Abstract
Purpose
Two mouse strains, BALB/c and C3H/HeOuJ, broadly used in the field of food allergy, were compared for the evaluation of the allergenic potential of ovalbumin (OVA).
Methods
Sensitization was made by administering 2 different OVA doses (1 and 5 mg), with cholera toxin as Th2-polarizing adjuvant. Antibody levels, severity of anaphylaxis, and Th1 and Th2 responses induced by the allergen were assessed. In addition, because the mice selected had functional toll-like receptor 4, the influence of contamination with lipopolysaccharide (LPS) on the immunostimulating capacity of OVA on spleen cells was also evaluated.
Results
Both strains exhibited similar susceptibility to OVA sensitization. The 2 protein doses generated similar OVA-specific IgE and IgG1 levels in both strains, whereas C3H/HeOuJ mice produced significantly more IgG2a. Oral challenge provoked more severe manifestations in C3H/HeOuJ mice as indicated by the drop in body temperature and the severity of the anaphylactic scores. Stimulation of splenocytes with OVA led to significantly higher levels of Th2 and Th1 cytokines in BALB/c, and these were less affected by protein contamination with LPS.
Conclusions
The antibody and cytokine levels induced by OVA in BALB/c mice and the observation that BALB/c spleen cell cultures were more resistant than those of C3H/HeOuJ mice to the stimulus of LPS make this strain prone to exhibit Th2-mediated food allergic reactions and very adequate for the study of the features of OVA that make it allergenic.
Keywords: Allergy, BALB/c, C3H/HeOuJ, cytokines, ovalbumin, lipopolysaccharide
INTRODUCTION
Murine models are broadly used in the field of food allergy to ascertain etiology, mechanisms, and preventive or therapeutic strategies through studies which would otherwise not be possible in human patients.1 Induction of oral sensitization to food proteins in mice requires the use of adjuvants, such as cholera toxin (CT) or staphylococcal enterotoxin B (SB), to overcome their strong tendency to develop oral tolerance by promoting Th2-polarized immune responses over Th1 responses, which produce antigen-specific IgE.2 Subsequent oral challenge with the food or allergen can cause gastrointestinal or systemic signs, such as diarrhea and shock syndrome, respectively.3
Two main mouse strains with well-defined genetic backgrounds: BALB/c and C3H have been applied to stablish the induction and effector mechanisms of common food allergens. In addition, there are available congenic mice of both strains carrying a mutation in toll-like receptor 4 (TLR4), which makes them insensitive to lipopolysaccharide (LPS), and thus, to the influence of gram-negative bacteria in the gastrointestinal tract.4 Studies conducted with these animal models have allowed testing experimentally the intrinsic properties of proteins that promote oral sensitization, the differential capacity of allergens to trigger the manifestations of food allergy and the influence of the food matrix and processing in their allergenic potential.5,6,7
However, recognition of proteins as immunogens is strain-dependent, leading to IgE or IgG-mediated responses.8 In fact, there are 2 different pathways of systemic murine anaphylaxis whose relative importance also depends on the route of administration and on the characteristics and amounts of antigen used to induce the antibody response and the anaphylactic reaction.3 Furthermore, several studies have documented that susceptibility of mice to orally induced anaphylaxis varies with the genetic background.9 In this respect, it should be taken into account that the presence of a functional LPS receptor does not correlate with the predisposition to sensitization or the severity of anaphylaxis, which in turn depend greatly on the allergen used.4 Therefore, previous knowledge underlines the need for selecting the most appropriate mouse strain for accurate estimation of the sensitizing and eliciting capacity of a particular allergen.
Ovalbumin (OVA, Gal d 2) is the most abundant protein in egg white and one of its major allergens.10 The importance of OVA stems not only from the high prevalence of egg allergy, the second more frequent food allergy in children below the age of 3, which affects up to 1.7% of children and adults,11 but also because OVA is normally used as a model protein to investigate the molecular and cellular mechanisms of allergic sensitization and tolerance.12,13,14,15
The aim of the present study was to compare the utility of 2 mouse strains: BALB/c and C3H/HeOuJ for the evaluation of the allergenic potential of OVA. For this purpose, IgE, IgG1, and IgG2a antibody levels, severity of anaphylaxis, and Th1 and Th2 responses induced by OVA were assessed. In addition, because the mice selected had functional TLR4, we investigated the influence of LPS contamination on the immunostimulating capacity of OVA using spleen cell cultures from naïve and sensitized mice of both strains.
