .0001), and surgery treatment (p = .051) but not to have either problem drinking (p = .0002) or radiation (p = .009) or chemotherapy (p = .003).
Univariate and Multivariate Survival Analyses
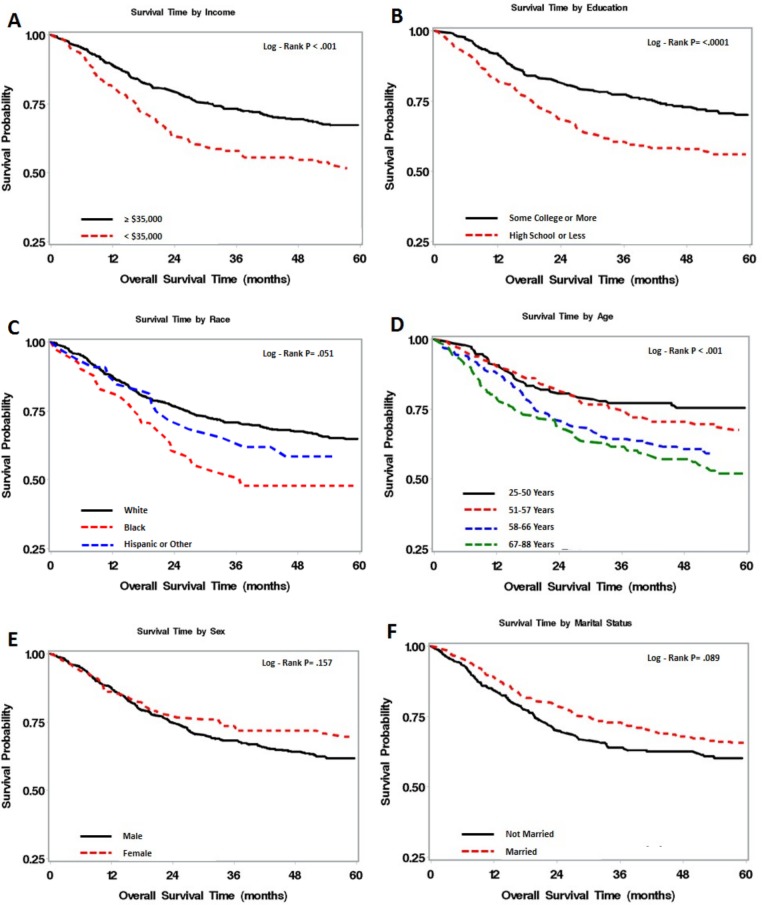
Fig 1 shows the Kaplan-Meier survival curves for the independent variables of income, educational level, ethnicity/race, age, sex, and marital status. Those in the lowest income quartile had the worst survival compared to all others (p < .001). Patients with a high school education or less had poorer survival compared to those with some college or more education (p < .0001). Blacks had worse survival compared to either white or Hispanic/other race groups (p = .051). The Kaplan-Meier curve revealed a significant association with survival for age, with those in the oldest age quartile having the worst survival (p < .001). Survival rates were similar for males and females for the first 2 years, but then diverged with a trend for men having worse survival than women (p = .157). Those who were not married trended toward poorer survival compared to those who were, albeit not significant (p = .089).
Fig 1. Kaplan-Meier survival curves for the independent variables.
Table 3 shows the univariate and multivariate hazard ratios of overall survival for each of the independent and covariates. Univariate analysis revealed that income, education, ethnicity/race, age, smoking status, problem drinking, BMI, cancer sites, cancer stage, and comorbidities were all significantly associated with overall survival. Not being married trended toward poorer survival in the univariate models, but along with sex, depressive symptoms, and treatment (surgery, radiation, and chemotherapy),was not significantly associated.
Table 3. Univariate and multivariate Cox proportional hazards model for 5-year overall survival.
(N = 634 [216 events, 418 censored]).
