Abstract
Purpose
Recent experimental evidence shows that extracellular vesicles (EVs) in indoor dust induce neurtrophilic pulmonary inflammation, which is a characteristic pathology in patients with severe asthma and chronic obstructive pulmonary disease (COPD). In addition, COPD is known to be an important risk factor for lung cancer, irrespective of cigarette smoking. Here, we evaluated whether sensitization to indoor dust EVs is a risk for the development of asthma, COPD, or lung cancer.
Methods
Serum IgG antibodies against dust EVs were measured in 90 healthy control subjects, 294 asthmatics, 242 COPD patients, and 325 lung cancer patients. Serum anti-dust EV IgG titers were considered high if they exceeded a 95 percentile value of the control subjects. Age-, gender-, and cigarette smoke-adjusted multiple logistic regression analyses were performed to determine odds ratios (ORs) for asthma, COPD, and lung cancer patients vs the control subjects.
Results
In total, 4.4%, 13.6%, 29.3%, and 54.9% of the control, asthma, COPD, and lung cancer groups, respectively, had high serum anti-dust EV IgG titers. Adjusted multiple logistic regression revealed that sensitization to dust EVs (high serum anti-dust EV IgG titer) was an independent risk factor for asthma (adjusted OR, 3.3; 95% confidence interval [CI], 1.1-10.0), COPD (adjusted OR, 8.0; 95% CI, 2.0-32.5) and lung cancer (adjusted OR, 38.7; 95% CI, 10.4-144.3).
Conclusions
IgG sensitization to indoor dust EVs appears to be a major risk for the development of asthma, COPD, and lung cancer.
Keywords: Extracellular vesicles, IgG sensitization, indoor dust, asthma, COPD, lung cancer
INTRODUCTION
Asthma, chronic obstructive pulmonary disease (COPD), and lung cancer are major diseases that impose high burden and mortality worldwide.1,2 Biological contaminants in indoor air can induce immune dysfunction and inflammation, resulting in inflammatory pulmonary disorders, such as asthma and COPD.3,4 Mild and moderate asthma are related to eosinophilic inflammation, whereas severe asthma and COPD are associated with neutrophilic inflammation.5,6 Cluster analysis to identify asthma subtypes has shown that neutrophilic severe asthma is characterized by fixed airway obstruction, whereas the eosinophilic subtype is reversible.7,8 COPD and lung cancer have been suggested to share a common cause, namely, cigarette smoking. However, some patients with COPD or lung cancer have no history of exposure to cigarette smoking. In such cases, COPD or lung cancer may be caused by agents other than cigarette smoke, such as occupational exposure to gas or dust, indoor exposure to biomass fuel combustion, or exposure to an unknown etiological agent.9
During recent several decades, the prevalence of asthma and COPD has been increasing, and this increase may be related with the change of housing styles, which results in increased biological contaminants in indoor environments.10,11,12 Although as yet poorly researched, possible etiological agents may be biological ones in indoor dust. Indoor dust contains extracellular vesicles (EVs) that originate from microorganisms. Bacteria-derived EVs are spherical-shaped, nanometer-sized, lipid-bilayered nanoparticles, which are produced by gram-negative and some gram-positive bacteria.13,14 Animal experiments have shown that EVs from indoor dust can induce neutrophilic pulmonary inflammation15 and that airway exposure to bacteria-derived EVs, which are present in indoor dust, can also induce neutrophilic inflammation and subsequently emphysema in the lung.16,17 Asthma and COPD (with or without emphysema) are characterized by chronic inflammation in the airways.18 Cancer has also been associated with chronic inflammation.19 This evidence led us to the notion that exposure to EVs in indoor dust (dust EVs) may increase the risk of asthma, COPD, and lung cancer. We performed a cross-sectional association study to determine whether serum antibodies against dust EVs associate with the increased risk of asthma, COPD, and lung cancer.
MATERIALS AND METHODS
Ethics approval
The study was approved by the Research Ethics Committee of Asan Medical Center (approval number, IRB 2014-0360) and of Dankook University Hospital (approval number, IRG 2012-04-0140). Each study participant provided informed consent.
Study subjects
The study subjects were divided into 4 groups. The first group consisted of 294 patients who visited Asan Medical Center (Seoul) and were diagnosed with asthma by physicians on the basis of age (>40 years) and reversible airway obstruction (post-bronchodilator or post-treatment FEV1 increase >15% of baseline value), irrespective of cigarette smoking history. The second group consisted of 242 patients who visited Asan Medical Center and were diagnosed with COPD by physicians on the basis of age (>40 years) and irreversible airway obstruction (post-bronchodilator FEV1/FVC <0.7), regardless of cigarette smoking history. The third group consisted of 325 patients with lung cancer who were enrolled at a referral hospital (Dankook University Hospital) in Cheonan, on the basis of the histologic confirmation of lung cancer. The fourth group consisted of 90 healthy control subjects with no respiratory disease on the basis of medical checkup at Asan Health Screening and Promotion Center in Seoul.
