Abstract
Allergen-specific immunotherapy (AIT), although in clinical use for more than a century, is still the only causal treatment of allergic diseases. The safety and efficacy of AIT has been demonstrated in a large number of clinical trials. In addition to allergy symptom reduction AIT plays an essential role in preventing new allergies and asthma and shows long-term effects after discontinuation of treatment. Ideally, it is capable of curing allergy. However, AIT is not effective in all allergic individuals and is not equally effective in the treatment of various hypersensitivities to different allergens. For many years, the route of administration and the vaccine compositions have been evolving. Still there is a strong need for research in the field of new AIT modalities to increase its effectiveness and safety. Growing evidence on immunological effects of AIT, especially new T cell subsets involved in antigen/allergen tolerance, provides novel concepts for safer and more effective vaccination. Pharmacoeconomic studies have demonstrated a clear advantage of AIT over pharmacologic therapies.
Keywords: Allergen immunotherapy, allergen vaccines
INTRODUCTION
Allergen-specific immunotherapy (AIT) is currently the only known causal effective treatment of IgE-mediated allergy. However, the mechanisms of AIT, as well as the mechanisms of allergic diseases and other immunologically conditioned diseases, remain unclear and are the subject of detailed studies of clinicians and researchers around the world. AIT was introduced by Leonard Noon more than 100 years ago and currently is the only disease-modifying treatment in allergy. The pioneer clinical trials with the AIT were undertaken by Noon in 1911 and continued by Freeman in Europe in grass pollen-seasonal allergic rhinitis. The allergens used in the therapy were water-extracts from grass pollen mixtures. While therapy was effective, there was a high risk of serious adverse events in patients during the allergen desensitization phase.1
The use of AIT carries the risk of adverse effects, resulting from, among others, iatrogenic exposure to an allergen. This method is not equally effective in all patients and not equally effective in hypersensitivities to various allergens. For this reason, constant research to improve preparations of vaccines, establishing the optimal dose and route of administration, better selection of allergy patient subgroups should help develop the best clinical desensitization effect. In addition, long-term data allowing a comprehensive assessment of molecular changes in the immune system during the development of allergen tolerance in AIT are still lacking. Clarification of mechanisms responsible for the development of either hypersensitivity reactions or tolerance to antigens (allergens) will have a profound impact on new treatment approaches, based on the identification of the causative factors and modulation of the natural course of allergic diseases and asthma.
AIT is indicated for the treatment of patients with moderate-to-severe intermittent or persistent allergic rhinitis. Numerous double-blind, placebo-controlled (DBPC) studies showed significant improvement in nasal and ocular symptom scores, reduced need for symptomatic medication and improved quality of life both during and after discontinuation of AIT.2 Pharmaco-economic studies demonstrated a clear advantage of AIT over pharmacologic therapies as well.
Currently available well-standardized allergen extracts, which should be used for AIT, include grass, tree, and weed (ragweed and mugwort) pollens,3,4 house dust mites,5,6,7 several mold species,3,8 and animal dander.9
There is a substantial need for research to elucidate the currently unknown mechanisms of AIT, such as regulatory T (Treg)-cell induction, early molecular markers and predictors, and mechanisms of long-term maintenance of allergen tolerance.10 Reliable biomarkers could be selected and validated with the intention to select the patients who will benefit most from AIT. The immune profile that triggers hypersensitivity development to certain allergens (their epitopes) in an individual remains an area of extensive basic and clinical research as well.
Mechanisms of AIT
T cells play an important role in the pathogenesis of allergy.11 T-helper (Th) CD4+ cells in allergy produce large amounts of different cytokines responsible for the initiation and maintenance of allergic inflammation, like interleukin (IL)-4, IL-5, and IL-13 (Th2 cytokine profile) and small amounts or no interferon-gamma (IFN-γ), a Th1-cytokine profile. The cytokines IL-4 and IL-13 stimulate B cells to produce immunoglobulin E (IgE), while IL-5 initiates and maintains eosinophilic inflammation. Changes in the number of T cells arise during the course of AIT very early and precede an increase in IgG concentrations, which reaches peak value at 3 months.12,13 Both healthy individuals and effectively treated allergic patients with AIT showed reduced and balanced Th1 and Th2 responses. In this state of immune homeostasis, there are also potent suppression mechanisms, executed in part by specific CD4+ Treg cells.
