Abstract
Cervical cancer cells commonly harbour a defective G1/S checkpoint owing to the interaction of viral oncoproteins with p53 and retinoblastoma protein. The activation of the G2/M checkpoint may thus become essential for protecting cancer cells from genotoxic insults, such as chemotherapy. In 52 cervical cancer patients treated with neoadjuvant chemotherapy, we investigated whether the levels of phosphorylated Wee1 (pWee1), a key G2/M checkpoint kinase, and γ-H2AX, a marker of DNA double-strand breaks, discriminated between patients with a pathological complete response (pCR) and those with residual disease. We also tested the association between pWee1 and phosphorylated Chk1 (pChk1), a kinase acting upstream Wee1 in the G2/M checkpoint pathway. pWee1, γ-H2AX and pChk1 were retrospectively assessed in diagnostic biopsies by immunohistochemistry. The degrees of pWee1 and pChk1 expression were defined using three different classification methods, i.e., staining intensity, Allred score, and a multiplicative score. γ-H2AX was analyzed both as continuous and categorical variable. Irrespective of the classification used, elevated levels of pWee1 and γ-H2AX were significantly associated with a lower rate of pCR. In univariate and multivariate analyses, pWee1 and γ-H2AX were both associated with reduced pCR. Internal validation conducted through a re-sampling without replacement procedure confirmed the robustness of the multivariate model. Finally, we found a significant association between pWee1 and pChk1. The message conveyed by the present analysis is that biomarkers of DNA damage and repair may predict the efficacy of neoadjuvant chemotherapy in cervical cancer. Further studies are warranted to prospectively validate these encouraging findings.
Introduction
Eukaryotic cells are constantly exposed to endogenous and exogenous sources of DNA damage. The transmission of undamaged DNA to the offspring is ensured by a complex molecular network, the DNA damage response (DDR), which operates through the coordinated activity of cell cycle checkpoints, DNA repair mechanisms and apoptotic pathways [1, 2]. The presence of genetic lesions triggers checkpoint-mediated arrest of the cell cycle [2]. This event enables DNA repair effectors and apoptotic pathways to repair the lesion or eliminate irremediably damaged cells, respectively.
Cancer cells aberrantly use DNA repair mechanisms to survive stressful conditions, such as exposure to chemotherapy [2]. A common trait to a variety of tumors is the defective nature of the G1/S-phase checkpoint, stemming from mutational or functional inactivation of p53 or retinoblastoma protein (pRb) [3]. When this occurs, cancer cells become extremely dependent on the G2/M checkpoint for cell cycle arrest and DNA repair [3]. The ataxia telangiectasia and Rad3-related protein (ATR)-Checkpoint kinase 1 (Chk1)-Wee1-like protein kinase (Wee1) cascade represents the core of the G2/M checkpoint, whose activation leads to the inhibition of the cyclin-dependent kinase 1 and culminates into checkpoint-mediated cell cycle arrest [3]. In such a manner, cancer cells have the time to correct chemotherapy-induced DNA lesions, avoiding entry into a lethal mitosis known as mitotic catastrophe [4]. G2/M checkpoint dependency in a p53-defective molecular background is a concept currently exploited for the clinical development of synthetic lethality-based therapeutics. When G1/S-phase checkpoint-defective cells are exposed to chemotherapeutics, the concomitant pharmacological inhibition of G2/M checkpoint kinases is deleterious for cell fitness [3].
We reasoned that G2/M checkpoint “addiction” for compensating p53 or pRb defects upon exposure to genotoxic agents can be exploited in the search for predictive biomarkers foreseeing chemotherapy sensitivity/resistance. In this exploratory analysis we focused on cervical cancer, the prototype of p53- and pRb-defective tumors. Indeed, human papillomavirus E6 and E7 oncoproteins promote ubiquitin-mediated degradation of p53 and pRb, respectively [5]. We thus retrospectively investigated the association between the levels of DNA damage and repair biomarkers, assessed in bioptic samples collected from untreated patients at the time of diagnosis, and pathological complete response (pCR) after neoadjuvant chemotherapy, i.e., chemotherapy delivered in the timeframe between diagnostic biopsy and the surgical resection. All the patients were homogenously treated with paclitaxel, ifosfamide and cisplatin (TIP regimen). We focused on phosphorylated Wee1 (pWee1) as a proxy of G2/M checkpoint activation, and phosphorylated H2A Histone Family Member X (γ-H2AX) as a marker of DNA double-strand breaks. Phosphorylated Chk1 (pChk1) was tested in a fraction of samples for a signaling study.