MATERIAL AND METHODS
Mice and proteins
Five-week-old female specific-pathogen-free BALB/c and C3H/HeOuJ mice were purchased from Charles River Laboratories (Saint Germain sur l'Arbresle, France) and were kept for 1 week under acclimation at the animal facility before starting the experiment. Animals were housed in sterilized cages (5 mice per cage) in a controlled environment at 22℃ with 12-hour light and 12-hour dark cycles. Bedding was autoclaved and changed at least weekly, according to the experimental protocols. The cages were only opened inside a laminar flow cabinet to maintain the specific pathogen free status during the whole experiment. All the mice had ad libitum access to an egg-free autoclaved feed (SAFE, Route de Saint Bris, France) and water. Diet was composed of vegetable proteins, cereals, and a mixture of vitamin and mineral, and did not contain animal protein.
The animal facility is committed to complying with the current regulations regarding animal welfare, observation of the animals' health, and training of the staff for their care and handling. All protocols involving animals were approved by the Bioethical Committee of the CSIC and followed the current EU legislation (Directive 2010/63/EU).
Chemicals
OVA (grade VI, 99% purity) was obtained from Sigma (St. Louis, MO, USA), and its LPS level was quantified by the Pierce® LAL Chromogenic Endotoxin Quantitation Kit (Thermo scientific, Waltham, USA; limit of detection 1-0.1 UE/mg), according to the manufacturer's instructions. In order to purify OVA from LPS contamination, size exclusion chromatography was carried out.16 For this purpose, a Superdex 75 column (Hiload 26/60, AP biotech, Uppsala, Sweden) was loaded with 10 mg/mL OVA in ammonium acetate (0.15 M, pH 6.0) and elution was carried out with 2.5 mL/min of this buffer. Ultrafiltration with Amicon® (EMD Millipore Corporation, Billerica, MA, USA) was used to remove buffer salts. This procedure reduced the LPS content of OVA from 446 UE/mg (OVA-LPS) to 1-3 UE/mg (OVA-LPS-free).
Experimental design
Sensitization and challenge of mice were performed as described by López-Expósito et al.17 BALB/c and C3H/HeOuJ mice (5 per group) were sensitized once per week for 6 weeks, by gavage with 2 different doses of OVA-LPS-free (1 and 5 mg) dissolved in 0.5 mL of 0.2 M bicarbonate with 10 µg of cholera toxin (CT) (List Biologicals, Campbell, CA, USA). Naïve mice received 10 µg of CT in 0.5 mL of bicarbonate. In week 7, all the mice were orally challenged twice with 50 mg of OVA-LPS-free 30 minutes apart, followed by a systemic challenge with 100 µg of OVA-LPS-free intraperitoneally (i.p.) administered, in case severe symptoms (≥4) after oral challenge were not observed. The severity of anaphylaxis was evaluated by measuring the body temperature decrease (rectal thermometer; Panlab, Cornellá, Spain) and scoring clinical signs 30 minutes after each dose. Clinical signs were graded by a score scale adapted from those of Li et al.18 and Perrier et al.19 as follows: 0=no signs; 1=scratching nose and mouth less than 10 times in 15 minutes; 2=puffiness around eyes and mouth, scratching nose and mouth more than 10 times in 15 minutes; 3=wheezing and labored respiration, cyanosis around the mouth and tail, diarrhea and difficulty in walking normally; 4=no activity after prodding; and 5=death. Thirty minutes after the last challenge, mice were sacrificed. Blood samples were collected, and sera were recovered and stored at -80℃ until analysis. Spleens were aseptically removed and immediately processed for splenocyte cultures.