| Univariate models | Multivariate models | |||||
|---|---|---|---|---|---|---|
| Parameter | Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value |
| Low incomea | 1.70 | 1.28–2.26 | < .001* | 1. 48 | 1.09–2.01 | .013* |
| High school education or less | 1.72 | 1.31–2.26 | < .001* | 1. 41 | 1.05–1.88 | .022* |
| Ethnicity/Race (vs white) | ||||||
| Black | 1.77 | 1.14–2.76 | .011* | 1. 33 | 0.81–2.17 | .262 |
| Hispanic, other (Native American) | 1.01 | 0.47–2.14 | .985 | 0. 92 | 0.42–2.03 | .836 |
| Age (in decades) | 1.37 | 1.21–1.55 | < .001* | 1. 43 | 1.23–1.67 | < .001* |
| Female sex | 0.78 | 0.55–1.10 | .159 | 0. 74 | 0.51–1.09 | .132 |
| Married | 0.79 | 0.61–1.04 | .090 | 1.05 | 0.79–1.41 | .734 |
| Smoking statusb (vs never) | ||||||
| Current smoker | 3.52 | 2.10–5.90 | < .001* | 2.60 | 1.47–4.61 | .001* |
| Former smoker | 2.39 | 1.46–3.92 | < .001* | 1.94 | 1.15–3.26 | .013* |
| Problem drinkingc | 1.51 | 1.14–2.02 | .005* | 1.15 | 0.82–1.60 | .412 |
| Body mass index | 0.95 | 0.93–0.98 | < .001* | 0.97 | 0.95–1.00 | .053 |
| Depressiond | 1.26 | 0.97–1.66 | .088 | 1.14 | 0.86–1.51 | .356 |
| Cancer site (vs Other) | ||||||
| Oral Cavity | 0.93 | 0.62–1.41 | .738 | 1.39 | 0.86–2.24 | .179 |
| Oropharynx | 0.66 | 0.45–0.98 | .037* | 0.84 | 0.56–1.26 | .402 |
| Larynx | 0.79 | 0.52–1.20 | .264 | 0.85 | 0.54–1.34 | .481 |
| Stage (vs Stage I) | ||||||
| Stage II | 2.48 | 1.17–5.26 | .018* | 3.86 | 1.73–8.61 | .001* |
| Stage III | 2.42 | 1.19–4.94 | .015* | 3.98 | 1.82–8.71 | .001* |
| Stage IV | 2.90 | 1.53–5.49 | .001* | 4.54 | 2.20–9.39 | < .001* |
| ACE-27 comorbidity (vs None) | ||||||
| Mild | 1.35 | 0.93–1.97 | .113 | 1.14 | 0.77–1.68 | .524 |
| Moderate | 2.11 | 1.43–3.11 | < .001* | 1.59 | 1.04–2.44 | .031* |
| Severe | 3.09 | 1.98–4.82 | < .001* | 2.09 | 1.30–3.36 | .002* |
| Surgery | 0.82 | 0.62–1.07 | .135 | 0.79 | 0.58–1.09 | .158 |
| Radiation treatment | 1.25 | 0.80–1.94 | .322 | 0.74 | 0.43–1.28 | .282 |
| Chemotherapy treatment | 1.33 | 1.98–1.81 | .064 | 1.33 | 0.90–1.98 | .152 |
a Lowest quartile of income <$35,000.
b Includes cigarettes, cigars, and pipe tobacco.
c Alcohol Use Disorders Identification Test (AUDIT) ≥8.
d Geriatric Depressive Scale Short Form ≥4.
*p < .05.
Multivariate analysis revealed that lowest quartile income (hazard ratio [HR], 1.5; 95% confidence interval [CI], 1.1–2.0), high school education or less (HR, 1.4; 95% CI, 1.1–1.9), and age in decades (HR, 1.4; 95% CI, 1.2–1.7) remained significant independent predictors of overall survival among head and neck cancer patients, while ethnicity/race, female sex, and marital status were not significant. Among covariates, smoking status, cancer stage, and comorbidities were significant, while problem drinking, BMI, cancer sites and treatment were no longer significant in the multivariate analysis.
Similar to the findings from overall survival, lower income, lower educational levels, black race, advanced age, being unmarried, current/former smoking, problem drinking, lower BMI, advanced stage, more severe comorbidity, and having chemotherapy were associated with worse cancer-specific survival in the univariate models (Table 4). In the multivariate model, a high school education or less (HR, 1.4; 95% CI, 1.0–2.1) and advanced age (HR, 1.3; 95% CI, 1.1–1.6) decreased cancer-specific survival rates (Table 4). Current/former smoking, lower levels of BMI, and advanced stage were significant covariates that predicted worse cancer-specific mortality, while comorbidity and chemotherapy were no longer significant.
Table 4. Univariate and multivariate Cox proportional hazards model for 5-year cancer-specific survival (N = 634 [147 events, 487 censored]).