Materials and Methods
Study Participants and Procedures
Fifty-two histologically confirmed cervical cancer patients (stage Ib2-IIIa) who received neoadjuvant chemotherapy were included in this retrospective analysis. All patients were treated with the TIP regimen (paclitaxel 175 mg/m2 on day 1 + ifosfamide 2500 mg/m2 on days 1 and 2 + cisplatin 50 mg/m2 on day 2 every 21 days for three or four cycles) followed by radical surgery. Patients were considered eligible if they completed the planned treatment, data on clinical features and treatment outcomes were available, and the amount of biological materials in their biopsies was sufficient for molecular analyses. pCR was defined as no residual disease in surgical samples. The immunohistochemical assessment of pWee1, γ-H2AX, and pChk1 was performed in formalin-fixed paraffin-embedded (FFPE) tissues, obtained from the biological specimens collected through bioptic procedures in untreated patients, using the following antibodies: anti-phospho-H2AX (Ser139) (clone JBW301) mouse monoclonal antibody (MAb) (Upstate, NY, USA) at the dilution of 1:500, anti-phospho-Wee1 (Ser642) (clone D47G5) rabbit MAb (Cell Signaling, Danvers, MA, USA) at the dilution of 1:100, and anti-phospho-Chk1 (Ser345) (clone 133D3) rabbit MAb (Cell Signaling, Danvers, MA, USA) at the dilution of 1:100. Immunohistochemical staining was performed in an automated autostainer (BOND-III, Leica, Milan, Italy) by a biotin-free polymeric horseradish peroxidase (HRP)-linker antibody conjugate system (Leica, Milan, Italy). For each tumor, three different, 3 μm paraffin sections were analyzed and examined by light microscopy. Immunoreaction of tumor cells was counted in four high-power fields (400x magnification) per section. pWee1 and pChk1 were considered positive when ≥10% of the neoplastic cells showed a distinct nuclear immunoreactivity. pWee1 and pChk1 were graded on a four-grade scale based on staining intensity (0: negative, 1+: weak, 2+: moderate, 3+: strong). Tumors were classified as negative (0 = pWee1neg and pChk1neg) or positive (1–3 = pWee1pos and pChk1pos).The Allred scores were obtained as previously described [6], considering staining intensity and percentage of tumor-expressing cells, and reported according to a scale of 0 to 8. Tumors were classified as low expressing if the Allred score was ≤ 2 (pWee1allred low, pChk1allred low), or as high expressing if the Allred score was > 2 (pWee1allred high, pChk1allred high). The multiplicative scores were obtained by multiplying staining intensity x the percentage of tumor-expressing cells, and were expressed on a scale of 0 to 300. Tumors were classified as low expressing (pWee1multi low and pChk1multi low) or high expressing (pWee1multi high and pChk1multi high) using the median score of all tumors as a cut-off point. γ-H2AX expression was considered as the percentage of tumor-expressing cells and analyzed both as continuous (γ-H2AXcont) and as categorical variable, whose modality was defined using the median score of all tumors (γ-H2AX low and γ-H2AX high). Tumor samples were evaluated independently by two investigators (SB and MC) who were blinded to treatment outcomes, and discordant cases were reviewed (MM). This retrospective study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethic Committee of “Regina Elena” National Cancer Institute of Rome, the coordinating centre. Written informed consents were secured before chemotherapy.
Statistical analysis
Cancer- and patient-related features were descriptively characterized for all the patients included in the present analysis. Medians and ranges were used to report on continuous variables, while categorical variables were expressed by frequencies and percentage values. In order to assess the relationships between categorical variables the Pearson’s Chi-squared test of independence (2-tailed) and the Fisher Exact test were employed. The use of univariate logistic regression models helped identify variables potentially impacting treatment outcome. Multivariate logistic regression models were built by including variables testing significant at the univariate assessment or identified based on the clinical plausibility of their role in influencing pCR. To estimate the risk of an overfitted multivariate model and examine its stability, an internal validation was carried out using a re-sampling procedure without replacement. To this end, one hundred datasets were generated by randomly removing approximately 20% of the original sample. For each simulation, we repeated the multivariate logistic regression model and the Cohen's Kappa coefficient, the Positive Predictive Value (PPV), the Negative Predictive Value (NPV), Sensibility and Specificity were calculated. We considered statistically significant p values less than 0.05. Statistical analyses were carried out using SPSS software (SPSS version 21, SPSS Inc., Chicago, IL, USA).