Clinical phenotyping
To evaluate atopy, skin prick testing was performed with 55 common inhalant allergens (Allergopharma, Reinbeck, Germany) as previously described.20 Atopy was defined as a positive skin prick test response (allergen/histamine ratio >1 and mean wheal size >4 mm) to 1 or more allergens. To evaluate reversible airway obstruction, FEV1 was measured by spirometry. A positive reversible airway obstruction was defined as an FEV1 increase of >15% after bronchodilator or anti-asthma treatment. COPD severity was evaluated by the FFV1 value; the mean FEV1 of the 242 COPD patients enrolled was 50% of predicted value (standard deviation, 16%). COPD patients were classified as mild, moderate, or severe according to COPD severity as defined by using the FEV1 tertile values (57% and 41% of predicted value).
Measurement of serum anti-dust EV antibodies
Dust samples, collected from the bed mattress of 2 apartment houses in Seoul, were sieved through a gauze and centrifuged twice at 10,000 g for 15 minutes. Dust EVs were collected from a supernatant fraction using the filtration and ultracentrifugation method as previously described.21 To measure anti-dust EV IgG titers in serum samples, 50 ng of dust EVs were coated in 96-well plates overnight. To quantify anti-dust EV IgG titers, anti-human IgG antibody were coated instead of dust EVs. On the next day, dust EV-coated wells were blocked with PBS containing 5% skim milk and were added by sera samples diluted 1:1,000 with 5% skim milk. Horse radish peroxidase-conjugated anti-IgG (Abcam, Cambridge, UK) served as a detection antibody and were measured by using a microplate reader.
Definition of a high anti-dust EV IgG antibody threshold
IgG sensitization to dust EVs was defined on an arbitrary basis as a high anti-dust EV IgG in serum if it exceeded the 95 percentile value of the control subjects.
Statistical analysis
Group means were compared by using t tests for 2 groups, and 1-way ANOVA for more than 2 groups. The groups were compared in terms of categorical variables by using the chi-square test. Whether a variable had a normal distribution was tested using the Kolmogorov-Smirnov test. To identify variables associated independently with asthma, COPD and lung cancer, multiple logistic regression analysis was performed after adjustment for age, gender, and cigarette smoking history. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). A P value of less than 0.05 was considered statistically significant. All analyses were performed by using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
General characteristics of the study groups and their high anti-dust EV IgG levels in serum
A total of 90 control subjects, 294 asthmatics, 242 COPD patients, and 325 lung cancer patients were enrolled as shown in Table 1. Compared to the control subjects, COPD and lung cancer patients had a higher mean age (both P<0.001), whereas asthmatic patients had similar ages. COPD and lung cancer patients were more likely to be male (both P<0.001) and to smoke (both P<0.001), whereas asthmatic patients were less likely to be male (P<0.01) and to smoke (P<0.001 vs the control group).
Table 1. Chacteristics of the study subjects.
| Subjects | ||||
|---|---|---|---|---|
| Control | Asthma | COPD | Lung cancer | |
| Subjects No. | 90 | 294 | 242 | 325 |
| Age (year)* | 51±10 | 55±15 | 66±7 | 66±9 |
| Male (%) | 57 | 42 | 88 | 84 |
| Cigarette smoker (%) | 52 | 27 | 85 | 75 |
| Anti-EV IgG† (%) | 4.4 | 13.6 | 29.3 | 54.9 |
*Mean±SD; †The positive rate of IgG sensitization to dust EVs based on a high anti-dust EV IgG level in serum.