| Univariate models | Multivariate models | |||||
|---|---|---|---|---|---|---|
| Parameter | Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value |
| Low incomea | 1.55 | 1.09–2.20 | .014* | 1.37 | 0.94–2.00 | .103 |
| High school education or less | 1.69 | 1.22–2.35 | .002* | 1.44 | 1.01–2.06 | .042* |
| Ethnicity/race (vs white) | ||||||
| Black | 1.76 | 1.03–3.00 | .039* | 1.26 | 0.75–2.46 | .317 |
| Hispanic, other (Native American) | 1.05 | 0.43–2.58 | .908 | 0.97 | 0.38–2.49 | .954 |
| Age (in decades) | 1.25 | 1.07–1.45 | .004* | 1.33 | 1.11–1.60 | .002* |
| Female sex | 0.78 | 0.51–1.20 | .261 | 0.72 | 0.46–1.14 | .167 |
| Married | 0.72 | 0.52–0.99 | .045* | 0.92 | 0.65–1.31 | .657 |
| Smoking statusb (vs never) | ||||||
| Current smoker | 3.34 | 1.77–6.30 | < .001* | 2.37 | 1.18–4.75 | .015* |
| Former smoker | 2.52 | 1.38–4.60 | .003* | 2.12 | 1.13–3.99 | .019* |
| Problem drinkingc | 1.45 | 1.02–2.05 | .038* | 1.00 | 0.67–1.50 | .999 |
| BMI | 0.94 | 0.91–0.97 | < .001* | 0.96 | 0.93–0.99 | .021* |
| Depressiond | 1.37 | 0.99–1.90 | .060 | 1.21 | 0.87–1.70 | .263 |
| Cancer site (vs Other) | ||||||
| Oral Cavity | 1.03 | 0.62–1.71 | .904 | 0.77 | 0.99–3.17 | .056 |
| Oropharynx | 0.75 | 0.47–2.21 | .236 | 0.93 | 0.57–1.53 | .772 |
| Larynx | 0.78 | 0.46–1.33 | .366 | 0.98 | 0.56–1.71 | .935 |
| Stage (vs Stage I) | ||||||
| Stage II | 3.55 | 1.14–11.00 | .028* | 5.39 | 1.66–17.54 | .005* |
| Stage III | 3.72 | 1.27–10.93 | .017* | 5.91 | 1.88–18.57 | .002* |
| Stage IV | 5.28 | 1.95–14.32 | .001* | 7.69 | 2.61–22.62 | < .001* |
| ACE-27 comorbidity (vs None) | ||||||
| Mild | 1.06 | 0.70–1.61 | .789 | 0.92 | 0.59–1.43 | .710 |
| Moderate | 1.40 | 0.89–2.21 | .150 | 1.12 | 0.68–1.85 | .654 |
| Severe | 1.99 | 1.17–3.39 | .012* | 1.36 | 0.77–2.40 | .296 |
| Surgery | 0.77 | 0.56–1.07 | .120 | 0.71 | 0.48–1.05 | .089 |
| Radiation treatment | 1.34 | 0.77–2.32 | .302 | 0.62 | 0.32–1.21 | .158 |
| Chemotherapy treatment | 1.61 | 1.09–2.37 | .017* | 1.55 | 0.94–2.55 | .086 |
a Lowest quartile of income <$35,000.
b Includes cigarettes, cigars, and pipe tobacco.
c Alcohol Use Disorders Identification Test (AUDIT) ≥8.
d Geriatric Depressive Scale Short Form ≥4.
* p < .05.
With regard to disease-free survival, low income, low educational levels, and increased age were significantly associated with decreased disease-free survival in univariate models (Table 5). Ethnicity/race, sex, and marital status were not significant in relation to disease-free survival within 5 years post-diagnosis. In multivariate models, low income (HR, 1.4; 95% CI, 1.0–1.9), high school education or less (HR, 1.4; 95% CI, 1.1–1.9), and advanced age (HR, 1.2; 95% CI, 1.1–1.4) remained significant. Among covariates, former smoking at diagnosis, lower BMI, oral cavity cancer, advanced stage, and chemotherapy independently predicted lower disease-free survival.
Table 5. Univariate and multivariate Cox proportional hazards model for 5-year disease-free survival (N = 634 [207 events, 427 censored]).
| Univariate models | Multivariate models | |||||
|---|---|---|---|---|---|---|
| Parameter | Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value |
| Low incomea | 1.48 | 1.10–2.00 | .009* | 1.40 | 1.01–1.92 | .042* |
| High school education or less | 1.59 | 1.21–2.10 | .001* | 1.43 | 1.06–1.93 | .018* |
| Ethnicity/race (vs white) | ||||||
| Black | 1.40 | 0.85–2.30 | .186 | 1.10 | 0.64–1.90 | .732 |
| Hispanic, other (Native American) | 1.16 | 0.54–2.46 | .707 | 1.20 | 0.54–2.64 | .654 |
| Age (in decades) | 1.20 | 1.06–1.36 | .005* | 1.23 | 1.06–1.44 | .006* |
| Female sex | 0.92 | 0.65–1.30 | .639 | 0.93 | 0.64–1.34 | .689 |
| Married | 0.85 | 0.65–1.13 | .266 | 1.08 | 0.80–1.46 | .601 |
| Smoking statusb (vs never) | ||||||
| Current smoker | 2.46 | 1.46–4.12 | .001* | 1.69 | 0.96–3.00 | .071 |
| Former smoker | 2.28 | 1.41–3.68 | .001* | 1.94 | 1.17–3.20 | .010* |
| Problem drinkingc | 1.38 | 1.02–1.85 | .035* | 0.99 | 0.70–1.39 | .956 |
| BMI | 0.94 | 0.91–0.97 | < .001* | 0.95 | 0.92–0.98 | < .001* |
| Depressiond | 1.09 | 0.83–1.43 | .545 | 1.02 | 0.77–1.36 | .879 |
| Cancer site (vs Other) | ||||||
| Oral Cavity | 1.19 | 0.77–1.84 | .441 | 1.70 | 1.03–2.81 | .038* |
| Oropharynx | 0.85 | 0.56–1.29 | .451 | 0.97 | 0.63–1.50 | .893 |
| Larynx | 0.90 | 0.57–1.41 | .638 | 1.01 | 0.62–1.64 | .959 |
| Stage (vs Stage I) | ||||||
| Stage II | 1.72 | 0.85–3.47 | .134 | 1.89 | 0.89–4.03 | .099 |
| Stage III | 1.68 | 0.87–3.24 | .123 | 1.80 | 0.88–3.70 | .109 |
| Stage IV | 2.08 | 1.18–3.68 | .011* | 2.01 | 1.04–3.87 | .038* |
| ACE-27 comorbidity (vs None) | ||||||
| Mild | 1.01 | 0.71–1.43 | .949 | 0.91 | 0.63–1.31 | .598 |
| Moderate | 0.30 | 0.83–1.80 | .302 | 1.05 | 0.69–1.60 | .808 |
| Severe | 1.47 | 0.93–2.33 | .103 | 1.15 | 0.70–1.89 | .581 |
| Surgery | 1.04 | 0.79–1.37 | .766 | 1.07 | 0.77–1.49 | .704 |
| Radiation treatment | 1.48 | 0.92–2.37 | .106 | 0.93 | 0.51–1.70 | .821 |
| Chemotherapy treatment | 1.68 | 1.21–2.33 | .002* | 2.15 | 1.40–3.32 | < .001* |
a Lowest quartile of income <$35,000.