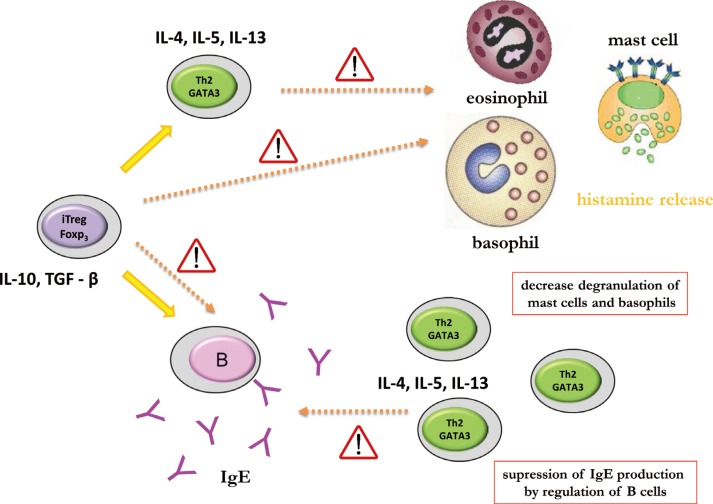
During AIT, peripheral tolerance is induced by the generation of allergen-specific Treg cells, which suppress proliferative and cytokine responses against the allergen of interest. Treg cells are characterized by IL-10 and TGF-beta (TGF-β) secretion as well as expression of important cell surface suppressive molecules, such as cytotoxic T lymphocyte antigen-4 and programmed death-1, that directly or indirectly influence effector cells of allergic inflammation, such as mast cells, basophils, and eosinophils. Treg cells, particularly IL-10 also have an influence on B cells, suppressing IgE production and inducing the production of blocking-type IgG4 antibodies. In addition, development of allergen-specific B regulatory (Breg) cells that produce IL-10 and develop into IgG4-producing plasma cells represents essential players in peripheral tolerance (Fig. 1).
Fig. 1. First administration of AIT vaccine causes decrease in degranulation of mast cells and basophils by blocking effect of Th2 and Treg cytokines. This decreases amounts of secreted histamine and other mediators by effector cells. IgE production is impaired as well by IL-10 and TGF-β cytokines produced by activated Treg cells.
AIT induces a shift in the numbers and function of IL-4-secreting Th2 cells toward IL-10-secreting inducible Treg (iTreg) cells showing the same allergen-epitope specificity.14 Different types of iTreg cells have been described. FOXP3+ (Forkhead box protein 3), adaptive Treg cells and FOXP3 negative but IL-10-producing type 1 regulatory T (Tr1) cells determine several facets of allergic inflammation. A significant correlation between improvement in symptoms and increases in Treg cell numbers during immunotherapy has been postulated. Identification of cytokine profile during AIT at the level of single cells may provide evidence of the formation of Treg populations producing IL-10 and/or production of IFN-γ, indicating immune deviation. As already mentioned, Treg cells play a central role in the induction of allergen-specific tolerance. Increased numbers of FOXP3+CD25+CD3+ cells were demonstrated in the nasal mucosa after immunotherapy with clinical efficacy and the suppression of seasonal allergic inflammation.15 The expression of FOXP3 correlates with the suppressive capacity of Treg cells.16 Increased FOXP3 expression negatively correlates with the serum levels of IgE and IFN-γ, and eosinophilia. Moreover, in asthma and atopic dermatitis patients the ratio of FOXP3+ T cells to total CD4+ T cells is significantly lower compared to healthy individuals.17
Furthermore, Breg-cell subsets may play a role in allergen tolerance in humans during AIT. Human type 1 regulatory B (Br1) cells produced high levels of IL-10 and potently suppressed antigen-specific CD4+ T-cell proliferation.18 Recently, the role of Breg cells characterized as CD73-CD25+CD71+ cells in tolerance-induction during AIT has been postulated.14 Breg cells induce isotype switch from IgE toward IgG4 and induce allergen-specific antibodies toward the non-anaphylactic and non-inflammatory type. However, the very long IgE life span is most probably responsible for the lack of correlation between the Ig isotype shift and the therapeutic effect of AIT. In addition, Breg cells also suppress allergen-specific T cells in both their regulatory and effector functions. T-cell suppression occurs both in secondary lymphoid organs and in affected tissues. Furthermore, iTreg cells are capable of suppression of innate effector cells of allergic inflammation (mast cells and basophils) and reduction in eosinophil numbers in mucosal tissues.