Serum anti-dust EV IgG levels
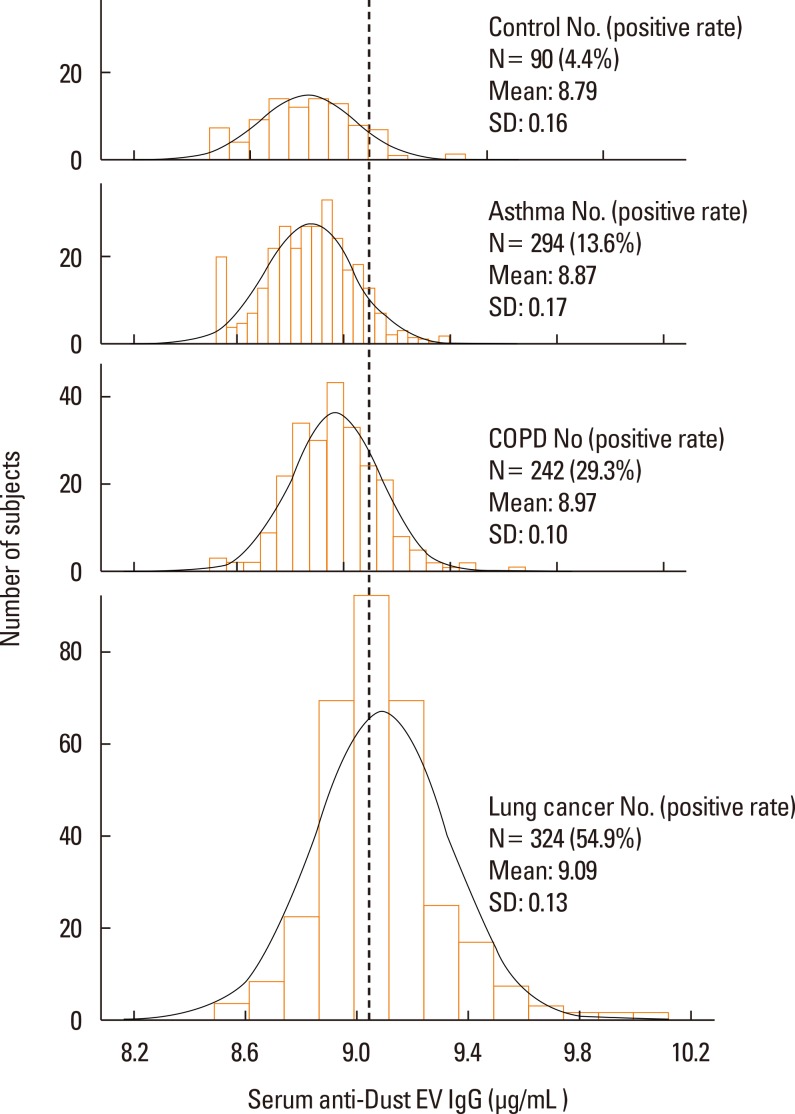
Serum anti-dust EV IgG concentrations of the control group had a normal (Gaussian) distribution (Fig. 1). Subgroup analyses revealed that serum anti-dust EV concentrations did not differ among the age subgroups of the control group This was also true when males and females, or smokers and non-smokers in the control group were compared (Fig. 2). Serum anti-dust EV IgG levels in the asthma, COPD, and lung cancer groups also had a normal distribution (Fig. 1). The positive rate of IgG sensitization to dust EVs was 13.6% in the asthma group, 29.3% in the COPD group, and 54.9% in the lung cancer group, whereas 4.4% in the control group (Table 1 and Fig. 1). Compared to the control subjects, asthmatic, COPD, and lung cancer patients significantly had a high anti-EV IgG antibody in serum (all P<0.001, Table 1 and Fig. 1).
Fig. 1. The distribution of serum anti-dust EV IgG concentrations in the healthy control, asthma, COPD, and lung cancer groups. The dashed vertical line indicates the cutoff value that represents high serum anti-dust EV IgG levels. This cutoff value was defined as the 95 percentile value of the control subjects. The histograms are presented with normal distribution curves. Serum anti-EV: the concentration of serum IgG reactive to EVs in indoor dust.
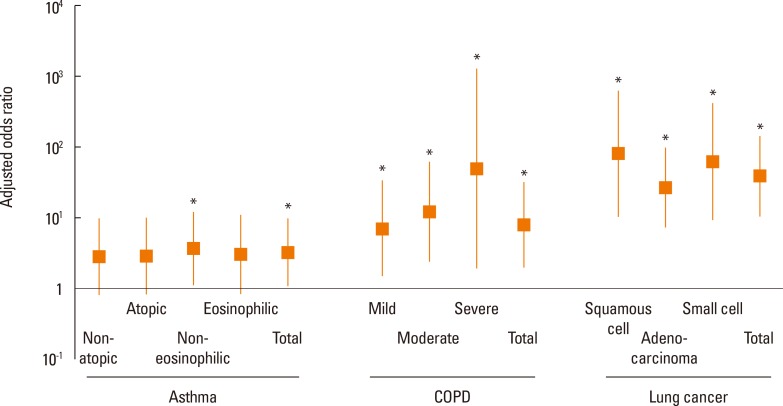
Fig. 2. Adjusted odds ratios showing the relationship between high serum anti-dust EV IgG levels and the risk of asthma subtypes/COPD severity/lung cancer subtypes. Multiple linear logistic regression analyses after adjustment for age, gender, and cigarette smoking history were performed on 294 asthma, 242 COPD, and 325 lung cancer patients vs 90 control subjects. Asthma was divided into non-atopic and atopic asthma according to positive skin prick test responses to common inhalant allergens, and into non-eosinophilic and eosinophilic asthma according to sputum eosinophil percentage (3%). COPD were divided into into tertile subgroups according to lung function. Lung cancer was classified as main cellular subtypes, namely, squamous cell carinoma, adenocarcinoma, and small cell lung cancer. Serum anti-dust EV IgG levels were deemed to be high when they exceeded the 95 percentile value of the control subjects. *P<0.05 vs the control group.