b Includes cigarettes, cigars, and pipe tobacco.
c Alcohol Use Disorders Identification Test (AUDIT) ≥8.
d Geriatric Depressive Scale Short Form ≥4.
*p < .05.
Discussion
Controlling for other socioeconomic variables, education consistently predicted all survival outcomes (overall, cancer-specific, and disease-free survival) among head and neck cancer patients. Those with a high school education or less had a 41% higher hazard of dying from all causes, 44% higher hazard of dying from cancer-specific causes, and a 43% higher hazard of recurrence than the higher education reference group. This finding is consistent with the literature, which has found an inverse relationship between educational level and cancer survival.[51]
The reason for the association between education and outcomes is not clear. While it has been suggested that education may be a marker for poor health behaviors, such as smoking and drinking,[51] that was not the case in these analyses, since smoking, problem drinking, and nutritional status (BMI) were covariates. Education may also be related to income, which may influence treatment options; however, this was also included as a covariate. It may be because those with less education lack knowledge about the disease progress and early manifestations of cancer recurrence, which can lead to non-adherence to follow-up visits and cause higher recurrence rates among this population.
Median household income was another strong predictor of overall and disease-free survival in this study. Lower income status showed a 48% higher hazard of dying from all-cause and 40% higher hazard of recurrence than the higher income reference group. Previous studies[21, 52] proposed that poverty decreases survival through inadequate access to care and even though most of the patients in this study were insured and there was no discrepancy in stage at diagnosis among those who were of lower income, access to care is affected by other factors including resources and desire to receive follow up care. Unfortunately, we did not have information on compliance with follow-up appointments and related factors such as availability of tangible support (e.g., transportation) and desire to receive follow up visits.
Another explanation for the relationship between income and survival is that the stress associated with poverty (e.g., food insecurity) and undernutrition may suppress the immune system and induce inflammatory markers associated with survival.[33, 53, 54] In fact, Fig 1 shows that it is those with the lowest income (earning less than $35,000 per year) who are at greatest risk for poor survival. However, since we used census tract data as a proxy for individual income level, there may be residual confounding factors not properly controlled for in the analysis.
This population is particularly interesting to study in terms of disparities because, unlike other studies where income and education are highly correlated, thereby causing multicollinearity, there was not a strong association between the two in this study. One explanation is that many VA patients are lower income but have some college education from the GI Bill, and many blacks from Henry Ford Hospital in the Detroit area have less education but a fair income and health insurance related to auto-industry jobs. Hence, low education (controlling for income) and low income (controlling for education) were independent predictors of poor survival.
Both low income and low education were associated with black race. Black race was significantly associated with both decreased overall survival and cancer-specific survival in the univariate analysis, but was not significant in multivariate analysis, which controlled for other SES-related and covariates. Consistent with other studies,[12, 55] who found when blacks receive similar cancer treatment and medical care as whites, they tend to have similar disease outcomes.
Blacks did not present at a later cancer stage and were equally likely to receive radiation and surgery, but less likely to receive chemotherapy. This may be because of concerning comorbid conditions, functional status, patient biases, and institutional treatment differences at the time of the study and/or other variables that were not measured. Nonetheless, blacks did not have decreased survival as a result of receiving less chemotherapy.
Both education and income levels were significantly associated with cancer site, with higher education and higher income groups more likely to have cancers of the oropharynx. Since higher SES patients were less likely to smoke, they may have more non-smoking–related human papilloma virus (HPV)-positive cancers, which are more common in the oropharynx and have a more favorable prognosis.[9, 15, 56] Unfortunately, information about HPV was not available, so we were unable to control for HPV status in the analyses.