Results
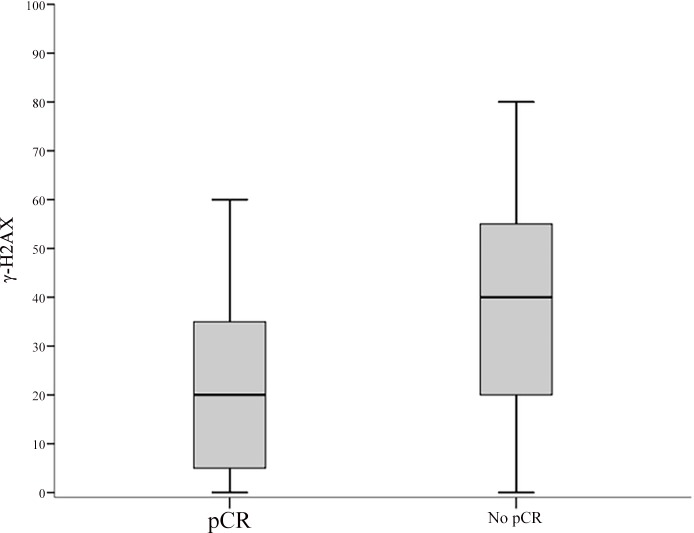
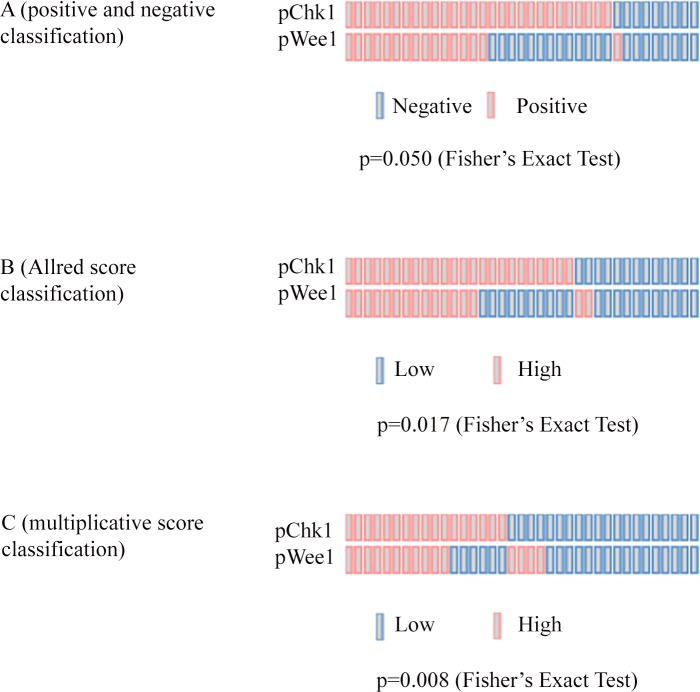
Baseline characteristics of the 52 patients included in this study are summarized in Table 1. Median time between diagnostic biopsy and radical surgery was 4.99 months [IQ Range: 4.01–5.83]. All the pre-chemotherapy samples, consisting in diagnostic biopsies, were examined for pWee1 and γ-H2AX, whereas pChk1 data were available for 37 samples. Median percentages of nuclear-expressing cells for pWee1, pChk1 and γ-H2AX were 40% (min/max 10/80), 30% (min/max 10/80) and 30% (min/max 0/80), respectively. Representative immunohistochemical staining patterns are illustrated in Fig 1. As shown in Table 2, we found a statistically significant association between elevated nuclear pWee1 expression and reduced pCR rate. The association tested significant for all the scoring methods investigated (pWee1pos vs pWee1neg, p = 0.016; pWee1allred high vs pWee1allred low, p = 0.016; pWee1multi high vs pWee1multi low, p = 0.034) (Table 2). Likewise, elevated nuclear levels of γ-H2AX were associated with reduced pCR rate, both when considered as categorical and continuous variable (p = 0.037 and p = 0.026 in Table 2 and Fig 2, respectively). When considering the combination of the two markers, only 1 patient out of 16 with double positive tumors experienced a pCR, 9 out of 16 patients with double negative tumors achieve a pCR, and an intermediate outcome was seen in patients whose tumors expressed only one biomarker (p = 0.009) (Table 3). Six out of 8 deaths were observed in double positive tumors (p = 0.013) (Table 3). In the univariate logistic regression model, pWee1 and γ-H2AX were directly associated with pCR (pWee1pos vs pWee1neg: Odds Ratio (OR) 5.31, 95% Confidence Interval (CI): 1.42–19.87, p = 0.013; γ-H2AXhigh vs γ-H2AXlow: OR 4.20, 95%CI:1.13–15.59, p = 0.032, respectively) (Table 4); the multivariate model confirmed the predictive role of pWee1 and γ-H2AX (Table 4). The internal validation performed through a re-sampling procedure confirmed the robustness of the multivariate model. Concordance, Positive Predictive Value, Negative Predictive Value, Sensitivity and Specificity are shown in Table 5. Finally, when investigating co-expression patterns, we did not observe any association between pWee1 and γ-H2AX (data available upon request), whereas a statistically significant association was reported between pWee1 and pChk1 (Fig 3).