Measurement of antigen-specific immunoglobulins and mast-cell degranulation
Blood samples were obtained on days 22 and 36 and after challenge (day 42). The specific murine IgE, IgG1, and IgG2a antibodies against OVA were quantified in sera by ELISA.20 Briefly, 96-well plates were coated with OVA or with rat anti-mouse IgE, IgG1, and IgG2a (BD Biosciences, San Diego, CA, USA) for the reference curves. After an overnight incubation at 4℃, plates were blocked and incubated overnight at 4℃ with serum samples (1/20 dilution for IgE, 1/5,000 dilution for IgG1, and 1/100 dilution for IgG2a) or serial dilutions of mouse IgE, IgG1, and IgG2a (BD Biosciences), respectively. Afterward, plates were incubated with biotinylated rat anti-mouse IgE, IgG1, and IgG2a (BD Biosciences), followed with avidin-horseradish peroxidase (BD Biosciences). The reactions were developed with ABTS (2,2'-azino-bis (3-ethylbenzthiazoline-6-sulfonate)) substrate (Roche, Mannheim, Germany) and read at 405 nm.
Mouse mast-cell degranulation was evaluated after challenge by measuring serum levels of mouse mast-cell protease 1 (mMCP-1), a marker of activation of intestinal mast cells, with a commercial ELISA kit (eBioscience, San Diego, USA), following the manufacturer's instructions.
Cytokines released following in vitro spleen-cell stimulation
Splenocytes from individual mice were cultured in RPMI 1640 medium containing 10% of fetal bovine serum, L-glutamine (2 mM), penicillin (50 U/mL), and streptomycin (50 µg/mL) (all from Biowest SAS, Nuaillé, France) at a cellular density of 4×106 cells/mL in 24-well plates. They were stimulated in triplicate with concanavalin A (2.5 µg/mL), OVA-LPS, or OVA-LPS-free (200 µg/mL) and culture medium as a control. Cells were maintained for 72 hours at 37℃ in 5% CO2. Afterward, plates were centrifuged and culture supernatants collected and stored at -80℃ until analysis. IFN-γ, TFN-α, IL-5, IL-10, and IL-13 were quantified using cytokine ELISA kits (eBioscience) as outlined by the manufacturers. The results were expressed in pg/mL as sample means for test replicates.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). Data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test, except for clinical sign scores, for which the Mann Whitney test was used. A t test was carried out to determine if the level of cytokines produced by splenocytes were different when stimulated with OVA-LPS-free or OVA-LPS. A P value of <0.05 was considered statistically significant.
RESULTS
Induction of OVA-specific IgE, IgG1, and IgG2a antibodies
Mice orally sensitized to OVA-LPS-free produced significantly higher amounts of IgE and IgG1 than naïve mice and, in general terms, there were no significant differences between the 2 doses of protein used for sensitization (1 or 5 mg) (Table). The levels of OVA-specific IgE did not change from day 22 after the initial sensitization, while maximum IgG1 production was detected following the last sensitization dose (day 36). IgE and IgG1 responses were similar in BALB/c and C3H/HeOuJ mice, whereas C3H/HeOuJ mice produced significantly more IgG2a than BALB/c mice. Unlike BALB/c mice, oral sensitization of C3H/HeOuJ led to a significant IgG2a response, which was independent of the sensitization dose and peaked on day 36 (Table).
Table. OVA-specific IgE, IgG1, and IgG2a values at days 22, 36, and 42.
| Day | 5 mg | 1 mg | Naïve | ||
|---|---|---|---|---|---|
| IgE | BALB/c | 22 | 977.04a±276.92 | 873.28a±128.40 | 0.00b |
| 36 | 1,194.12a±243.82 | 712.78a±193.11 | 0.00b | ||
| 42 | 1,606.00a±351.82 | 861.97a±279.86 | 0.00b | ||
| C3H/HeOuJ | 22 | 1,332.52a±175.56 | 925.15a±240.80 | 0.00b | |
| 36 | 926.80a±65.85 | 1,226.80a±105.72 | 0.00b | ||
| 42 | 850.10a±121.22 | 502.36a±45.58 | 0.00b | ||
| IgG1 | BALB/c | 22 | B61,770.05a±7,021.37 | B38,422.46b±4,290.84 | B759.36c±9.56 |
| 36 | A229,759.18a±9,217.51 | B201,502.29a±20,013.75 | A5,378.44b±23.64 | ||
| 42 | B120,493.13a±23,430.8 | AB106,204.12a±26,270.70 | A5,092.74b±30.42 | ||
| C3H/HeOuJ | 22 | B75,176.47a±2,766.60 | B22,797.79b±4,329.10 | B759.36c±8.79 | |
| 36 | A280,733.96a±2,683.72 | A219,853.21a±14,141.12 | A6,201.83b±420.19 | ||
| 42 | AB152,733.96a±16,125.7 | AB147,853.21±19,768.58 | A6,078.27b±356.33 | ||
| IgG2a | BALB/c | 22 | B121.93±15.21 | B111.89±7.10 | B74.86±0.97 |
| 36 | B2,145.90±535.27 | B884.90±73.94 | AB467.55±2.25 | ||
| 42 | B1,510.69a±176.78 | B671.07b±41.88 | AB465.10b±3.61 | ||
| C3H/HeOuJ | 22 | AB3,129.95±983.27 | B156.68±8.60 | B89.84±1.56 | |
| 36 | A43,321.23a±4,569.57 | A31,762.25a±3,773.39 | A1,135.86b±128.14 | ||
| 42 | A24,821.23a±4,153.53 | A21,786.15a±1,642.40 | A1,100.24b±120.99 |
Values are means (ng/mL±SEM, n=5 per group) obtained for each mouse strain (BALB/c and C3H/HeOuJ) sensitized with 5, 1, or 0 (naïve) mg of OVA.