The IOM report, “Unequal Treatment,”[57] contains several recommendations on how to reduce disparities, which are related to the study findings, yet the IOM report focused primarily on race/SES disparities and did not address age, sex, and marital status. In this study, older patients were less likely to receive surgery and chemotherapy; it is unclear whether this is due to provider bias, patient preferences, or decisions made jointly based on comorbidities, which are higher in older persons. Older persons were also more likely to come from the VA Ann Arbor Healthcare System and Henry Ford Hospital, where chemotherapy was provided less frequently.
The survival curve for sex (Fig 1) is interesting in that the survival rate for both males and females was about the same for the first 2 years after diagnosis, but thereafter, females began to mimic the survival advantage shown in both general and head and neck cancer patient population trends.[17, 18, 58] While not significant, this survival advantage for females persisted even though females had less education, a lower likelihood of being married, and more depressive symptoms. However, females did have higher rates of surgery and lower rates of chemotherapy and radiation (all common treatment trends among those with a more favorable prognosis). It may also be related to the finding that women are more likely to have frequent clinic visits than men,[59] which may lead to early detection of the tumor.
Studies have shown that unmarried cancer patients are diagnosed at a later stage, are more likely to be untreated, and have a higher risk of dying.[35, 60] However, in this study, there was no relationship between marital status and cancer stage or treatment. Marriage significantly predicted better cancer-specific survival in univariate analysis, yet it was no longer significant in multivariate analysis. It is possible that low social support (not being married) or cultural attitudes (e.g., mistrust physicians) may have contributed to less frequent follow-up visits and suboptimal detection of recurrence, thus resulting in poorer survival. Special outreach may be required for those lacking support, such as those who are unmarried, widowed, or separated, to obtain needed care.
In terms of covariates, it was not surprising that about one-quarter of the patients smoked at diagnosis, and smoking was associated with all three survival outcomes, which has been documented in other studies.[29, 61, 62] While problem drinking has been shown to decrease survival in other studies of head and neck cancer patients[38, 62] and was a significant predictor of overall survival in univariate models, problem drinking was not significant in multivariate models. As demonstrated in other studies,[39, 63] higher BMI independently predicted better cancer-specific and disease-free survival. This finding supports the evidence that those with higher BMI better sustain the adverse effects of cancer treatments, thus leading to better survival outcomes.[39] Moreover, higher BMI may reflect better nutritional status, which may influence treatment options. Consistent with other studies,[19, 41, 64–66] those with oral cancer, advanced cancer stage, and more severe comorbidities had poorer survival. Chemotherapy was associated with poor survival as patients with more progressed stages generally receive chemotherapy. While treatment modality was evaluated in relation to survival, more detailed data on treatment modality (e.g., types and dosages of therapeutic regimens, treatment intervals) were not available to analyze. While a power analysis was not conducted a priori for this secondary data analysis, the odds ratios in the univariate results are very close to the ones in the multivariate results, which indicate that the adjustment effects of covariates are not significant. Moreover, the width of the confidence intervals in multivariate analyses was narrow enough to provide enough information to be confident about the finding, particularly those with narrow intervals that are far from 1.0.
Conclusion
This longitudinal study of head and neck cancer patients, which controlled for a large number of confounders, showed that low income, low education, and advanced age predicted poor survival. This was true even though there was fairly equal access to care. Disparities in health habits, clinical characteristics, comorbidities, and treatment were also found among selected SES, age, sex, and marital status groups. Implementation of the recommendations of IOM report, “Unequal Treatment,” may reduce disparities among head and neck cancer patients.
Acknowledgments
This study was supported by the US National Institutes of Health through the University of Michigan’s Head and Neck Cancer SPORE (grant #P50 CA97248). The authors wish to thank Suzan McCormick, Chelsea Hughes, Allison Dubois, Elizabeth Knight, and the staffs of the University of Michigan Hospital, VA Ann Arbor Healthcare System, and Henry Ford Hospital Otolaryngology clinics for their assistance with recruitment and data collection.
Data Availability
The VA data is very sensitive and would require a number of approvals before it could be released. Similar approvals would also apply at the University of Michigan. There is a large amount of HIPPA data that would need to be de-identified. If someone wants the data, they would have to contact Sonia Duffy for Veterans Affairs data and Carol Bradford for University of Michigan data, and procedures (e.g., data sharing agreements, sign off from privacy officers, de-identification, etc.) would then need to be followed to obtain the data.