Table 1. Baseline characteristics and treatment outcome of cervical cancer patients treated with neoadjuvant chemotherapy (N = 52).
| Characteristics | N (%) | |
|---|---|---|
| Age at diagnosis Median (range) | 45.5 (37.2–56.0) | |
| Stage | ||
| I | 17 (32.7) | |
| II-III | 35 (67.3) | |
| Histology | ||
| Squamous cell carcinoma | 43 (82.7) | |
| Adenocarcinoma | 9 (17.3) | |
| Number of chemotherapy cycles | ||
| 3 | 28 (53.8) | |
| 4 | 24 (46.2) | |
| Pathological complete response | ||
| Yes | 16 (30.8) | |
| No | 36 (69.2) |
Fig 1. Representative examples of immunohistochemical expression of DNA damage and repair biomarkers in cervical cancer patients.
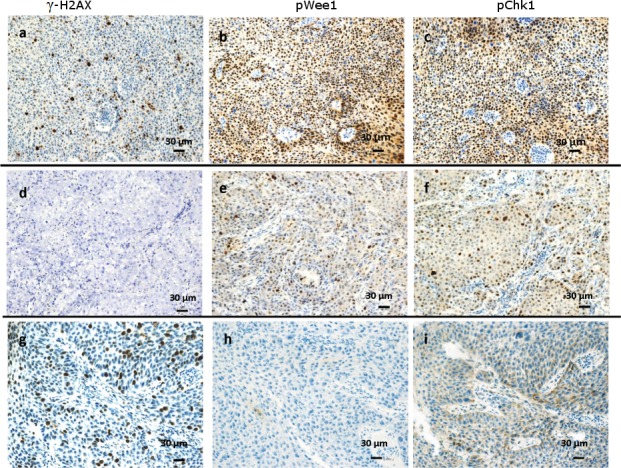
Three consecutive sections for each tumor are showed. (A-C) A triple positive tumor with nuclear γ-H2AX(A), pWee1(B) and pChk1 (C) immunoreactivity.(D-F) A tumor that did not express γ-H2AX (D), and that co-expressed pWee1(E) and pChk1 (F). (G-I) A tumor expressing nuclear γ-H2AX (G) that lacked both pWee1(H) and pChk1 (I) expression.
Table 2. Association between biomarkers of DNA damage and repair (pWee1 and γ-H2AX) and pathological complete response in cervical cancer patients treated with neoadjuvant chemotherapy (N = 52).
| Pathological complete response | |||
|---|---|---|---|
| No | Yes | Fisher's Exact Test | |
| Biomarker | N (%) | N (%) | P-value |
| pWee1neg | 13 (52.0) | 12 (48.0) | 0.016 |
| pWee1pos | 23 (85.2) | 4 (14.8) | |
| pWee1allred low | 13 (52.0) | 12 (48.0) | 0.016 |
| pWee1allred high | 23 (85.2) | 4 (14.8) | |
| pWee1multi low | 14 (53.8) | 12 (46.2) | 0.034 |
| pWee1multi high | 22 (84.6) | 4 (15.4) | |
| γ-H2AXcat low | 15 (55.6) | 12 (44.4) | 0.037 |
| γ-H2AXcat high | 21 (84.0) | 4 (16.0) | |
pWee1, phosphorylated Wee1-like protein kinase; γ-H2AX, phosphorylated H2A Histone Family Member X
Fig 2. Box plot of the distribution of γ-H2AX values by pathologic complete response.
In the figure: the upper horizontal line of the box is the 75th percentile; the lower horizontal line of the box is the 25th percentile; the horizontal bar within box is the median value; the upper horizontal bar outside the box is the maximum value; the lower horizontal bar outside the box is the minimum values.