a-cDifferent lowercase superscript letters indicate significant differences (P<0.01) within rows; A-BDifferent uppercase superscript letters indicate significant differences (P<0.05) within columns for each antibody (IgE, IgG1, or IgG2a).
Anaphylactic responses to OVA
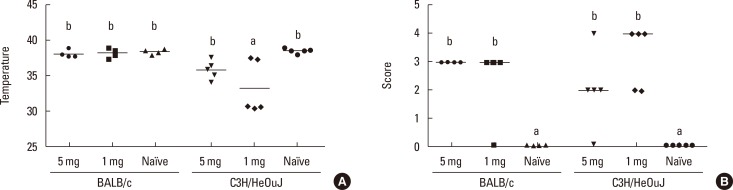
Oral challenge with OVA-LPS-free led to a drop in body temperature in C3H/HeOuJ mice which was significantly more pronounced in those sensitized with the lowest dose (1 mg) (Fig. 1A). The body temperature of orally challenged BALB/c mice was higher and not significantly different from that of their naïve counterparts. In fact, BALB/c mice only showed a significant temperature drop when the allergen was i.p. administered (not shown).
Fig. 1. Anaphylaxis in BALB/c and C3H/HeOuJ mice sensitized with 5, 1, or 0 (naïve) mg of OVA. Body temperature (A) and symptom scores (B), after the second oral challenge with 50 mg of OVA. Horizontal bars represent mean values for temperature and median values for scores (n=5). Different letters indicate statistically significant differences (P<0.05).
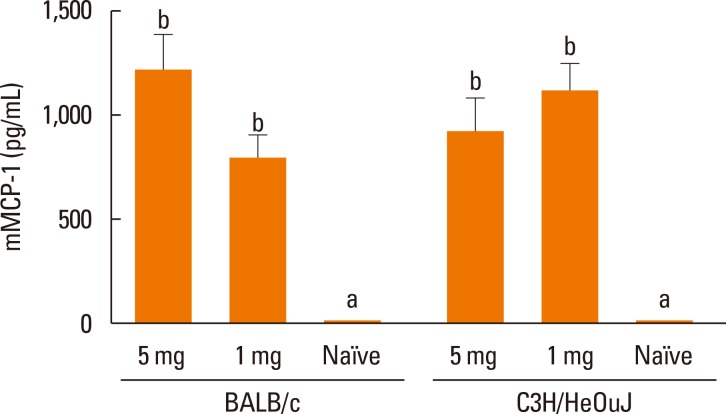
Significant anaphylaxis signs were detected in sensitized BALB/c and C3H/HeOuJ mice following both oral challenges (Fig. 1B). Symptoms consisted of decreased activity, labored respiration, and difficulty in walking normally in both strains, but the responses were characterized by diarrhea in BALB/c mice, and scratching and puffiness around nose and mouth in C3H/HeOuJ mice. The anaphylactic scores were similar in both strains of mice regardless of the dose used for sensitization (Fig. 1B), and there were no statistical differences in the severity of the symptoms elicited by the subsequent oral challenges or the i.p. challenge (data not shown). Similarly, the serum levels of mMCP-1 in sensitized mice were significantly higher than in naïve mice, but they did not change with the strain or the sensitization dose (Fig. 2).