Allergen-specific IgE levels increase initially after the start of AIT and decrease to pretreatment levels during the maintenance phase. AIT particularly induces allergen-specific antibodies of the IgG4 subclass. Similarly, natural exposure to an allergen in non-allergic individuals, such as beekeepers, is often associated with an increase in specific IgG4. However, there is a poor correlation between the levels of allergen-specific IgG and clinical protection, and much better correlation with allergen dose that has been administered. Therefore, IgG4 antibodies can be viewed as a marker of the dose of introduced allergen during AIT. Analysis of IgG subtypes induced by AIT has shown increases in allergen-specific IgG4 and IgG1, with 10- to 100-fold increases in their serum levels.12 Specific IgG4 in serum shows a relatively early and rapid increase and continues to increase during the whole course of AIT. Treg cells, via secretion of the immunoregulatory cytokine IL-10, affect immunoglobulin synthesis through strong suppression of allergen-specific IgE, while increasing IgG4 production.19 A decrease in serum IgE appears much later than clinical tolerance, which occurs relatively early during the course of AIT and does not correlate with the magnitude of clinical improvement after treatment.
Cellular mechanisms (at the level of T cells) that play a role in successful AIT are probably the same as those responsible for the development of classical immunological tolerance: anergy associated with the absence of co-stimulation, cell death due to activation (clonal deletion), shift in Th function from Th2 profile toward Th0/Th1 profile with increased production of IFN-γ (immune deviation), induction of Treg cells, or a combination of these mechanisms.20,21,22 The shift in the balance between allergen- specific Th2 and Treg cells is central to either development of allergen tolerance or allergic status or even the recovery from allergic disease.19,23,24
The understanding of the AIT mechanisms is of utmost importance in characterization of early and late diagnostic biomarkers to select the best responders and to optimize the treatment.25 These findings together with new biotechnological approaches create a platform for the development of advanced vaccines.26
Vaccine development
Novel vaccines should meet increasing needs for reduction in adverse effects, costs, and duration of treatment. The vaccines have to induce long-term tolerance to allergens. Better selection of patients should be made to identify those who can benefit the most from the appropriate vaccine. Use of multiple vaccines at the same time should be possible in multisensitized patients.
Currently used allergen extracts are standardized for total allergenic activity, and they consist of major allergens. On the other hand, they contain many proteins which are not allergens. One particular allergen source usually contains more than 1 clinically relevant allergen and constitutes a set of major and minor allergens of different clinical importance.27
Compared to allergen extracts, recombinant allergen cocktails can be formulated with high quality and precise quantity of relevant allergen as well as with high technical reproducibility of the mixture. The component- resolved immunotherapy preparations may be based on individually composed mixtures of allergenic epitopes.28
Recombinant allergen-based vaccination strategies arose from a strong need to improve safety and enhance efficacy of AIT. A number of successful clinical studies with both wild-type and hypoallergenic derivatives of recombinant allergen vaccines have been reported during the last decade. They showed profiles with high efficacy and safety as well as very strong modulation of T and B cell responses to specific allergens.29 The first clinical studies with recombinant allergens have delivered very encouraging results.30 Jutel et al.,12 evaluated the use of mixture of 5 different wild-type recombinant allergens of timothy grass in the treatment of seasonal allergic rhinitis in a DBPC clinical trial. The study was successful, and all treated patients developed high allergen-specific IgG1 and IgG4 antibody response.