A high serum anti-dust EV IgG level as a potential risk factor for asthma
Multiple logistic regression analysis was performed to evaluate risk factors for asthma after adjustment for age, gender, cigarette smoking history, and dust EV sensitization (Table 2). This evaluation revealed that adjusted ORs for asthma were 1.03 (95% CI 1.01-1.05, P<0.01) for every 1-year increase in age, 0.7 (95% CI 0.3-1.5, P>0.05) for female gender, 0.3 (95% CI 0.1-0.6, P=0.001) for cigarette smoking history, and 3.3 (95% CI 1.1-10.0, P<0.05) for dust EV sensitization. This finding indicates that, in addition to aging, IgG sensitization to dust EVs may be an independent risk factor for asthma, whereas cigarette smoking is a protective factor for asthma.
Table 2. Association of IgG sensitization to dust EVs with asthma, COPD, and lung cancer by multivariable analysis.
| Asthma | COPD | Lung cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value† | Odds ratio | 95% CI | P value† | Odds ratio | 95% CI | P value† | |
| Aging | 1.03 | 1.01-1.05 | 0.002 | 1.2 | 1.1-1.2 | <0.001 | 1.2 | 1.1-1.2 | <0.001 |
| Female gender | 0.7 | 0.3-1.5 | 0.352 | 1.0 | 0.3-2.8 | 0.93 | 0.7 | 0.3-1.8 | 0.45 |
| Cigarette smoking | 0.3 | 0.1-0.6 | 0.001 | 3.7 | 1.4-10.1 | 0.01 | 2.7 | 1.1-7.0 | 0.03 |
| Anti-EV IgG* | 3.3 | 1.1-10.0 | 0.039 | 8.0 | 2.0-3.25 | 0.003 | 38.7 | 10.4-144.3 | <0.001 |
*The positive rate of IgG sensitization to dust EVs, based on a high anti-dust EV IgG level in serum; †Logistic regression analysis vs the control group.
A high serum anti-dust EV IgG level as a potential risk factor for COPD
Multiple logistic regression analysis was performed to evaluate risk factors for COPD after adjustment for age, gender, cigarette smoking history, and dust EV sensitization (Table 2). This study showed that adjusted ORs for COPD were 1.2 (95% CI 1.1-1.2, P<0.001) for every 1-year increase in age, 1.0 (95% CI 0.3-2.8, P>0.05) for female gender, 3.7 (95% CI 1.4-10.1, P=0.01) for cigarette smoking history and 8.0 (95% CI 2.0-32.5, P=0.003) for dust EV sensitization. This finding indicates that, in addition to aging and cigarette smoking, IgG sensitization to dust EVs is an independent risk factor for COPD.
A high serum anti-dust EV IgG level as a potential risk factor for lung cancer
Multiple logistic regression analysis was performed to evaluate risk factors for lung cancer (Table 2). This study showed that adjusted ORs for lung cancer after adjustment for age, gender, cigarette smoking history, and dust EV sensitization were 1.2 (95% CI 1.1-1.2, P<0.001) for every 1-year increase in age, 0.7 (95% CI 0.3-1.8, P>0.05) for female gender, 2.7 (95% CI 1.1-7.0, P=0.03) for cigarette smoking history, and 38.7 (95% CI 10.4-144.3, P<0.001) for dust EV sensitization. This finding indicates that, in addition to aging and cigarette smoking, IgG sensitization to dust EVs may be an independent major risk factor for lung cancer.
Association between high serum anti-dust EV IgG levels and asthma subtypes
Asthma subtypes were categorized as non-atopic and atopic asthma based on skin prick test responses to common inhalant allergens, and non-eosinophilic and eosinophilic asthma based on the 3% cutoff value of sputum eosinophil percentage. When we compared between non-atopic and atopic asthma using the t test, the mean values of serum anti-dust EV IgG in serum from nonatopic and atopic asthma were 8.86 and 8.87, respectively, and there was no significant difference in anti-dust EV IgG levels between the 2 subtypes (P=0.952) (Table 3). When non-eosinopilic asthma was compared to eosinophilic asthma 1, the mean values of serum anti-dust EV IgG were 8.89 and 8.86 from the 2 subtypes, respectively, and there is no significant difference in anti-dust EV IgG levels between the 2 subtypes (P=0.556) (Table 3). When multiple logistic regression analysis was performed after adjustment for age, gender, and cigarette smoking history in each of the 4 asthma subtypes vs the control group, a high serum anti-dust EV IgG level was found to be an independent risk factor for non-eosinophilic asthma (Fig. 2 and Table 4). Adjusted ORs for the non-atopic, atopic, non-eosinophilic, and eosinophilic asthma subgroups vs the control group were 2.8 (95% CI 0.8-9.6, P=0.1), 2.9 (95% CI 0.9-9.8, P=0.08), 3.7 (95% CI 1.1-12.3, P=0.03), and 3.1 (95% CI 0.8-11.4, P=0.10), respectively.