Table 3. Association between the co-expression of pWee1 and γ-H2AX and A) Pathological complete response (N = 52), B) Death (N = 8).
| Pathological complete response | |||
| A) N = 52 | No | Yes | Chi2 |
| N (%) | N (%) | P-value | |
| pWee1neg / γ-H2AXcat low | 7 (43.8) | 9 (56.3) | 0.009 |
| pWee1neg /γ-H2AXcat high or pWee1pos /γ-H2AXcat low | 14 (70.0) | 6 (30.0) | |
| pWee1pos /γ-H2AXcat high | 15 (93.8) | 1 (6.2) | |
| Death | |||
| B) N = 8 | No | Yes | Chi2 |
| N (%) | N (%) | P-value | |
| pWee1neg /γ-H2AXcat low | 15 (93.8) | 1 (6.2) | 0.013 |
| pWee1neg /γ-H2AXcat high or pWee1pos /γ-H2AXcat low | 19 (95.0) | 1 (5.0) | |
| pWee1pos /γ-H2AXcat high | 10 (62.5) | 6 (37.5) | |
pWee1, phosphorylated Wee1-like protein kinase; γ-H2AX, phosphorylated H2A Histone Family Member X
Table 4. Uni and multivariate logistic regression models of patient- and disease-related features and pathological complete response.
| Univariate logistic regression model | Multivariate logistic regression model* | ||||
|---|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | ||
| Variables | |||||
| Age | >45.5 vs ≤45.5 | 0.70 (0.21–2.28) | 0.549 | ||
| Stage | II-III vs I | 1.36 (0.40–4.69) | 0.623 | ||
| Histology | AC vs SCC | Not applicable | Not applicable | ||
| CT cycles | 4 vs 3 | 2.46 (0.71–8.52) | 0.156 | ||
| γ-H2AX | high vs low | 4.20 (1.13–15.59) | 0.032 | 7.14 (1.30–39.29) | 0.024 |
| pWee1 | pos vs neg | 5.31 (1.42–19.87) | 0.013 | 8.92 (1.68–47.26) | 0.010 |
* Adjusted for age, stage, number of chemotherapy cycles.
AC, Adenocarcinoma; SCC: Squamous cell carcinoma; CT, Chemotherapy.
Table 5. Replication stability of the multivariate analysis after internal validation with a re-sampling procedure.
One hundred less-powered simulation datasets were generated, each approximately 80% of the original size.
| Mean | Median | Minimum | Maximum | Standard Deviation | |
|---|---|---|---|---|---|
| Cohen's kappa coefficient | 0.575 | 0.581 | 0.408 | 0.715 | 0.06 |
| Positive predictive value (PPV) | 0.741 | 0.742 | 0.600 | 0.888 | 0.05 |
| Negative predictive value (NPV) | 0.858 | 0.857 | 0.800 | 0.933 | 0.02 |
| Sensitivity | 0.660 | 0.667 | 0.440 | 0.800 | 0.06 |
| Specificity | 0.898 | 0.899 | 0.833 | 0.966 | 0.03 |
Fig 3. OncoPrints showing the association between pWee1 and pChk1 in 37 cervical cancer samples.
(A) Association according to staining intensity-based classification (positive vs negative). (B) Association according to Allred score classification (high vs low). (C) Association according to a multiplicative score classification (high vs low).
Discussion
In the present study we retrospectively explored the predictive significance of pWee1 and γ-H2AX expression, evaluated in diagnostic biopsies related 52 cervical cancer patients who received neoadjuvant chemotherapy. We also investigated the association between pWee1 and pChk1 in order to provide clues on whether Wee1 activation in cervical cancer is mediated by Chk1. To our knowledge, this is the first study reporting on DNA damage and repair biomarkers in cervical cancer that exploited the concept of the defective nature of the G1/S-phase checkpoint. Overall, we observed a statistically significant association between elevated expression of pWee1 and γ-H2AX and reduced rate of pCR. Thus, we provided first hints that the elevated expression of DDR biomarkers in diagnostic samples might be associated with suboptimal efficacy of chemotherapy, evaluated through pCR in surgically resected tumors. We also observed a positive association between pWee1 and pChk1 expression that suggests effective G2/M checkpoint activation. We are aware that our results are hypothesis-generating in nature given the retrospective design of the study. Nevertheless, beyond the straightforward analytical approach, our study has some important strengths.