In the first DBPC immunotherapy study with recombinant allergen preparations, 2 different hypoallergenic derivatives of the major birch pollen allergen Bet v 1 and placebo were compared. The active treatment induced protective IgG antibodies, and recombinant allergen preparations were well tolerated.31 A comparison between recombinant birch pollen allergen vaccine, standard birch pollen extract, natural purified birch pollen allergen, and placebo was published in 2008. The patients were immunized subcutaneously over 2 years. All the treated groups demonstrated significant and equal improvement in symptoms, medication use and skin test reactivity compared to the placebo group. Interestingly, the recombinant vaccine-treated group presented greater increases in specific IgG1, IgG2, and IgG4 levels and decrease in the skin test reactivity.32 Recombinant vaccines for grass pollen, birch pollen, and house dust mite represent the major focus in the future development. Vaccines for other allergens may be very expensive due to a large number of minor allergens.29
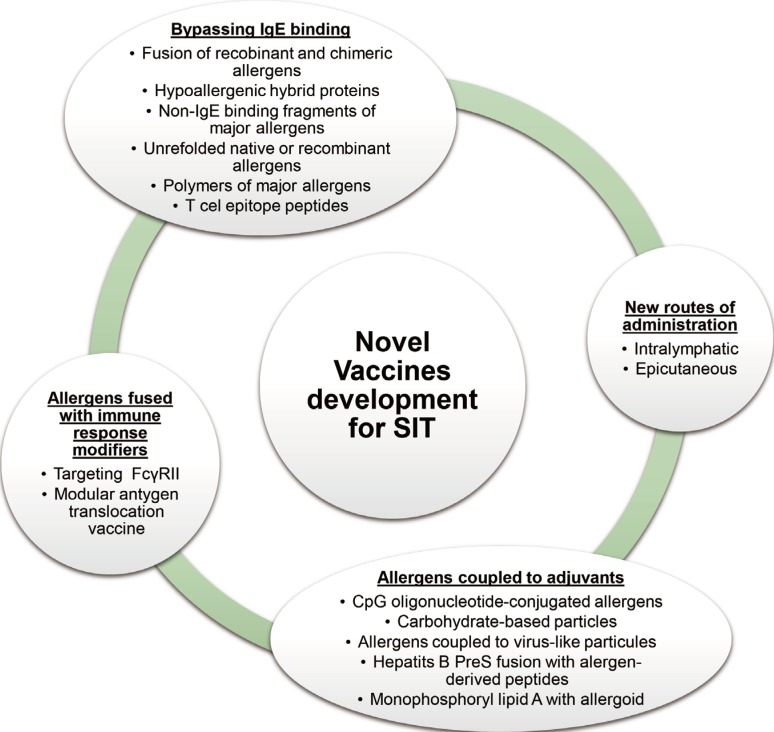
Another approach includes bypassing IgE binding to avoid IgE-mediated adverse effects and also inducing T-cell tolerance.33 Allergen fragments, fusions, hybrids and chimeras are used to avoid recognition by conformation dependent B cell epitopes and to utilize linear amino acid sequence of T-cell epitopes. These approaches provide the possibility to enhance the tolerogenic T cell-dependent signal due to the administration of higher doses of preparation with the low risk of anaphylaxis.33,34 The prominent example of this approach is peptide immunotherapy that utilizes linear T-cell epitope peptides.35,36
Peptide vaccines have been applied in cat dander and bee venom allergy. In some studies, vaccination improved tolerance to cats and pulmonary function in cat-allergic patients.37 Moreover, short allergen-derived peptides can directly initiate a major histocompatibility complex-restricted T cell-dependent late asthmatic reaction, without requirement for an early IgE/mast cell-dependent response in Fel d 1-sensitised asthmatic patients.38 To evaluate the effect of T-cell peptide therapy on the allergen-induced cutaneous late phase reaction, the skin biopsies were studied before and after T-cell peptide therapy. Treatment resulted in allergen-dependent recruitment to the skin of CD4+/IFNγ+, and CD3+/CD25+ cells concluded as Th1 profile, but not Th2/Treg cells.39 The application of peptides representing linear T-cell epitopes has recently been investigated in clinical trials.40,41,42 Moreover, IgE cross-linking was profoundly reduced using a recombinant chimeric protein which comprises the entire amino acid sequences of 3 bee venom major allergens.43 Hypoallergenic hybrid molecules were used as house dust mite allergy treatment, generating a higher T-cell proliferation response and greater reduction in IgE reactivity.44 Hypoallergenic derivatives of the major birch pollen allergen, Bet v 1, were developed in 2004. Active treatment induced protective IgG antibodies and reduced increases in specific IgE induced by seasonal birch pollen exposure.31
Another attempt is to physically couple allergens to modifiers of the innate immune response. For example, fusion of allergens with human FcγR has been reported to inhibit allergen-induced basophil and mast cell degranulation by cross-linking FcγR and FcεRI receptors.45
