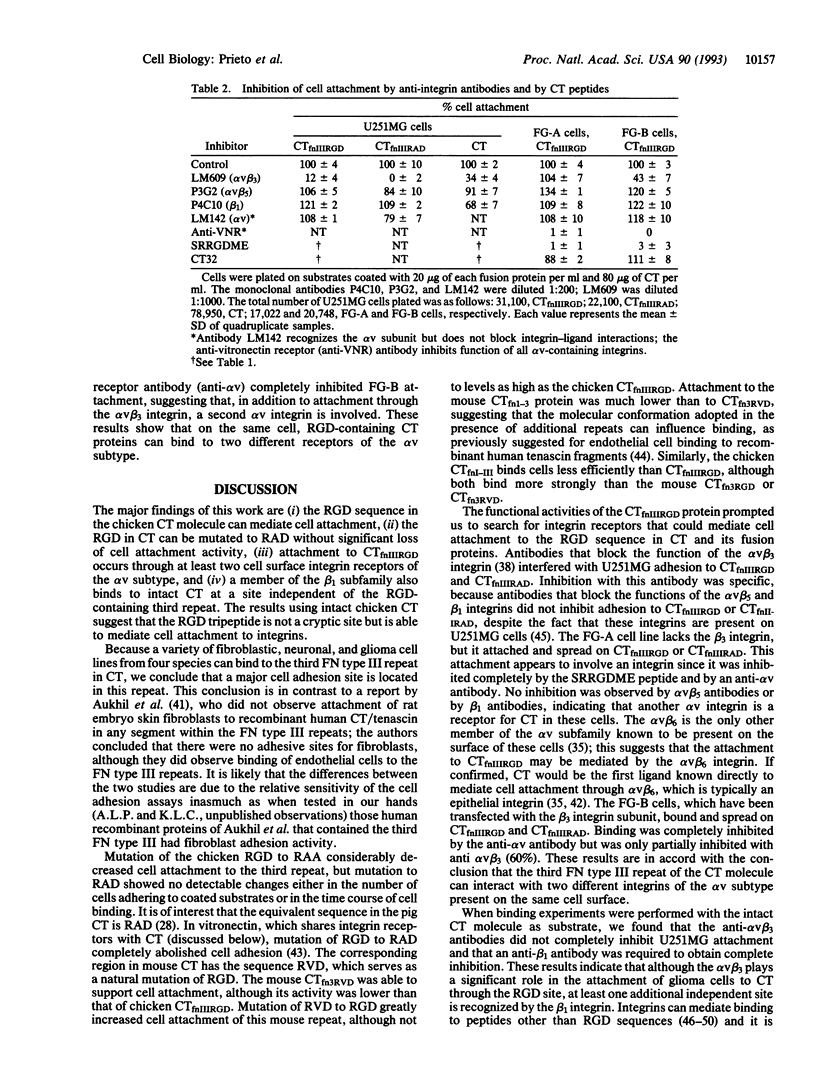
Abstract
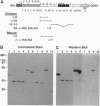
To identify potential cell surface receptors for chicken cytotactin (CT), we have characterized the ability of recombinant fusion proteins spanning the proximal fibronectin (FN) type III repeats of the molecule to support attachment of glioma and carcinoma cell lines. The third FN type III repeat, which contains the RGD tripeptide, supported cell attachment and cell spreading; however, mutation of RGD to RAD did not result in significant loss of either activity. In addition, the same repeat of mouse CT, which contains a natural mutant, RVD, also supported cell attachment and spreading, although at a lower level; both activities were increased by mutation of the RVD sequence to RGD. Studies utilizing RGD-containing peptides and well-characterized antibodies to integrins indicated that cell attachment to the third FN type III repeat was mediated by at least two different integrin receptors of the alpha v subtype. Additional cellular receptors may also be involved in cell attachment to CT. For example, an antibody to the beta 1 subfamily of integrins partially inhibited binding of cells to intact CT but did not inhibit cell binding to the third FN type III repeat. These findings suggest that the RGD site in CT is able to mediate cell attachment to integrins and thus is not a cryptic adhesion site. They also open the possibility that the functions of CT in processes such as counteradhesion, cell migration, cell proliferation, and cell differentiation may be mediated in part by interaction with multiple integrins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991 Nov;115(3):829–841. doi: 10.1083/jcb.115.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukhil I., Joshi P., Yan Y., Erickson H. P. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993 Feb 5;268(4):2542–2553. [PubMed] [Google Scholar]
- Bourdon M. A., Ruoslahti E. Tenascin mediates cell attachment through an RGD-dependent receptor. J Cell Biol. 1989 Mar;108(3):1149–1155. doi: 10.1083/jcb.108.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busk M., Pytela R., Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992 Mar 25;267(9):5790–5796. [PubMed] [Google Scholar]
- Calof A. L., Lander A. D. Relationship between neuronal migration and cell-substratum adhesion: laminin and merosin promote olfactory neuronal migration but are anti-adhesive. J Cell Biol. 1991 Nov;115(3):779–794. doi: 10.1083/jcb.115.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Cherny R. C., Honan M. A., Thiagarajan P. Site-directed mutagenesis of the arginine-glycine-aspartic acid in vitronectin abolishes cell adhesion. J Biol Chem. 1993 May 5;268(13):9725–9729. [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A., Beck K., Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988 May 6;53(3):383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Chiquet M., Vrucinić-Filipi N., Schenk S., Beck K., Chiquet-Ehrismann R. Isolation of chick tenascin variants and fragments. A C-terminal heparin-binding fragment produced by cleavage of the extra domain from the largest subunit splicing variant. Eur J Biochem. 1991 Jul 15;199(2):379–388. doi: 10.1111/j.1432-1033.1991.tb16134.x. [DOI] [PubMed] [Google Scholar]