First, the neoadjuvant setting offers multiple advantages for the identification and development of cancer biomarkers: i) the analysis of potential markers in a molecular background not “polluted” by the exposure of previous anticancer treatments, ii) the identification of predictive markers to select patients who will more likely benefit from chemotherapy, iii) the identification of biomarkers that also hold prognostic significance, even though evidence on the association between pCR and long-term survival outcomes in cervical cancer is not as robust as it is in breast cancer [7, 8].
Second, thus far, in cervical cancer the search for predictive biomarkers linked to the increased ability of cancer cells to protect their genome when challenged with chemotherapy or radiotherapy has been exclusively focused on nucleotide excision repair (NER) proteins, and in particular on the excision repair cross-complementation group1 (ERCC1) protein [9–13]. NER is deputed to correct bulky helix-distorting lesions, such as those inflicted on the DNA by platinum-based therapy. However, within the context of the DDR, NER is one of the many distal effectors assigned to maintain genome integrity. A number of molecular networks safeguard the genome, albeit their engagement depends on the type of lesion. DNA repair pathways also include base excision repair (BER), mismatch repair (MMR), direct repair, and the double-strand break (DSB) recombinational repair. This latter encompasses the error-free homologous recombination repair (HRR) and the error-prone non-homologous end-joining (NHEJ) [1]. Therefore, the level of biologic complexity of the DDR might be underestimated when exclusively considering one, or few, components collocated in a specific repair network. Moreover, concerns were raised on the reliability, and biological significance, of ERCC1 detection methods [14, 15]. Conversely, our study focused on master DDR components, whose activation is known to be particularly efficient in cervical cancer.
Next, we hypothesized that endogenous levels of DNA damage, mirrored by γ-H2AX, should have been paralleled by increased expression of pWee1 and pChk1.Even though the ATR-Chk1-Wee1 axis is primarily activated by stretched of single-stranded DNAs, these abnormal structures may generate DSBs upon replication fork collapse [16]. Moreover, we reasoned that the activation of the G2/M checkpoint should be particularly proficient in the presence of high basal levels of endogenous DNA damages, representing an adaptive mechanism through which cancer cells counteract oncogene-induced replication stress [16]. Indeed, it is known that ATR and Chk1 suppress the apoptotic response following DNA replication stress [17], and that tumors characterized by elevated levels of replicative stress, such as Myc-driven cancers, are extremely vulnerable to the pharmacological targeting of G2/M checkpoint kinases [18–23]. We did not observe any association between pWee1 and γ-H2AX, but rather these endpoints were independently associated with pCR. We can speculate that two independent repair avenues, particularly efficient in cervical cancer, were captured in this study. A suitable candidate is the Ataxia-telangiectasia mutated (ATM)-Checkpoint kinase 2 (Chk2) pathway, which is mainly activated by DSBs [16]. An extensive cooperation exists between the ATM-Chk2 pathway and ATR-Chk1-Wee1 signaling, and ATM also phosphorylates H2AX [16].
Another aspect emerging from this study relates to the association between pWee1 and pChk1 expression. Wee1 is placed downstream Chk1 [3], and Wee1 phosphorylation at Ser642 increases its stability in the nucleus and promotes cell cycle arrest at the G2/M transition [24, 25]. However, to our knowledge formal proof that this regulatory mechanism, namely Chk1-driven phosphorylation of Wee1 at Ser642, operates in mammalian cells is still lacking. Current evidence mostly stems from studies using Xenopus extracts and Schizosaccharomyces pombe as model systems [26, 27]. Even though our study was not designed to generate mechanistic insights into the dynamics governing Wee1 activation, its results provide a suggestion for future preclinical investigations.
A final point that deserves consideration refers to the protective role of G2/M checkpoint activation in the context of cancer stem cells [28]. Activation of the axis has been associated with therapeutic resistance in different cancer stem cell models, including brain, lung and colon cancers [29–31]. Multiplying the efforts for establishing a collection of patient-derived cervical cancer stem cells for comprehensive molecular characterization is a strategy that should be pursued to further dissect the relationship existing between G2/M checkpoint activation and chemoresistant features. The relevance of this approach is even more evident when considering the need for more accurate cellular and animal models in light of the number of Chk1 and Wee1 inhibitors that entered clinical development [3, 32]. For instance, a phase I trial with the first-in-class Wee1 inhibitor AZD1775 (MK1775) in association with cisplatin and radiation therapy is ongoing (ClinicalTrials.gov Identifier: NCT01958658), and a phase I/II trial in combination with topotecan/cisplatin results as completed (ClinicalTrials.gov Identifier:NCT01076400). Moreover, a phase I-II trial of AZD1775 in combination with chemotherapy has been initiated in patients with TP53-mutated epithelial ovarian, fallopian tube, or primary peritoneal cancer [33].