Current clinical data with recombinant or peptide vaccinations are very promising. The large diversity of future approaches relies on infinite possibilities for combinations of multiple immune stimulators and methods for coupling.26 Apart from the physical fusion, the novel methods of AIT can be combined with immune-modifying biological therapies. Several studies show that anti-IgE therapy combined with AIT is effective.46 Additional benefits which had been shown were reduced adverse effects with decreased anaphylactic reaction, improvement in rescue medication scores (decreased requirement for rescue medication). A short course of peptide immunotherapy with a fixed dose of peptides may be able to achieve therapeutic effects comparable with subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT).40 Novel vaccine approaches are outlined in Fig. 2.
Fig. 2. Novel vaccine development for AIT26.
Route of administration
Currently under development are new routes of administration of allergen vaccines to patients undergoing allergen immunotherapy. The new routes should reduce the risk of anaphylactic reactions and allow more rapid up-dosing, while minimizing costs of treatment and inconvenience to the patients linked with reduced number and duration of visit in the clinic.
AIT is given mainly by subcutaneous injections whose frequency depends on the type of allergy and the individual scheme proposed to the patient. The conventional schedule for SCIT using allergen extracts consists of a dose build-up by injection once weekly, followed by maintenance dose injections at 4- to 8-week intervals. Using modified allergenic proteins as allergoids can decrease the number of build-up injections. There is also a possibility to use cluster or rush schedules. In cluster schedule, multiple injections (2-3 times) are given on non-consecutive days, where in rush protocol multiple injections are given on consecutive days, reaching the maintenance phase in 2-3 days. It is shown that in cluster method, there is no increase in systemic reactions and rapid improvement in symptoms severity.47 In rush schedule, there were more systemic reactions.48,49 The conventional duration of AIT is 3-5 years. Prospective studies using SCIT in grass pollen-allergic patients and house dust mite allergic asthmatics suggest that 3 years of AIT gives prolonged remission of symptoms after discontinuation.50,51 A prospective study of SLIT with house dust mite extracts in patients with allergic rhinitis demonstrated remission lasting 7 years after 3 years of treatment and 8 years after 4 years of treatment.52 In the 1980s, a number of fatal adverse reactions were reported, which led to many concerns about the safety of SCIT. This stimulated the exploration of safer routes of administration.48 Over the last 2 decades, there has been increasing use of SLIT. In SLIT, the build-up period is short or not-needed. Evidence supports a better response to daily SLIT administration.53,54 It has been well documented in DBPC trials that SLIT is clinically less effective than SCIT.55
There are other new approaches under investigation using different routes of administration, including epicutaneous and intralymphatic routes of administration of allergen extracts.56 Both routes showed similar efficacy to SCIT in grass pollen allergy, but what is worth highlighting is that fewer applications and lower total doses of allergen were required.57,58,59 Besides different routes of administration, the modifications of allergen preparations using recombinant technology to produce modified proteins and peptides can enhance the desirable immune response to the allergens.