Funding Statement
This study was supported by the US National Institutes of Health through the University of Michigan's Head and Neck Cancer SPORE (grant #P50 CA97248). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Field TS, Buist DSM, Doubeni C, Enger S, Fouayzi H, Hart G, et al. Disparities and survival among breast cancer patients. Journal of the National Cancer InstituteMonographs. 2005;2005(35):88–95. 10.1093/jncimonographs/lgi044 [DOI] [PubMed] [Google Scholar]
- 2.Albain KS, Unger JM, Crowley JJ, Coltman JCA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. Journal of the National Cancer Institute. 2009;101(14):984–92. 10.1093/jnci/djp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang S, Melvin T- A, Calzada G, Friduss M, Johnson C. PS2-34: Disparities in Head and Neck Cancer Patient Survival Relative to Race and Gender. Clinical Medicine & Research. 2014;12(1–2):79–80. 10.3121/cmr.2014.1250.ps2-34 [DOI] [Google Scholar]
- 4.Chang T-S, Hou S-J, Su Y-C, Chen L-F, Ho H-C, Lee M-S, et al. Disparities in oral cancer survival among mentally ill patients. PloS one. 2013;8(8):e70883 10.1371/journal.pone.0070883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina MA, Cheung MC, Perez EA, Byrne MM, Franceschi D, Moffat FL, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113(10):2797–806. Epub 2008/10/08. 10.1002/cncr.23889 . [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services. Healthy People 2020 Washington, DC. USA: Office of Disease Prevention and Health Promotion; [cited 2015 12/4]. Available from: http://www.healthypeople.gov/.
- 7.Betancourt JR, Maina AW. The Institute of Medicine report "Unequal Treatment": implications for academic health centers. Mt Sinai J Med. 2004;71(5):314–21. . [PubMed] [Google Scholar]
- 8.Subramanian S, Chen A. Treatment patterns and survival among low-income Medicaid patients with head and neck cancer. JAMA Otolaryngology–Head & Neck Surgery. 2013;139(5):489–95. [DOI] [PubMed] [Google Scholar]
- 9.Jiron J, Sethi S, Ali-Fehmi R, Franceschi S, Struijk L, van Doorn L-J, et al. Racial disparities in Human Papillomavirus (HPV) associated head and neck cancer. American Journal of Otolaryngology. 2014;35(2):147–53. 10.1016/j.amjoto.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. The Laryngoscope. 2006;116(7):1093–106. 10.1097/01.mlg.0000224939.61503.83 [DOI] [PubMed] [Google Scholar]
- 11.Moore CE. Head and Neck Cancer Disparity in Underserved Communities: Probable Causes and the Ethics Involved. Journal of health care for the poor and underserved. 2012;23(4). [DOI] [PubMed] [Google Scholar]
- 12.Ragin CC, Langevin SM, Marzouk M, Grandis J, Taioli E. Determinants of head and neck cancer survival by race. Head & neck. 2011;33(8):1092–8. 10.1002/hed.21584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfisterer MJ, Vazquez A, Mady LJ, Khan MN, Baredes S, Eloy JA. Squamous cell carcinoma of the parotid gland: A population-based analysis of 2545 cases. American Journal of Otolaryngology. 2014;35(4):469–75. 10.1016/j.amjoto.2014.03.003. 10.1016/j.amjoto.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhang Y, Ma S. Racial Differences in Nasopharyngeal Carcinoma in the United States. Cancer epidemiology. 2013;37(6):10.1016/j.canep.2013.08.008. 10.1016/j.canep.2013.08.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrank TP, Han Y, Weiss H, Resto VA. Case-matching analysis of head and neck squamous cell carcinoma in racial and ethnic minorities in the United States—Possible role for human papillomavirus in survival disparities. Head & neck. 2011;33(1):45–53. 10.1002/hed.21398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullen K. Abstract IA02: Racial and gender-based survival disparities in head and neck cancer. Cancer Epidemiology Biomarkers & Prevention. 2015;24(10 Supplement):IA02–IA. [Google Scholar]
- 17.Osazuwa-Peters N, Massa S, Christopher K, Walker R, Varvares M. Race and sex disparities in long-term survival of oral and oropharyngeal cancer in the United States. J Cancer Res Clin Oncol. 2015:1–8. 10.1007/s00432-015-2061-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dilling TJ, Bae K, Paulus R, Watkins-Bruner D, Garden AS, Forastiere A, et al. Impact of gender, partner status, and race on locoregional failure and overall survival in head and neck cancer patients in three radiation therapy oncology group trials. International Journal of Radiation Oncology* Biology* Physics. 2011;81(3):e101–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morse DE, Kerr AR. Disparities in oral and pharyngeal cancer incidence, mortality and survival among black and white Americans. The Journal of the American Dental Association. 2006;137(2):203–12. 10.14219/jada.archive.2006.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrogi AJ. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiology, Biomarkers & Prevention. 2006;15(1):25–31. [DOI] [PubMed] [Google Scholar]
- 21.Mehta R, Gillan AS, Ming ZY, Rai BP, Byrne D, Nabi G, et al. Socio-economic deprivation and outcomes following radical nephroureterectomy for clinically localized upper tract transitional cell carcinoma. World Journal of Urology. 2015;33(1):41–9. 10.1007/s00345-014-1262-0 [DOI] [PubMed] [Google Scholar]