- Crossin K. L. Cytotactin binding: inhibition of stimulated proliferation and intracellular alkalinization in fibroblasts. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11403–11407. doi: 10.1073/pnas.88.24.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Grumet M., Thiery J. P., Edelman G. M. Site-restricted expression of cytotactin during development of the chicken embryo. J Cell Biol. 1986 May;102(5):1917–1930. doi: 10.1083/jcb.102.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988 Nov;104(3):369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- Friedlander D. R., Hoffman S., Edelman G. M. Functional mapping of cytotactin: proteolytic fragments active in cell-substrate adhesion. J Cell Biol. 1988 Dec;107(6 Pt 1):2329–2340. doi: 10.1083/jcb.107.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladson C. L., Cheresh D. A. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991 Dec;88(6):1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Marton L. S., Stefansson K. An alternatively spliced region of the human hexabrachion contains a repeat of potential N-glycosylation sites. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1588–1592. doi: 10.1073/pnas.86.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Crossin K. L., Edelman G. M. Molecular forms, binding functions, and developmental expression patterns of cytotactin and cytotactin-binding proteoglycan, an interactive pair of extracellular matrix molecules. J Cell Biol. 1988 Feb;106(2):519–532. doi: 10.1083/jcb.106.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Burgoon M. P., Hoffman S., Crossin K. L., Cunningham B. A., Edelman G. M. A cDNA clone for cytotactin contains sequences similar to epidermal growth factor-like repeats and segments of fibronectin and fibrinogen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2186–2190. doi: 10.1073/pnas.85.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Hoffman S., Cunningham B. A., Edelman G. M. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplony A., Zimmermann D. R., Fischer R. W., Imhof B. A., Odermatt B. F., Winterhalter K. H., Vaughan L. Tenascin Mr 220,000 isoform expression correlates with corneal cell migration. Development. 1991 Jun;112(2):605–614. doi: 10.1242/dev.112.2.605. [DOI] [PubMed] [Google Scholar]
- Lane T. F., Sage E. H. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990 Dec;111(6 Pt 2):3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy D. J., Hendrickson W. A., Aukhil I., Erickson H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992 Nov 6;258(5084):987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Leavesley D. I., Ferguson G. D., Wayner E. A., Cheresh D. A. Requirement of the integrin beta 3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992 Jun;117(5):1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike J. D., Sodeik B., Cao L., Leucona S., Weitz J. I., Detmers P. A., Wright S. D., Silverstein S. C. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Tucker R. P., Halfter W., Chiquet-Ehrismann R., Epperlein H. H. The distribution of tenascin coincides with pathways of neural crest cell migration. Development. 1988 Jan;102(1):237–250. doi: 10.1242/dev.102.1.237. [DOI] [PubMed] [Google Scholar]