Fig. 2. Mucosal mast-cell activation in BALB/c and C3H/HeOuJ mice sensitized with 5, 1, or 0 (naïve) mg of OVA. Values are means (n=5) and SEM. Different letters indicate statistically significant differences (P<0.05).
Cytokine responses to OVA and influence of LPS contamination
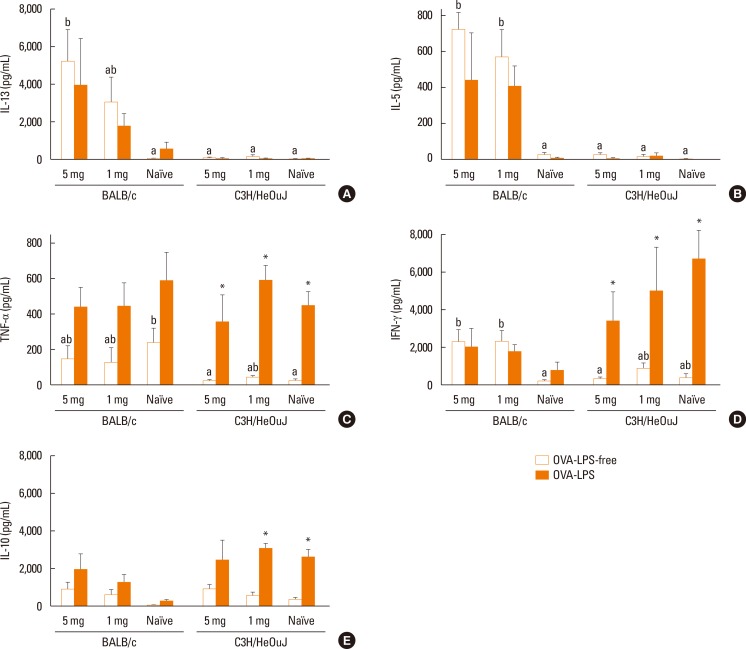
Spleen-cell cultures from naïve mice of both strains produced low levels of cytokines following stimulation with OVA-LPS-free (Fig. 3A-E; white bars). In sensitized mice, stimulation of splenocytes with OVA-LPS-free induced the production of significantly more Th2-related cytokines, such as IL-13 and IL-5 (Fig. 3A and B), and the Th1-related cytokine IFN-γ (Fig. 3D) in BALB/c mice than in C3H/HeOuJ mice, without a significant influence of the dose used for sensitization. OVA-LPS-free did not induce a significant release of TNF-α or IL-10 from spleen cells of sensitized BALB/c and C3H/HeOuJ mice and, in this case, there were no significant differences in the amount of these cytokines produced by both strains, except in the levels of TNF-α generated by the spleen cells from naïve mice (Fig. 3C and E).
Fig. 3. Cytokine levels―IL-13 (A), IL-5 (B), TNF-α (C), INF-γ (D), and IL-10 (E) ― produced by spleen cells of BALB/c and C3H/HeOuJ mice sensitized with 5, 1, or 0 (naïve) mg of OVA and incubated in the presence of OVA-LPS-free and OVA-LPS for 72 hours. Values are means and SEM. Different letters indicate statistically significant differences (P<0.05) between spleen cells cultured with OVA-LPS-free. *indicates P<0.05 between OVA-LPS-free and OVA-LPS.
Stimulation of splenocytes with OVA-LPS did not generate more Th2-related cytokines than OVA-LPS-free in any mouse strain (Fig. 3A and B). However, OVA-LPS significantly enhanced the production of the Th1-related cytokines TNF-α and IFN-γ in naïve and sensitized C3H/HeOuJ mice (Fig. 3C and D). Similarly, LPS-OVA stimulated the splenocytes of naïve and sensitized C3H/HeOuJ mice to produce IL-10, leading to higher levels of this cytokine than those induced by OVA-LPS-free (Fig. 3E).