AIT is usually recommended in subjects over 5 years of age, however, sublingual AIT is safe and effective even in children as young as 3 years of age. The recommended duration of AIT for allergic rhinitis is 3 years, both in SCIT and SLIT.60
Future perspectives
Continuous development of immunology and bioengineering help improve patient diagnostics as well as quality and composition of vaccines including novel compounds. Novel diagnostic biomarkers will help select the best responders for correctly chosen patient-specific treatment. New routes of administration also provide a promising alternative to current treatment. However, new regulations, especially in Europe and in the USA, demand the conductance of a large number of very expensive clinical trials, especially in children. This is limiting the development of novel clinical modalities which does not keep pace with rapid technological improvement animated by progress in immunology and biotechnology. Large investments in the allergy vaccine market are necessary to improve this situation.
CONCLUSIONS
Major problems which need to be solved by development of new vaccines for AIT include: reduction in adverse effect risk during therapy, early identification of subjects who are potentially not responding to the treatment, achieving sustained, life-long allergen tolerance or complete cure of allergic disease after AIT discontinuation, reduction in the burden for patients related with visits to the doctor's office, and last but not least reduction in costs of therapy.
Another important issue involves the role of AIT in sensitization or allergy prevention. Such strategies are currently under evaluation; however, validation of the use of specific tools, especially biomarkers, that will provide help to identify subjects who can potentially benefit from such modalities is still lacking.
Footnotes
The authors are supported by Polish National Science Centre grants No. 2012/04/M/NZ6/00355 and 2012/04/A/NZ6/00407
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Ring J, Gutermuth J. 100 years of hyposensitization: history of allergen-specific immunotherapy (ASIT) Allergy. 2011;66:713–724. doi: 10.1111/j.1398-9995.2010.02541.x. [DOI] [PubMed] [Google Scholar]
- 2.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–1296.e3. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Pfaar O, Biedermann T, Klimek L, Sager A, Robinson DS. Depigmented-polymerized mixed grass/birch pollen extract immunotherapy is effective in polysensitized patients. Allergy. 2013;68:1306–1313. doi: 10.1111/all.12219. [DOI] [PubMed] [Google Scholar]
- 4.Mailhol C, Didier A. Specific immunotherapy in grass pollen allergy. Hum Vaccin Immunother. 2012;8:1544–1547. doi: 10.4161/hv.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun T, Yin K, Wu LY, Jin WJ, Li Y, Sheng B, et al. A DNA vaccine encoding a chimeric allergen derived from major group 1 allergens of dust mite can be used for specific immunotherapy. Int J Clin Exp Pathol. 2014;7:5473–5483. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao BB, Diao JD, Liu ZM, Li CP, Jiang YX. Generation of a chimeric dust mite hypoallergen using DNA shuffling for application in allergen-specific immunotherapy. Int J Clin Exp Pathol. 2014;7:3608–3619. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015;7:221–229. doi: 10.4168/aair.2015.7.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res. 2015;7:205–220. doi: 10.4168/aair.2015.7.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raap U, Wagenmann M, Pfaar O. Allergen-specific immunotherapy in pet allergy - an update. Hautarzt. 2011;62:657–662. doi: 10.1007/s00105-011-2159-0. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos NG, Agache I, Bavbek S, Bilo BM, Braido F, Cardona V, et al. Research needs in allergy: an EAACI position paper, in collaboration with EFA. Clin Transl Allergy. 2012;2:21. doi: 10.1186/2045-7022-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romagnani S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol. 2000;105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 12.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Müller UR, Jutel M, Reimers A, Zumkehr J, Huber C, Kriegel C, et al. Clinical and immunologic effects of H1 antihistamine preventive medication during honeybee venom immunotherapy. J Allergy Clin Immunol. 