Table 3. Association of IgG sensitization to dust EVs with asthma subtypes.
| Non-atopic (n=131) | Atopic (n=100) | P value | Non-eosinophilic† (n=132) | Eosinophilic (n=117) | P value | |
|---|---|---|---|---|---|---|
| Anti-EV IgG (µg/mL)* | 8.86±0.18 | 8.87±0.18 | 0.952 | 8.89±0.17 | 8.86±0.16 | 0.556 |
*Mean±SD; †The percentage of eosinophils in sputum <3%.
Table 4. Risk of IgG sensitization to dust EVs to asthma subtype expression.
| Non-atopic (n=115) | Atopic (n=87) | Non-eosinophilic‡ (n=115) | Eosinophilic (n=104) | |||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value† | Odds ratio (95% CI) | P value† | Odds ratio (95% CI) | P value† | Odds ratio (95% CI) | P value† | |
| Aging | 1.1 (1.0-1.1) | <0.001 | 1.0 (1.0-1.0) | 0.27 | 1.0 (1.0-1.0) | 0.01 | 1.0 (1.0-1.0) | 0.01 |
| Female gender | 1.8 (0.7-4.6) | 0.25 | 0.5 (0.2-2.2) | 0.14 | 0.8 (0.3-1.8) | 0.53 | 0.6 (0.2-1.4) | 0.20 |
| Cigarette smoking | 0.6 (0.2-1.6) | 0.29 | 0.3 (0.1-0.6) | <0.001 | 0.3 (0.1-0.8) | 0.01 | 0.2 (0.1-0.6) | <0.001 |
| Anti-EV IgG* | 2.8 (0.8-9.6) | 0.1 | 2.9 (0.9-9.8) | 0.08 | 3.7 (1.1-12.3) | 0.03 | 3.1 (0.8-11.4) | 0.10 |
*High serum IgG against dust EVs; †Linear logistic regression analysis vs the control group; ‡The percentage of eosinophils in sputum < 3%.
Association between high serum anti-dust EV IgG levels and COPD severity
The COPD patients enrolled were classified as mild (n=80), moderate (n=82), or severe (n=80) COPD according to COPD severity as defined by using the FEV1 tertile values (57% and 41% of predicted normal value). The mean values of serum anti-dust EV IgG were 8.95, 8.86, and 9.01 in mild, moderate and severe COPD subtypes, respectively, and there were no significant differences in anti-dust EV IgG levels among the 3 subtypes (P=0.065 using the 1-way ANOVA test) (Table 5). When multiple logistic regression analysis was performed after adjustment for age, gender, and cigarette smoking history in each of the 3 COPD tertile subgroups vs the control group, a high anti-dust EV IgG level in serum was found to be an independent risk factor for each of the 3 COPD subgroups (Fig. 2 and Table 6). Adjusted ORs for mild, moderate, and severe COPD subgroups vs the control group were 7.1 (95% CI 1.5-33.4, P=0.01), 12.2 (95% CI 2.4-61.5, P=0.002), and 50.3 (95% CI 1.9-1316.0, P=0.02), respectively.
Table 5. Association of IgG sensitization to dust EVs with COPD severity.
| Mild (n=80) |
Moderate (n=82) |
Severe (n=80) |
P value | |
|---|---|---|---|---|
| Anti-EV IgG (µg/mL)* | 8.95±0.19 | 8.86±0.15 | 9.01±0.16 | 0.065 |
*Mean±SD.
Table 6. Risk of IgG sensitization to dust EVs to the expression of COPD severity.
| Mild (n=80) | Moderate (n=82) | Severe (n=80) | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) |
P value† | Odds ratio (95% CI) |
P value† | Odds ratio (95% CI) |
P value† | |
| Aging | 1.2 (1.1-1.2) | <0.001 | 1.2 (1.1-1.2) | <0.001 | 1.2 (1.1-1.3) | <0.001 |
| Female gender | 0.6 (0.2-2.1) | 0.44 | 1.8 (0.4-7.6) | 0.45 | 0.3 (0.03-3.1) | 0.31 |
| Cigarette smoking | 1.6 (0.5-4.9) | 0.42 | 8.9 (2.2-36.2) | 0.002 | 119.0 (5.9-2,389.8) | 0.002 |
| Anti-dust EV IgG* | 7.1 (1.5-33.4) | 0.01 | 12.2 (2.4-61.5) | 0.002 | 50.3 (1.9-1,316.0) | 0.02 |
*High serum IgG against dust EVs; †Linear logistic regression analysis vs the control group.