DISCUSSION
The present study assessed the antibody levels, severity of anaphylaxis, and Th1 and Th2 responses induced by OVA in 2 different mouse strains of CT-primed, IgE-mediated food allergy: BALB/c and C3H/HeOuJ. The utility of different mouse models for the evaluation of the allergenic potential of proteins has been compared using i.p. sensitization;21,22 but even if exposure to food allergens through non-oral routes, particularly through the skin, is increasingly being recognized as a factor which promotes sensitization over tolerance,23 the oral route is generally accepted as the most relevant. However, to the best of our knowledge, very few studies have made such a comparison using oral sensitization, most of them with cow's milk and peanut proteins administered to C3H/HeJ mice, which do not express TLR4.4,9,24 Considering the high shipping price to Europe of the C3H/HeJ mouse, broadly used in many food allergy studies,18,25 the use of its congenic C3H/HeOuJ, with functional TLR4, appears as a convenient alternative. Finally, because the mice selected were sensitive to the adjuvant activity of LPS, which is usually present in commercial proteins as a contaminant, we investigated the influence of LPS on the immunostimulating capacity of OVA in spleen cells of naïve and sensitized mice of both strains.
The 2 doses of OVA (1 and 5 mg) generated similar OVA-specific IgE and IgG1 levels in BALB/c and C3H/HeOuJ mice. However, we detected significantly higher OVA-specific IgG2a levels in C3H/HeOuJ mice, which suggests a Th1 bias in this mouse strain.26 According to Berin et al.,4 there are no differences between BALB/c and C3H/HeOuJ mice in the IgE and IgG1 responses to lactoglobulin (β-Lg), although C3H/HeOuJ mice produce higher peanut protein-specific IgE and IgG1 levels than BALB/c mice. However, Smit et al.24 reported higher concentrations of peanut-specific IgE and IgG1 in BALB/c mice and higher concentrations of IgG2a in C3H/HeOuJ, a discrepancy that was attributed to differences in the peanut sensitization protocols.
While OVA seemed equally immunogenic in both mouse strains, as judged by its capacity to generate IgE and IgG1 antibodies, oral challenge induced a more pronounced temperature drop in C3H/HeOuJ than in BALB/c mice. Regarding the severity of clinical signs following oral challenge with OVA, there were no significant differences between the mouse strains or the 2 oral doses used for sensitization in the anaphylaxis scores, although there was a non-significant tendency to higher scores in the C3H/HeOuJ strain sensitized with the lowest dose. It should be noted that there were differences in the type of clinical symptoms developed, which suggests the convenience of using different scoring systems for both strains. Similarly, serum levels of mMCP-1, a protease released by activated intestinal mast cells, which is indicative of mast-cell degranulation, were significantly increased in the sensitized mice as compared to their naïve controls, but there were no significant differences between C3H/HeOuJ and BALB/c mice.
In view of our own results and of previous findings, it can be pointed out that whereas systemic symptoms are strain-dependent, they also vary with the antigen used for immunization.22 Thus, orally sensitized BALB/c mice are totally resistant to peanut protein-induced anaphylaxis even in the presence of high specific serum IgE levels.4,9,24 Anaphylactic reactions to β-Lg are also less severe in BALB/c mice than in C3H/HeOuJ mice.4 Sensitized C3H/HeOuJ mice develop OVA-specific IgE and anaphylaxis on challenge,7 but there are also examples of BALB/c mice successfully sensitized to OVA by the gastrointestinal route with the aid of CT,14,19,27 anti-acid medication28 or oil emulsion plus salicylate,29 which develop clinical symptoms of IgE- or IgG1-mediated anaphylaxis upon oral challenge.
Stimulation of spleen cells with OVA led to a significantly higher production of Th2 (IL-5 and IL-13) and Th1 (IFN-γ) cytokines in the BALB/c strain than in the C3H/HeOuJ strain. Berin et al.4 and Smit et al.24 also reported that spleen cells of peanut-sensitized BALB/c mice generated more IL-4, IL-5, IL-13, and IFN-γ than those of C3H/HeOuJ mice. Moreover, the secretion of high amounts of IFN-γ together with Th2 cytokines, following OVA stimulation of sensitized animals, is considered a characteristic feature of BALB/c mice.19 As expected, the presence of LPS contamination in the stimulating protein induced the production of IFN-γ and TNF-α and thus it led to a skewing of the cytokine response to a Th1 phenotype, although it also promoted IL-10. LPS is a TLR4 ligand that enhances Th1 or Th17 responses but suppresses Th2 immunity.2 It is a common contaminant of commercial protein preparations, and it is documented that it promotes the release of TNF-α, IL-6, IL-1β, and IL-10 by spleen cells of naïve BALB/c mice.16 It is noteworthy that the influence of LPS was much more pronounced in naïve/sensitized C3H/HeOuJ mice than in BALB/c mice. Therefore, while factors different from TLR4 functionality influence the susceptibility to sensitization and allergic manifestation in mice with a BALB/c or a C3H background,4 our results indicate that, in the presence of a functional LPS receptor, the presence of contaminant LPS affected the immunostimulating capacity of proteins differently depending on the mouse strain. The antibody and cytokine profiles induced by OVA in BALB/c mice, with low levels of OVA-specific IgG2 and high levels of IL-13 and IL-5, argue against a predominant Th1 response in this strain that could inhibit Th2-mediated food allergic reactions.9 Furthermore, BALB/c spleen cells proved to be more resistant than those of C3H/HeOuJ mice to the stimulus of a Th1-polarizing agent such as LPS.