- Maruta H., Holden J., Sizeland A., D'Abaco G. The residues of Ras and Rap proteins that determine their GAP specificities. J Biol Chem. 1991 Jun 25;266(18):11661–11668. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Lightner V. A., Aukhil I., Yan Y. Z., Erickson H. P., Hök M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991 Nov;115(4):1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen D., Thiery J. P. Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res. 1980;211(2):269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- Nishi T., Weinstein J., Gillespie W. M., Paulson J. C. Complete primary structure of porcine tenascin. Detection of tenascin transcripts in adult submaxillary glands. Eur J Biochem. 1991 Dec 5;202(2):643–648. doi: 10.1111/j.1432-1033.1991.tb16418.x. [DOI] [PubMed] [Google Scholar]
- Onda H., Poulin M. L., Tassava R. A., Chiu I. M. Characterization of a newt tenascin cDNA and localization of tenascin mRNA during newt limb regeneration by in situ hybridization. Dev Biol. 1991 Nov;148(1):219–232. doi: 10.1016/0012-1606(91)90331-v. [DOI] [PubMed] [Google Scholar]
- Prieto A. L., Andersson-Fisone C., Crossin K. L. Characterization of multiple adhesive and counteradhesive domains in the extracellular matrix protein cytotactin. J Cell Biol. 1992 Nov;119(3):663–678. doi: 10.1083/jcb.119.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto A. L., Jones F. S., Cunningham B. A., Crossin K. L., Edelman G. M. Localization during development of alternatively spliced forms of cytotactin mRNA by in situ hybridization. J Cell Biol. 1990 Aug;111(2):685–698. doi: 10.1083/jcb.111.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg C., Postigo A. A., Sikorski E. E., Butcher E. C., Pytela R., Erle D. J. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol. 1992 Apr;117(1):179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Funk S. E., Everitt E. A., Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989 Jul;109(1):341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A. Transmembrane signalling by integrins. Trends Cell Biol. 1992 Oct;2(10):304–308. doi: 10.1016/0962-8924(92)90120-c. [DOI] [PubMed] [Google Scholar]
- Sheppard D., Rozzo C., Starr L., Quaranta V., Erle D. J., Pytela R. Complete amino acid sequence of a novel integrin beta subunit (beta 6) identified in epithelial cells using the polymerase chain reaction. J Biol Chem. 1990 Jul 15;265(20):11502–11507. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Fok K. F., Zutter M. M., Adams S. P., Rodriguez B. A., Santoro S. A. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991 Apr 25;266(12):7363–7367. [PubMed] [Google Scholar]
- Tan S. S., Crossin K. L., Hoffman S., Edelman G. M. Asymmetric expression in somites of cytotactin and its proteoglycan ligand is correlated with neural crest cell distribution. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7977–7981. doi: 10.1073/pnas.84.22.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A., Beck S., Ekblom P. Amino acid sequence of mouse tenascin and differential expression of two tenascin isoforms during embryogenesis. J Cell Biol. 1991 Jan;112(2):355–362. doi: 10.1083/jcb.112.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Adhesive recognition sequences. J Biol Chem. 1991 Jul 15;266(20):12809–12812. [PubMed] [Google Scholar]