Association between high anti-dust EV levels and cellular subtypes of lung cancer
The cellular subtypes of 325 lung cancer patients were squamous cell carcinoma (n=94), adenocarcinoma carcinoma (n=90), small cell carcinoma (n=70), adenosquamous carcinoma (n=6), large cell carcinoma (n=4), and non-small cell carcinoma without any specific cellular subtype (n=60). The mean values of serum anti-dust EV IgG were 9.07, 9.07, and 9.15 in the subtypes squamous cell carcinoma, adenocarcinoma, and small cell carcinoma, respectively, but there were no significant differences in anti-dust EV IgG levels among the subtypes (P=0.082, by the 1-way ANOVA test) (Table 7). Multiple logistic regression analysis was performed after adjustment for age, gender, and cigarette smoking history in subjects with squamous cell carcinoma, adenocarcinoma, and small cell lung carcinoma vs the control subjects. This evaluation showed that a high serum anti-dust EV IgG level was an independent risk factor for all the 3 lung cancer subtypes (Fig. 2 and Table 8). Adjusted ORs for the subtypes squamous cell carcinoma, adenocarcinoma, and small carcinoma vs the control group were 81.1 (95% CI 10.4-631.2, P<0.001), 27.2 (95% CI 7.4-99.7, P<0.001), and 61.6 (95% CI 9.0-421.6, P<0.001), respectively.
Table 7. Association of IgG sensitization to dust EVs with lung cancer subtypes.
| Squamous cell (n=94) |
Adenocarcinoma (n=90) |
Small cell (n=70) |
P value | |
|---|---|---|---|---|
| Anti-EV IgG (µg/mL)* | 9.07±0.18 | 9.07±0.19 | 9.15±0.34 | 0.082 |
*Mean±SD.
Table 8. Risk of IgG sensitization to dust EVs to lung cancer subtype expression.
| Squamous cell (n=94) | Adenocarcinoma (n=90) | Small cell (n=70) | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) |
P value† | Odds ratio (95% CI) |
P value† | Odds ratio (95% CI) |
P value† | |
| Aging | 1.2 (1.1-1.3) | <0.001 | 1.2 (1.1-1.2) | <0.001 | 1.2 (1.1-1.3) | <0.001 |
| Female gender | 0.1 (0.0-0.8) | 0.03 | 1.8 (0.5-6.8) | 0.37 | 0.3 (0.1-1.5) | 0.15 |
| Cigarette smoking | 6.5 (1.5-29.0) | 0.01 | 2.8 (0.7-10.1) | 0.13 | 9.1 (2.0-41.3) | 0.004 |
| Anti-EV IgG (+)* | 81.1 (10.4-631.2) | <0.001 | 27.2 (7.4-99.7) | <0.001 | 61.6 (9.0-421.6) | <0.001 |
*High serum IgG against dust EVs; †Linear logistic regression analysis vs the control group.
DISCUSSION
The current study showed that the serum anti-dust EV IgG levels were significantly higher in patients with asthma, COPD, or lung cancer than in the control subjects. Moreover, a high serum anti-dust EV IgG level was found to be an independent risk factor for asthma, especially non-eosinophilic asthma, COPD with mild, moderate, or severe severity, and lung cancer, irrespective of cellular subtypes, after adjustment for age, gender, and cigarette smoking history. Thus, IgG sensitization to dust EVs may increase the risk of non-eosinophilic asthma, COPD, and lung cancer expression and/or development.
The 2014 Global Strategy for Asthma Management and Prevention defines asthma as a heterogeneous disease, usually characterized by chronic airway inflammation. Recent evidence suggests that non-eosinophilic asthma represents a stable phenotype associated with distinct lower airway pathology and structure.22 Specifically, patients are observed to have severe and persistent asthma in the absence of eosinophilic inflammation, and may experience an exacerbation of asthma symptoms without an increase in eosinophilic inflammation.23 In addition, persistent asthma may be associated with the presence of significant neutrophilic inflammation.24 Our previous data also showed that dust EVs induce neutrophilic inflammation in the airways.21 The present data revealed that IgG sensitization to dust EVs is associated with the increased risk of asthma, especially non-eosinophilic asthma. Considering that lower airway inflammation in non-eosinophilic asthma develops in response to etiologic factors acting through immune responses other than inhalant allergens,22 dust EVs might be an important etiological agent of non-eosinophilic asthma.
To the best of our knowledge, there have been few studies directly assessing the relationship between exposure to dust EVs and lung cancer. However, COPD is known to be the risk factor of lung cancer,18 and there is evidence to support that chronic inflammation may promote the development of cancer.19,25 Moreover, asthma is also suggested to be a risk factor for lung cancer development in subjects with never-smoking history. Indeed, a recent meta-analysis showed a 1.8-fold increase in lung cancer risk among asthmatics.26 In addition, an epidemiologic study revealed a 1.82-fold risk for lung cancer development in asthmatic patients vs healthy subjects.27 Thus, we postulated that exposure to dust EVs could evoke neutrophilic pulmonary inflammation, which in turn may promote the development of lung cancer. This possibility is supported by the observation that carcinogenesis in organs other than the lung may be promoted by microorganism-induced chronic inflammation.28 For example, it has been proven that Helicobacter pylori causes chronic gastritis and possibly gastric cancer.