In our study, there was no clear relationship between the sensitization dose and the antibody, anaphylactic, and cytokine responses in any of the strains. In this respect, Li et al.18,25 reported that lower oral doses of cow's milk and peanut proteins plus CT were more effective than higher doses in inducing higher specific IgE concentrations and more severe anaphylactic reactions in mice.
In conclusion, we were able to induce allergy to OVA in BALB/c and C3H/HeOuJ mice, establishing appropriate sensitization and challenge doses. The results showed that both strains exhibited similar susceptibility to OVA sensitization, although oral challenge provoked more severe manifestations in C3H/HeOuJ mice than in BALB/c mice. Stimulation of spleen cells with OVA led to significantly higher levels of Th2 and Th1 cytokines in the BALB/c strain than in the C3H/HeOuJ strain, and these were less affected by protein contamination with LPS. The response of the splenocytes from BALB/c to OVA stimulation, releasing higher concentrations of Th2- and Th1-related cytokines, renders this strain more adequate than the C3H/HeOuJ strain to study the features of OVA that make it immunogenic and the factors promoting sensitization, as well as to understand the mechanisms underlying the efficacy of immunotherapy treatments with this allergen. In addition, the observation of measurable clinical symptoms (in particular, diarrhea) following oral and systemic challenges of BALB/c mice with OVA also points at its suitability to estimate the safety of those treatments.
ACKNOWLEDGMENTS
Financial support was received from AGL2014 59771-R project from MINECO (Spain). The authors are participants in the COST-Action ImpARAS FA1402. A. P-T and D. L-O are recipients of FPI and FPU fellowships from MINECO and MECD, respectively. I. L-E thanks CSIC for his JAE-Doc contract.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: insights into etiology, prevention, and treatment provided by murine models. J Allergy Clin Immunol. 2014;133:309–317. doi: 10.1016/j.jaci.2013.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berin MC, Shreffler WG. T(H)2 adjuvants: implications for food allergy. J Allergy Clin Immunol. 2008;121:1311–1320. doi: 10.1016/j.jaci.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006;61:64–71. doi: 10.1111/j.1398-9995.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 5.van Wijk F, Nierkens S, Hassing I, Feijen M, Koppelman SJ, de Jong GA, et al. The effect of the food matrix on in vivo immune responses to purified peanut allergens. Toxicol Sci. 2005;86:333–341. doi: 10.1093/toxsci/kfi187. [DOI] [PubMed] [Google Scholar]
- 6.Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008;63:882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 7.Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Węgrzyn A. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol. 2011;127:990–997.e1-2. doi: 10.1016/j.jaci.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dearman RJ, Kimber I. Animal models of protein allergenicity: potential benefits, pitfalls and challenges. Clin Exp Allergy. 2009;39:458–468. doi: 10.1111/j.1365-2222.2008.03194.x. [DOI] [PubMed] [Google Scholar]
- 9.Morafo V, Srivastava K, Huang CK, Kleiner G, Lee SY, Sampson HA, et al. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003;111:1122–1128. doi: 10.1067/mai.2003.1463. [DOI] [PubMed] [Google Scholar]
- 10.Mine Y, Yang M. Recent advances in the understanding of egg allergens: basic, industrial, and clinical perspectives. J Agric Food Chem. 2008;56:4874–4900. doi: 10.1021/jf8001153. [DOI] [PubMed] [Google Scholar]