2008;122:1001–1007.e4. doi: 10.1016/j.jaci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 15.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–1472. 1472.e1. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Klunker S, Chong MM, Mantel PY, Palomares O, Bassin C, Ziegler M, et al. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009;206:2701–2715. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orihara K, Narita M, Tobe T, Akasawa A, Ohya Y, Matsumoto K, et al. Circulating Foxp3+CD4+ cell numbers in atopic patients and healthy control subjects. J Allergy Clin Immunol. 2007;120:960–962. doi: 10.1016/j.jaci.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 18.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Meiler F, Zumkehr J, Klunker S, Rückert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–2898. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 22.Jutel M, Akdis CA. T-cell regulatory mechanisms in specific immunotherapy. Chem Immunol Allergy. 2008;94:158–177. doi: 10.1159/000155000. [DOI] [PubMed] [Google Scholar]
- 23.Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008;63:1455–1463. doi: 10.1111/j.1398-9995.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 24.Möbs C, Ipsen H, Mayer L, Slotosch C, Petersen A, Würtzen PA, et al. Birch pollen immunotherapy results in long-term loss of Bet v 1-specific TH2 responses, transient TR1 activation, and synthesis of IgE-blocking antibodies. J Allergy Clin Immunol. 2012;130:1108–1116.e6. doi: 10.1016/j.jaci.2012.07.056. [DOI] [PubMed] [Google Scholar]
- 25.Jutel M, Akdis CA. Novel immunotherapy vaccine development. Curr Opin Allergy Clin Immunol. 2014;14:557–563. doi: 10.1097/ACI.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 26.Jutel M, Van de Veen W, Agache I, Azkur KA, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and novel ways for vaccine development. Allergol Int. 2013;62:425–433. doi: 10.2332/allergolint.13-RAI-0608. [DOI] [PubMed] [Google Scholar]
- 27.Aalberse RC, Crameri R. IgE-binding epitopes: a reappraisal. Allergy. 2011;66:1261–1274. doi: 10.1111/j.1398-9995.2011.02656.x. [DOI] [PubMed] [Google Scholar]
- 28.Jutel M, Solarewicz-Madejek K, Smolinska S. The future of allergen-specific immunotherapy. Drug Inf J. 2012;46:683–687. [Google Scholar]
- 29.Jutel M, Solarewicz-Madejek K, Smolinska S. Recombinant allergens: the present and the future. Hum Vaccin Immunother. 2012;8:1534–1543. doi: 10.4161/hv.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeiler T, Taivainen A, Rytkönen M, Rautiainen J, Karjalainen H, Mäntyjärvi R, et al. Recombinant allergen fragments as candidate preparations for allergen immunotherapy. J Allergy Clin Immunol. 1997;100:721–727. doi: 10.1016/s0091-6749(97)70264-1. [DOI] [PubMed] [Google Scholar]
- 31.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Akdis CA, Blaser K. Bypassing IgE and targeting T cells for specific immunotherapy of allergy. Trends Immunol. 2001;22:175–178. doi: 10.1016/s1471-4906(01)01862-2. [DOI] [PubMed] [Google Scholar]
- 34.Pree I, Reisinger J, Focke M, Vrtala S, Pauli G, van Hage M, et al. Analysis of epitope-specific immune responses induced by vaccination with structurally folded and unfolded recombinant Bet v 1 allergen derivatives in man. J Immunol. 2007;179:5309–5316. doi: 10.4049/jimmunol.179.8.5309. [DOI] [PubMed] [Google Scholar]
- 35.Norman PS, Ohman JL, Jr, Long AA, Creticos PS, Gefter MA, Shaked Z, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–1628. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 36.Müller U, Akdis CA, Fricker M, Akdis M, Blesken T, Bettens F, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101:747–754. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 37.Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999;93:222–231. doi: 10.1006/clim.1999.4795. [DOI] [PubMed] [Google Scholar]
- 38.Haselden BM, Kay AB, Larché M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander C, Ying S, B Kay A, Larché M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced latephase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005;35:52–58. doi: 10.1111/j.1365-2222.2005.02143.x. [DOI] [PubMed] [Google Scholar]