A standardized method for evaluating chronic exposure to dust EVs has not yet been established. In our previous study, we used anti-dust EV IgG antibody as a surrogate marker for dust EV exposure. That study showed that children with atopic asthma have higher serum anti-dust EV IgG levels than age-matched atopic children with rhinitis or dermatitis.21 Haneberg et al.29 measured serum antibodies specific for meningococcal EVs and confirmed that vaccination with meningococcal EVs induces an effective immune response. They measured anti-meningococcal EV antibodies by ELISA in serum samples incubated in EV-coated 96-well plates. Their findings support that our present method for measuring anti-dust EV antibodies is valid.
The present study has some limitations. First, we were not able to confirm a causal relationship between exposure to dust EVs and the development of asthma, COPD, or lung cancer because of the cross-sectional design of the present study. To confirm such a causal relationship, a cohort study will be needed. Although an animal study has shown that dust EVs induce neutrophilic inflammation in the lung,30 the degree of exposure to dust EVs would have to be measured in a proposed cohort study to ensure that higher exposure to dust EVs increases the risk for developing asthma, COPD, or lung cancer and that the anti-dust EV IgG level is an appropriate surrogate for dust EV exposure. Second, in the present study, the control subjects were younger than those with COPD or lung cancer; they were also more likely to be female and non-smokers. However, these differences may insignificantly have affected our results because subgroup analysis of the control subjects revealed that serum anti-dust EV IgG levels did not differ between different age, males and females, or smokers and non-smokers. Moreover, our multivariate analysis revealed that a high anti-dust EV IgG level in serum remained an independent risk for COPD and lung cancer, after adjustment for age, gender, and cigarette smoking history. Third, we did not evaluate risk factors that may promote the development of COPD and lung cancer, such as occupational exposure to gas/dust or second-hand exposure to smoke. Fourth, the age- and gender-adjusted ORs of cigarette smoking for lung cancer were lower in the current study than in previous studies. This may reflect the characteristics of recruited subjects in the present study. In particular, the control subjects were on average 15 years younger than the recruited subjects with COPD or lung cancer. Thus, it is possible that, in case 50% of the control subjects were smokers, they may have developed COPD or lung cancer at a later age. In other words, some of the control subjects may have been erroneously included in the control group because they may actually develop COPD or lung cancer in next 15 or more years. The lower OR in the lung cancer group in the current study vs other publications may also be supported by a recent Korean study, which showed that the risk of cigarette smoking for lung cancer may not be as high as previously reported: the adjusted relative risks for lung cancer in subjects who smoked 11-15, 16-20, 21-34, and 35 or more pack-years were 1.99, 3.16, 3.20, and 8.55, respectively.31 In the future, prospectively designed studies will be needed to evaluate the precise role of cigarette smoking in the development of lung cancer, based on the evidence that bacteria-derived EVs in indoor dust induce neutrophilic pulumonary inflammation and emphysema.17,21
In conclusion, the present study showed that anti-dust EV IgG antibody titers in serum were significantly higher in patients with non-eosinophilic asthma, COPD, or lung cancer than in healthy control subjects. Multivariable analysis showed that a high serum anti-dust EV IgG concentration was an independent risk factor for non-eosinophilic asthma, COPD, and lung cancer, after adjustment for age, gender, and cigarette smoking. Additional studies of cohort design will be needed to determine whether dust EV exposure has a causal relationship with asthma, COPD, and lung cancer. Nevertheless, the present findings provide an insight into the pathogenesis of non-eosinophilic asthma, COPD, and lung cancer, as well as a clue to developing novel diagnostic and/or therapeutic modalities.