- 11.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Hilmenyuk T, Bellinghausen I, Heydenreich B, Ilchmann A, Toda M, Grabbe S, et al. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology. 2010;129:437–445. doi: 10.1111/j.1365-2567.2009.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Untersmayr E, Diesner SC, Oostingh GJ, Selzle K, Pfaller T, Schultz C, et al. Nitration of the egg-allergen ovalbumin enhances protein allergenicity but reduces the risk for oral sensitization in a murine model of food allergy. PLoS One. 2010;5:e14210. doi: 10.1371/journal.pone.0014210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuercher AW, Holvoet S, Weiss M, Mercenier A. Polyphenol-enriched apple extract attenuates food allergy in mice. Clin Exp Allergy. 2010;40:942–950. doi: 10.1111/j.1365-2222.2010.03460.x. [DOI] [PubMed] [Google Scholar]
- 15.Dearman RJ, Beresford L, Foster ES, McClain S, Kimber I. Characterization of the allergenic potential of proteins: an assessment of the kiwifruit allergen actinidin. J Appl Toxicol. 2014;34:489–497. doi: 10.1002/jat.2897. [DOI] [PubMed] [Google Scholar]
- 16.Brix S, Bovetto L, Fritsché R, Barkholt V, Frøkiaer H. Immunostimulatory potential of β-lactoglobulin preparations: effects caused by endotoxin contamination. J Allergy Clin Immunol. 2003;112:1216–1222. doi: 10.1016/j.jaci.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 17.López-Expósito I, Chicón R, Belloque J, López-Fandiño R, Berin MC. In vivo methods for testing allergenicity show that high hydrostatic pressure hydrolysates of β-lactoglobulin are immunologically inert. J Dairy Sci. 2012;95:541–548. doi: 10.3168/jds.2011-4646. [DOI] [PubMed] [Google Scholar]
- 18.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–214. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 19.Perrier C, Thierry AC, Mercenier A, Corthésy B. Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice. Clin Exp Allergy. 2010;40:153–162. doi: 10.1111/j.1365-2222.2009.03329.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, Huang CK, Zhang TF, Schofield BH, Burks AW, Bannon GA, et al. Oral administration of IL-12 suppresses anaphylactic reactions in a murine model of peanut hypersensitivity. Clin Immunol. 2001;101:220–228. doi: 10.1006/clim.2001.5122. [DOI] [PubMed] [Google Scholar]
- 21.Thomas K, Herouet C, Bannon G, Ladics G, MacIntosh S, Privalle L, et al. Evaluation of IP mouse models for assessing the allergenic potential of proteins. J Allergy Clin Immunol. 2005;115:S250. [Google Scholar]
- 22.Ladics GS, Knippels LM, Penninks AH, Bannon GA, Goodman RE, Herouet-Guicheney C. Review of animal models designed to predict the potential allergenicity of novel proteins in genetically modified crops. Regul Toxicol Pharmacol. 2010;56:212–224. doi: 10.1016/j.yrtph.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol. 2013;23:R389–R400. doi: 10.1016/j.cub.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smit JJ, Willemsen K, Hassing I, Fiechter D, Storm G, van Bloois L, et al. Contribution of classic and alternative effector pathways in peanut-induced anaphylactic responses. PLoS One. 2011;6:e28917. doi: 10.1371/journal.pone.0028917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–158. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 26.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Yang C, Mine Y. Multiple T cell epitope peptides suppress allergic responses in an egg allergy mouse model by the elicitation of forkhead box transcription factor 3- and transforming growth factor-beta-associated mechanisms. Clin Exp Allergy. 2010;40:668–678. doi: 10.1111/j.1365-2222.2009.03442.x. [DOI] [PubMed] [Google Scholar]
- 28.Diesner SC, Knittelfelder R, Krishnamurthy D, Pali-Schöll I, Gajdzik L, Jensen-Jarolim E, et al. Dose-dependent food allergy induction against ovalbumin under acid-suppression: a murine food allergy model. Immunol Lett. 2008;121:45–51. doi: 10.1016/j.imlet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shindo T, Kanazawa Y, Saito Y, Kojima K, Ohsawa M, Teshima R. Effective induction of oral anaphylaxis to ovalbumin in mice sensitized by feeding of the antigen with aid of oil emulsion and salicylate. J Toxicol Sci. 2012;37:307–315. doi: 10.2131/jts.37.307. [DOI] [PubMed] [Google Scholar]