- 22.Richardson MA, Tarsi EJ, Replogle WH, Jefferson GD. Socioeconomic Disparities in Mississippi Head and Neck Cancer Patients. Otolaryngology—Head and Neck Surgery. 2013;149(2 suppl):P195 10.1177/0194599813496044a161 [DOI] [Google Scholar]
- 23.Du XL, Liu C-C. Racial/Ethnic Disparities in Socioeconomic Status, Diagnosis, Treatment and Survival among Medicare-insured Men and Women with Head and Neck Cancer. Journal of health care for the poor and underserved. 2010;21(3):913–30. 10.1353/hpu.0.0331 [DOI] [PubMed] [Google Scholar]
- 24.Movva S, Noone AM, Banerjee M, Patel DA, Schwartz K, Yee CL, et al. Racial differences in cervical cancer survival in the Detroit metropolitan area. Cancer. 2008;112(6):1264–71. Epub 2008/02/08. 10.1002/cncr.23310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(8):1950–62. Epub 2008/08/19. 17/8/1950 [pii] 10.1158/1055-9965.EPI-07-2774 . [DOI] [PubMed] [Google Scholar]
- 26.Brenner H, Arndt V. Recent increase in cancer survival according to age: higher survival in all age groups, but widening age gradient. Cancer causes & control: CCC. 2004;15(9):903–10. [DOI] [PubMed] [Google Scholar]
- 27.Goodman DM. Treatment Patterns and Survival Among Low-Income Medicaid Patients With Head and Neck Cancer. JAMA: the journal of the American Medical Association. 2013;310(3):328. [Google Scholar]
- 28.Camilon PR, Stokes WA, Nguyen SA, Lentsch EJ. The prognostic significance of age in oropharyngeal squamous cell carcinoma. Oral Oncology. 2014;50(5):431–6. 10.1016/j.oraloncology.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 29.Pytynia KB, Grant JR, Etzel CJ, Roberts D, Wei Q, Sturgis EM. Matched analysis of survival in patients with squamous cell carcinoma of the head and neck diagnosed before and after 40 years of age. Arch Otolaryngol Head Neck Surg. 2004;130(7):869–73. [DOI] [PubMed] [Google Scholar]
- 30.de Cassia Braga Ribeiro K, Kowalski LP, Latorre Mdo R. Perioperative complications, comorbidities, and survival in oral or oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2003;129(2):219–28. [DOI] [PubMed] [Google Scholar]
- 31.de Graeff A, de Leeuw JR, Hordijk GJ, Blijham GH, Winnubst JA. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. European journal of cancer. 2001;37(3):332–9. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JC, Li G, Reitzel LR, Wei Q, Sturgis EM. No Evidence of Sex-Related Survival Disparities among Head and Neck Cancer Patients Receiving Similar Multidisciplinary Care: A Matched-Pair Analysis. Clinical Cancer Research. 2010;16(20):5019–27. 10.1158/1078-0432.ccr-10-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113(4):750–7. 10.1002/cncr.23615 [DOI] [PubMed] [Google Scholar]
- 34.Vercelli M, Lillini R, Capocaccia R, Micheli A, Coebergh JW, Quinn M, et al. Cancer survival in the elderly: effects of socio-economic factors and health care system features (ELDCARE project). European journal of cancer. 2006;42(2):234–42. [DOI] [PubMed] [Google Scholar]
- 35.Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273–8. 10.1002/cncr.29171 [DOI] [PubMed] [Google Scholar]
- 36.Konski A, Berkey BA, Kian Ang K, Fu KK. Effect of education level on outcome of patients treated on Radiation Therapy Oncology Group Protocol 90‐03. Cancer. 2003;98(7):1497–503. 10.1002/cncr.11661 [DOI] [PubMed] [Google Scholar]
- 37.Wells JC, Samuel TA, Morton K, Looney SW. Tobacco use and race as copredictors of overall survival in patients with breast cancer. Journal of Clinical Oncology. 2013;31(26). [Google Scholar]
- 38.López RVM, López RVM, Zago MA, Eluf-Neto J, Curado MP. Education, tobacco smoking, alcohol consumption, and IL-2 and IL-6 gene polymorphisms in the survival of head and neck cancer. Brazilian journal of medical and biological research. 2011;44(10):1006–12. 10.1590/S0100-879X2011007500097 [DOI] [PubMed] [Google Scholar]
- 39.Pai P-C, Chuang C-C, Tseng C-K, Tsang N-M, Chang K-P, Yen T-C, et al. Impact of Pretreatment Body Mass Index on Patients With Head-and-Neck Cancer Treated With Radiation. International Journal of Radiation Oncology Biology Physics. 2012;83(1):e93–e100. 10.1016/j.ijrobp.2011.11.071. [DOI] [PubMed] [Google Scholar]
- 40.Bassett WW, Cooperberg MR, Sadetsky N, Silva S, DuChane J, Pasta DJ, et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: Data from CaPSURE. Urology. 2005;66(5):1060–5. 10.1016/j.urology.2005.05.040 [DOI] [PubMed] [Google Scholar]
- 41.Lazure KE, Lydiatt WM, Denman D, Burke WJ. Association between depression and survival or disease recurrence in patients with head and neck cancer enrolled in a depression prevention trial. Head & neck. 2009;31(7):888–92. 10.1002/hed.21046 [DOI] [PubMed] [Google Scholar]
- 42.Patel UA, Brennan TE. Disparities in head and neck cancer: assessing delay in treatment initiation. The Laryngoscope. 2012;122(8):1756–60. 10.1002/lary.23357 [DOI] [PubMed] [Google Scholar]
- 43.Saunders J, Aasland O, Babor T, de la Fuente J, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88(Suppl 6):791–804. [DOI] [PubMed] [Google Scholar]
- 44.Brower KJ, Severin JD. Alcohol and other drug-related problems In: Knesper DJ, Riba MB, Schwenk TL, editors. Primary Care Psychiatry. 1st ed. Philadelphia: W. B. Saunders Company; 1997. [Google Scholar]
- 45.National Center for Cost Containment. Geropsychology Assessment Resource Guide. Milwaukee: National Center for Cost Containment, Department of Veterans Affairs; 1996 1996, Revision.