ACKNOWLEDGMENTS
We thank the members of the KOLD Study Groups for providing the KOLD Cohort data and samples. This study was supported by grants from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI14C2628 and HI10C2020), a grant from Asan Institute for Life Sciences (14-306), and a grant from the National Research Foundation of Korea Grant funded by the Korean Government (No. 2011-0000879).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, et al. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007;178:5375–5382. doi: 10.4049/jimmunol.178.8.5375. [DOI] [PubMed] [Google Scholar]
- 4.Jeon SG, Oh SY, Park HK, Kim YS, Shim EJ, Lee HS, et al. TH2 and TH1 lung inflammation induced by airway allergen sensitization with low and high doses of double-stranded RNA. J Allergy Clin Immunol. 2007;120:803–812. doi: 10.1016/j.jaci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Kim YM, Kim YS, Jeon SG, Kim YK. Immunopathogenesis of allergic asthma: more than the th2 hypothesis. Allergy Asthma Immunol Res. 2013;5:189–196. doi: 10.4168/aair.2013.5.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang AS, Kim SH, Kim TB, Park HW, Kim SH, Chang YS, et al. Impact of atopy on asthma and allergic rhinitis in the cohort for reality and evolution of adult asthma in Korea. Allergy Asthma Immunol Res. 2013;5:143–149. doi: 10.4168/aair.2013.5.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 10.Shendell DG, Mizan SS, Yamamoto N, Peccia J. Associations between quantitative measures of fungi in home floor dust and lung function among older adults with chronic respiratory disease: a pilot study. J Asthma. 2012;49:502–509. doi: 10.3109/02770903.2012.682633. [DOI] [PubMed] [Google Scholar]
- 11.Hansel NN, McCormack MC, Belli AJ, Matsui EC, Peng RD, Aloe C, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:1085–1090. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husman T. Health effects of indoor-air microorganisms. Scand J Work Environ Health. 1996;22:5–13. doi: 10.5271/sjweh.103. [DOI] [PubMed] [Google Scholar]
- 13.Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics. 2007;7:3143–3153. doi: 10.1002/pmic.200700196. [DOI] [PubMed] [Google Scholar]
- 14.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 15.Kim MR, Hong SW, Choi EB, Lee WH, Kim YS, Jeon SG, et al. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses. Allergy. 2012;67:1271–1281. doi: 10.1111/all.12001. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Lee EY, Kim SH, Kim DK, Park KS, Kim KP, et al. Staphylococcus aureus extracellular vesicles carry biologically active beta-lactamase. Antimicrob Agents Chemother. 2013;57:2589–2595. doi: 10.1128/AAC.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YS, Lee WH, Choi EJ, Choi JP, Heo YJ, Gho YS, et al. Extracellular vesicles derived from Gram-negative bacteria, such as Escherichia coli, induce emphysema mainly via IL-17A-mediated neutrophilic inflammation. J Immunol. 2015;194:3361–3368. doi: 10.4049/jimmunol.1402268. [DOI] [PubMed] [Google Scholar]
- 18.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YK, Cho SH, Koh YY, Son JW, Jee YK, Lee MH, et al. Skin reactivity to inhalant allergens, total serum IgE levels, and bronchial responsiveness to methacholine are increased in parents of nonatopic asthmatic children. J Allergy Clin Immunol. 1999;104:311–316. doi: 10.1016/s0091-6749(99)70372-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, et al. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy. 2013;43:443–454. doi: 10.1111/cea.12085. [DOI] [PubMed] [Google Scholar]
- 22.Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol. 2007;119:1043–1052. doi: 10.1016/j.jaci.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Turner MO, Hussack P, Sears MR, Dolovich J, Hargreave FE. Exacerbations of asthma without sputum eosinophilia. Thorax. 1995;50:1057–1061. doi: 10.1136/thx.50.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 25.Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9:375–383. doi: 10.1016/j.coph.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santillan AA, Camargo CA, Jr, Colditz GA. A meta-analysis of asthma and risk of lung cancer (United States) Cancer Causes Control. 2003;14:327–334. doi: 10.1023/a:1023982402137. [DOI] [PubMed] [Google Scholar]
- 27.Gorlova OY, Zhang Y, Schabath MB, Lei L, Zhang Q, Amos CI, et al. Never smokers and lung cancer risk: a case-control study of epidemiological factors. Int J Cancer. 2006;118:1798–1804. doi: 10.1002/ijc.21561. [DOI] [PubMed] [Google Scholar]
- 28.Qadri Q, Rasool R, Gulzar GM, Naqash S, Shah ZA. H. pylori infection, inflammation and gastric cancer. J Gastrointest Cancer. 2014;45:126–132. doi: 10.1007/s12029-014-9583-1. [DOI] [PubMed] [Google Scholar]
- 29.Haneberg B, Dalseg R, Wedege E, Høiby EA, Haugen IL, Oftung F, et al. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect Immun. 1998;66:1334–1341. doi: 10.1128/iai.66.4.1334-1341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi DS, Yang JS, Choi EJ, Jang SC, Park S, Kim OY, et al. The protein interaction network of extracellular vesicles derived from human colorectal cancer cells. J Proteome Res. 2012;11:1144–1151. doi: 10.1021/pr200842h. [DOI] [PubMed] [Google Scholar]
- 31.Bae JM, Li ZM, Shin MH, Kim DH, Lee MS, Ahn YO. Lung cancer incidence by smoking status in Korean men: 16-years of observations in the Seoul Male Cancer Cohort study. J Korean Med Sci. 2013;28:636–637. doi: 10.3346/jkms.2013.28.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]