Conclusions
To sum up, pWee1 andγ-H2AX expression in pre-chemotherapy samples showed ability to foresee pCR in cervical cancer patients treated with neoadjuvant paclitaxel, ifosfamide and cisplatin. Based on the extremely promising results herein presented prospective validation or, alternatively, ancillary molecular analyses in the context of prospective trials is warranted to better characterize the predictive ability of these biomarkers.
Acknowledgments
We thank Tania Merlino for technical assistance.
Abbreviations
- ATM
Ataxia-telangiectasia mutated
- ATR
ataxia telangiectasia and Rad3-related protein
- Chk1
Checkpoint kinase 1
- Chk2
Checkpoint kinase 2
- DDR
DNA damage response
- DSB
double-strand break
- pCR
pathological complete response
- pChk1
hosphorylated Chk1
- γ-H2AX
phosphorylated H2A Histone Family Member X
- pRb
retinoblastoma protein
- pWee1
phosphorylated Wee1
- Wee1
Wee1-like protein kinase
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. [DOI] [PubMed] [Google Scholar]
- 2.Medema RH, Macurek L. Checkpoint control and cancer. Oncogene. 2012;31:2601–2613. 10.1038/onc.2011.451 [DOI] [PubMed] [Google Scholar]
- 3.Maugeri-Saccà M, Bartucci M, De Maria R. Checkpoint kinase 1 inhibitors for potentiating systemic anticancer therapy. Cancer Treat Rev. 2013;39:525–533. 10.1016/j.ctrv.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 4.Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–392. 10.1038/nrm3115 [DOI] [PubMed] [Google Scholar]
- 5.Vici P, Mariani L, Pizzuti L, Sergi D, Di Lauro L, Vizza E, et al. Emerging biological treatments for uterine cervical carcinoma. J Cancer. 2014;5:86–97. 10.7150/jca.7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leake R, Barnes D, Pinder S, Ellis I, Anderson L, Anderson T, et al. Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. UK Receptor Group, UK NEQAS, The Scottish Breast Cancer Pathology Group, and The Receptor and Biomarker Study Group of the EORTC. J Clin Pathol. 2000;53:634–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buda A, Lissoni AA, Floriani I,Biagioli E, Gerardi C, Bonazzi C, et al. Long-Term Clinical Benefits of Neoadjuvant Chemotherapy in Women With Locally Advanced Cervical Cancer: Validity of Pathological Response as Surrogate Endpoint of Survival. Int J Gynecol Cancer. 2015;;25:1468–1475 10.1097/IGC.0000000000000515 [DOI] [PubMed] [Google Scholar]
- 8.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 9.Bajpai D, Banerjee A, Pathak S,Thakur B, Jain SK, Singh N. Single nucleotide polymorphisms in the DNA repair genes in HPV-positive cervical cancer. Eur J Cancer Prev. 2015March 25. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Muallem MZ, Marnitz S, Richter R,Köhler C, Sehouli J, Arsenic R. ERCC1 expression as a predictive marker of cervical cancer treated with cisplatin-based chemoradiation. Anticancer Res. 2014;34:401–406. [PubMed] [Google Scholar]
- 11.Bai ZL, Wang YY, Zhe H, He JL, Hai P. ERCC1 mRNA levels can predict the response to cisplatin-based concurrent chemoradiotherapy of locally advanced cervical squamous cell carcinoma. Radiat Oncol. 2012;7:221 10.1186/1748-717X-7-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JS, Jeon EK, Chun SH, Won HS, Lee A, Hur SY, et al. ERCC1 (excision repair cross-complementation group 1) expression as a predictor for response of neoadjuvant chemotherapy for FIGO stage 2B uterine cervix cancer. Gynecol Oncol. 2011;120:275–279. 10.1016/j.ygyno.2010.10.034 [DOI] [PubMed] [Google Scholar]
- 13.Chung HH, Kim MK, Kim JW, Park NH, Song YS, Kang SB, Lee HP. XRCC1 R399Q polymorphism is associated with response to platinum-based neoadjuvant chemotherapy in bulky cervical cancer. Gynecol Oncol. 2006;103:1031–1037. [DOI] [PubMed] [Google Scholar]
- 14.Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101–1110. 10.1056/NEJMoa1214271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olaussen KA, Dunant A, Fouret P,Brambilla E, André F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. [DOI] [PubMed] [Google Scholar]