- 46.Edge SB. AJCC cancer staging manual London: Springer; 2010. [Google Scholar]
- 47.American Joint Committee on Cancer, editor. AJCC cancer staging manual, 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 48.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel ELJ. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA: the journal of the American Medical Association. 2004;291(20):2441–7. [DOI] [PubMed] [Google Scholar]
- 49.Piccirillo J, Costas I, Claybour P, Borah A, Grove L, Jeffe D. The measurement of comorbidity by cancer registries. J Registry Manage. 2003;30(1):8–15. [Google Scholar]
- 50.Stevens JP. Applied multivariate statistics for the social sciences. 3rd ed. NJ: Erlbaum: Mahwah; 1996. [Google Scholar]
- 51.Steenland K, Henley J, Thun M. All-cause and cause-specific death rates by educational status for two million people in two American Cancer Society cohorts, 1959–1996. American journal of epidemiology. 2002;156(1):11–21. 10.1093/aje/kwf001 [DOI] [PubMed] [Google Scholar]
- 52.Freeman HP. Cancer in the socioeconomically disadvantaged. CA Cancer J Clin. 1989;39(5):266–88. [DOI] [PubMed] [Google Scholar]
- 53.Dowd JB, Palermo TM, Aiello AE. Family poverty is associated with cytomegalovirus antibody titers in US Children. Health Psychology. 2012;31(1):5 10.1037/a0025337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. 10.1038/nature10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA: the journal of the American Medical Association. 2002;287(16):2106–13. Epub 2002/04/23. jma10055 [pii]. . [DOI] [PubMed] [Google Scholar]
- 56.Ringstrom E, Peters E, Hasegawa M, Posner M, Liu M, Kelsey KT. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8(10):3187–92. [PubMed] [Google Scholar]
- 57.Smedley B, Stith A, Nelson A, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care Washington D.C.: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 58.Arias E. United States life tables, 2004. Hyattsville, MD: National Center for Health Statistics, 2007 2007. Report No. [PubMed]
- 59.Redondo-Sendino A, Guallar-Castillón P, Banegas JR, Rodríguez-Artalejo F. Gender differences in the utilization of health-care services among the older adult population of Spain. BMC public health. 2006;6(1):155–. 10.1186/1471-2458-6-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41–7. Epub 2005/09/27. 10.1007/s10549-005-3702-4 . [DOI] [PubMed] [Google Scholar]
- 61.Browman GP, Mohide EA, Willan A, Hodson I, Wong G, Grimard L, et al. Association between smoking during radiotherapy and prognosis in head and neck cancer: a follow-up study. Head & neck. 2002;24(12):1031–7. [DOI] [PubMed] [Google Scholar]
- 62.Do KA, Johnson MM, Doherty DA, Lee JJ, Wu XF, Dong Q, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States). Cancer causes & control: CCC. 2003;14(2):131–8. [DOI] [PubMed] [Google Scholar]
- 63.Park SM. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. Journal of clinical oncology. 2006;24(31):5017–24. 10.1200/JCO.2006.07.0243 [DOI] [PubMed] [Google Scholar]
- 64.Baatenburg de Jong RJ, Hermans J, Molenaar J, Briaire JJ, le Cessie S. Prediction of survival in patients with head and neck cancer. Head & neck. 2001;23(9):718–24. [DOI] [PubMed] [Google Scholar]
- 65.Woodard TD, Oplatek A, Petruzzelli GJ. Life after total laryngectomy: a measure of long-term survival, function, and quality of life. Arch Otolaryngol Head Neck Surg. 2007;133(6):526–32. [DOI] [PubMed] [Google Scholar]
- 66.Pugliano FA, Piccirillo JF, Zequeira MR, Fredrickson JM, Perez CA, Simpson JR. Clinical-severity staging system for oral cavity cancer: Five-year survival rates. Otolaryngology-Head and Neck Surgery. 1999;120(1):38–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The VA data is very sensitive and would require a number of approvals before it could be released. Similar approvals would also apply at the University of Michigan. There is a large amount of HIPPA data that would need to be de-identified. If someone wants the data, they would have to contact Sonia Duffy for Veterans Affairs data and Carol Bradford for University of Michigan data, and procedures (e.g., data sharing agreements, sign off from privacy officers, de-identification, etc.) would then need to be followed to obtain the data.