- 40.Worm M, Lee HH, Kleine-Tebbe J, Hafner RP, Laidler P, Healey D, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. 97.e1–97.e14. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 41.Patel D, Couroux P, Hickey P, Salapatek AM, Laidler P, Larché M, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–109.e1-7. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 42.Pellaton C, Perrin Y, Boudousquié C, Barbier N, Wassenberg J, Corradin G, et al. Novel birch pollen specific immunotherapy formulation based on contiguous overlapping peptides. Clin Transl Allergy. 2013;3:17. doi: 10.1186/2045-7022-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karamloo F, Schmid-Grendelmeier P, Kussebi F, Akdis M, Salagianni M, von Beust BR, et al. Prevention of allergy by a recombinant multi-allergen vaccine with reduced IgE binding and preserved T cell epitopes. Eur J Immunol. 2005;35:3268–3276. doi: 10.1002/eji.200425522. [DOI] [PubMed] [Google Scholar]
- 44.Asturias JA, Ibarrola I, Arilla MC, Vidal C, Ferrer A, Gamboa PM, et al. Engineering of major house dust mite allergens Der p 1 and Der p 2 for allergen-specific immunotherapy. Clin Exp Allergy. 2009;39:1088–1098. doi: 10.1111/j.1365-2222.2009.03264.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002;8:518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125:383–389. doi: 10.1016/j.jaci.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Tabar AI, Echechipía S, García BE, Olaguibel JM, Lizaso MT, Gómez B, et al. Double-blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 2005;116:109–118. doi: 10.1016/j.jaci.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Hejjaoui A, Ferrando R, Dhivert H, Michel FB, Bousquet J. Systemic reactions occurring during immunotherapy with standardized pollen extracts. J Allergy Clin Immunol. 1992;89:925–933. doi: 10.1016/0091-6749(92)90214-m. [DOI] [PubMed] [Google Scholar]
- 49.Temiño VM, Wu P, Konig J, Fahrenholz JM. Safety of multiple aeroallergen rush immunotherapy using a modified schedule. Allergy Asthma Proc. 2013;34:255–260. doi: 10.2500/aap.2013.34.3651. [DOI] [PubMed] [Google Scholar]
- 50.Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 51.Des Roches A, Paradis L, Knani J, Hejjaoui A, Dhivert H, Chanez P, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract V Duration of the efficacy of immunotherapy after its cessation. Allergy. 1996;51:430–433. doi: 10.1111/j.1398-9995.1996.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 52.Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Long-lasting effects of sublingual immunotherapy according to its duration: a 15-year prospective study. J Allergy Clin Immunol. 2010;126:969–975. doi: 10.1016/j.jaci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 53.Bordignon V, Parmiani S. Variation of the skin end-point in patients treated with sublingual specific immunotherapy. J Investig Allergol Clin Immunol. 2003;13:170–176. [PubMed] [Google Scholar]
- 54.Bauer CS, Rank MA. Comparative efficacy and safety of subcutaneous versus sublingual immunotherapy. J Allergy Clin Immunol. 2014;134:765–765.e2. doi: 10.1016/j.jaci.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 55.Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12. doi: 10.1111/j.1398-9995.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 56.von Moos S, Johansen P, Tay F, Graf N, Kündig TM, Senti G. Comparing safety of abrasion and tape-stripping as skin preparation in allergen-specific epicutaneous immunotherapy. J Allergy Clin Immunol. 2014;134:965–967.e4. doi: 10.1016/j.jaci.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 57.Senti G, Prinz Vavricka BM, Erdmann I, Diaz MI, Markus R, McCormack SJ, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105:17908–17912. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Senti G, von Moos S, Tay F, Graf N, Sonderegger T, Johansen P, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: a double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–135. doi: 10.1016/j.jaci.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 59.Hylander T, Latif L, Petersson-Westin U, Cardell LO. Intralymphatic allergen-specific immunotherapy: an effective and safe alternative treatment route for pollen-induced allergic rhinitis. J Allergy Clin Immunol. 2013;131:412–420. doi: 10.1016/j.jaci.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 60.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]