- 16.Larsson LG. Oncogene- and tumor suppressor gene-mediated suppression of cellular senescence. Semin Cancer Biol. 2011;21:367–376. 10.1016/j.semcancer.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 17.Myers K, Gagou ME, Zuazua-Villar P,Rodriguez R, Meuth M. ATR and Chk1 suppress a caspase-3-dependent apoptotic response following DNA replication stress. PLoS Genet. 2009;5:e1000324 10.1371/journal.pgen.1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole KA, Huggins J, Laquaglia M, Hulderman CE, Russell MR, Bosse K, et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc Natl Acad Sci U S A. 2011;108:3336–3341. 10.1073/pnas.1012351108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murga M, Campaner S, Lopez-Contreras AJ, Toledo LI, Soria R, Montaña MF, et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat Struct Mol Biol. 2011;18:1331–1335. 10.1038/nsmb.2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Höglund A, Nilsson LM, Muralidharan SV,Hasvold LA, Merta P, Rudelius M, et al. Therapeutic implications for the induced levels of Chk1 in Myc-expressing cancer cells. Clin Cancer Res. 2011;17:7067–7079. 10.1158/1078-0432.CCR-11-1198 [DOI] [PubMed] [Google Scholar]
- 21.Ferrao PT, Bukczynska EP, Johnstone RW, McArthur GA. Efficacy of CHK inhibitors as single agents in MYC-driven lymphoma cells. Oncogene. 2012;31:1661–1672. 10.1038/onc.2011.358 [DOI] [PubMed] [Google Scholar]
- 22.Brooks K, Oakes V, Edwards B, Ranall M, Leo P, Pavey S, et al. A potent Chk1 inhibitor is selectively cytotoxic in melanomas with high levels of replicative stress. Oncogene. 2013; 32:788–796. 10.1038/onc.2012.72 [DOI] [PubMed] [Google Scholar]
- 23.Saini P, Li Y, Dobbelstein M. Wee1 is required to sustain ATR/Chk1 signaling upon replicative stress. Oncotarget. 2015;6:13072–13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothblum-Oviatt CJ, Ryan CE, Piwnica-Worms H. 14-3-3 binding regulates catalytic activity of human Wee1 kinase. Cell Growth Differ. 2001;12:581–589. [PubMed] [Google Scholar]
- 25.Wang Y, Jacobs C, Hook KE,Duan H, Booher RN, Sun Y. Binding of 14-3-3beta to the carboxyl terminus of Wee1 increases Wee1 stability, kinase activity, and G2-M cell population. Cell Growth Differ. 2000;11:211–219. [PubMed] [Google Scholar]
- 26.Lee J, Kumagai A, Dunphy WG. Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol Biol Cell. 2001;12:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maugeri-Saccà M, Bartucci M, De Maria R. DNA damage repair pathways in cancer stem cells. Mol Cancer Ther. 2012;11:1627–1636. 10.1158/1535-7163.MCT-11-1040 [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. [DOI] [PubMed] [Google Scholar]
- 30.Bartucci M, Svensson S, Romania P, Dattilo R, Patrizii M, Signore M, et al. Therapeutic targeting of Chk1 in NSCLC stem cells during chemotherapy. Cell Death Differ. 2012;19:768–778. 10.1038/cdd.2011.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallmeier E, Hermann PC, Mueller MT, Machado JG, Ziesch A, De Toni EN, et al. Inhibition of ataxia telangiectasia- and Rad3-related function abrogates the in vitro and in vivo tumorigenicity of human colon cancer cells through depletion of the CD133(+) tumor-initiating cell fraction. Stem Cells. 2011;29:418–429. 10.1002/stem.595 [DOI] [PubMed] [Google Scholar]
- 32.Do K, Doroshow JH, Kummar S. Wee1 kinase as a target for cancer therapy. Cell Cycle.2013;12:3159–3164. 10.4161/cc.26062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore KN, McMeekin DS, Hamilton EP,Strickland DK, Fields Jones S, Stults DM, et al. Multicenter randomized Phase II study of AZD1775 plus chemotherapy versus chemotherapy alone in patients with platinum-resistant TP53-mutated epithelial ovarian, fallopian tube, or primary peritoneal cancer.J Clin Oncol 33, 2015 (suppl; abstr TPS5608). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.