Abstract
The expression of and role played by RecA in protecting sporulating cells of Bacillus subtilis from DNA damage has been determined. Results showed that the DNA-alkylating agent Mitomycin-C (M-C) activated expression of a PrecA-gfpmut3a fusion in both sporulating cells’ mother cell and forespore compartments. The expression levels of a recA-lacZ fusion were significantly lower in sporulating than in growing cells. However, M-C induced levels of ß-galactosidase from a recA-lacZ fusion ~6- and 3-fold in the mother cell and forespore compartments of B. subtilis sporangia, respectively. Disruption of recA slowed sporulation and sensitized sporulating cells to M-C and UV-C radiation, and the M-C and UV-C sensitivity of sporangia lacking the transcriptional repair-coupling factor Mfd was significantly increased by loss of RecA. We postulate that when DNA damage is encountered during sporulation, RecA activates the SOS response thus providing sporangia with the repair machinery to process DNA lesions that may compromise the spatio-temporal expression of genes that are essential for efficient spore formation.
Introduction
Sporulation in Bacillus subtilis is a developmental process that is triggered by nutrient depletion and high cell density, and serves as a strategy for population survival. An early morphological event in sporulation is the asymmetric cell division that gives rise to a two-compartment sporangium [1]. Distinct programs of gene expression are then directed in these two cells by compartment-specific RNA polymerase sigma (σ) factors [2]. The spores produced in this process exhibit minimal if any metabolism, but are highly resistant to heat, radiation, desiccation and chemical agents [3, 4]. Consequently, these spores can survive for very long periods [5, 6], until they encounter an appropriate environment allowing spore germination and resumption of cell growth [7].
Spontaneous or induced chromosomal damage in either compartment of the sporangium may compromise the successful accomplishment of the sporulation program in B. subtilis. Consequently, sporulating cells deploy a variety of mechanisms to sense and process DNA lesions that could interfere with proper spore formation [8–11]. These mechanisms include: 1) the DNA-damage scanning protein (DisA) which operates early in sporulation delaying this developmental program until genomic lesions have been eliminated; and 2) the nucleotide excision repair (NER) pathway that processes DNA-distorting or -crosslinking lesions produced by ultraviolet irradiation and mitomycin-C (M-C), respectively. Interestingly, the efficiency of the NER system is enhanced by the transcription-coupling repair (TCR) factor, Mfd, during B. subtilis sporulation [11]. Moreover, the UV-endonuclease YwjD, which possibly works in concert with the error-prone polymerases YqjH and/or YqjW also operates during sporulation to eliminate UV-promoted DNA damage [9, 10]. Of note, genes encoding UvrA and UvrB repair proteins as well as YqjW are part of the SOS regulon, a gene circuitry that is under transcriptional control by RecA and DinR [12, 13]. In exponentially growing B. subtilis cells, the presence of strand breaks promote the formation of RecN foci; moreover, viability is compromised in growing RecA-deficient cells [14]. Interestingly, sporangia lacking RecA exhibit aberrant morphologies, which likely affect nucleoid condensation and ultimately result in a low sporulation frequency [15]. Low levels of RecA have been detected in dormant spores; however, the RecA concentration increases during spore outgrowth and its contribution to DNA repair during this developmental stage has been demonstrated [16]. Thus, spores lacking RecA exhibit decreased resistance to DNA damaging factors such as heat, UV irradiation, hydrogen peroxide, ultrahigh vacuum desiccation and ionizing radiation [16–19]. A recent study revealed that YabT, a Hanks-type protein kinase, phosphorylates RecA during sporulation promoting the formation of RecA foci that can assemble into a nucleofilament in response to DNA damage. Interestingly, persistence of this protein structure beyond late sporulation results in abortive spore morphogenesis, suggesting that phosphorylated RecA may contribute to a checkpoint that delays or prevents the completion of spore development in sporangia with severely damaged chromosomes [20].
Although it has been shown that DNA replication is important for assembly of RecA in response to DNA damage and likely for SOS induction [21], there is no current information regarding the role of this protein when the progress of the transcription complex becomes impaired due to DNA damage. This is important since during endospore formation, B. subtilis does not replicate its chromosomes, although the transcriptional program that is essential for endospore development continues [2, 22, 23]. We report here that expression of recA is induced by DNA-damaging factors during sporulation, and in both sporangium compartments, and that the activation of the SOS response by RecA is required to eliminate DNA lesions inflicted by UV-C and M-C in B. subtilis sporangia.
Materials and Methods
Bacterial strains and growth conditions
The B. subtilis strains and plasmids used in this study are described in Table 1. Bacterial cultures were grown at 37°C in Luria-Bertani medium (LB, [24]), Penassay broth (PAB) (antibiotic medium 3; Difco Laboratories, Sparks, MD) or Difco sporulation medium (DSM, [25]) with shaking at 250 rpm. When appropriate, spectinomycin (100 μg/mL), neomycin (12.5 μg/mL) or erythromycin (5 μg/mL) was added to media.
Table 1. B. subtilis strains and plasmids used in this study.
| Strain | Genotype and descriptiona | Construction or sourceb |
|---|---|---|
| 168 | Wild type, trpC2 | Laboratory stock |
| SL7360 | ΔrecA::neo (Neo) | Patrick Piggot |
| PERM938 | Δmfd::tet (Tet) | [11] |
| PERM1030 | ΔrecA::neo (Neo) | SL7360→168 |
| PERM1033 | Δmfd::tet, ΔrecA::neo (Tet Neo) | SL7360→PERM938 |
| PERM1233 | spoVFA::lacZ (Ery) | Laboratory stock |
| PERM1238 | amyE::PrecA-gfpmut3a (Spc) | pPERM1237→168 |
| PERM1238a | amyE::-gfpmut3a (Spc) | pPERM1237a→168 |
| PERM1280 | ΔrecA::neo, spoVFA::lacZ (Neo Ery) | SL7360→PERM1233 |
| YB3001 | amyE::recA-lacZ (Cm) | Ron Yasbin |
| LAS600 | Δupp | [31] |
| LAS523 | +dinR3 [lexA(Ind-)] | [31] |
| PERM1486 | LAS600 amyE::recA-lacZ (Cm) | YB3001 → LAS600 |
| PERM1487 | LAS523 amyE::recA-lacZ (Cm) | YB3001 → LAS523 |
| Plasmid | ||
| pAD123 | Shuttle gfpmut3a fusion vector (Amp Cm) | BGSC |
| pDR111 | amyE::Phyper-spank promoter (Phs) (Amp Spc) | David Rudner |
| pPERM1236 | pAD123 containing the PrecA-gfpmut3a construct (Amp Cm) | This study |
| pPERM1237 | pDR111 containing the PrecA-gfpmut3a construct (Amp Spc) | This study |
aSelection markers are in parentheses. Antibiotics were used at the following concentrations: Neo, neomycin (12.5 μg/mL); Tet, tetracycline (10 μg/mL); Ery, erythromycin (5 μg/mL); Spc, spectinomycin (100 μg/mL); Amp, ampicillin (100 μg/mL) and Cm, chloramphenicol (5 μg/mL).
b “X” → “Y” indicates that “strain Y” was transformed with DNA from “source X”.
BGSC, Bacillus Genetic Stock Center.
Strains construction
Standard techniques were used for strain construction, including isolation of chromosomal and plasmid DNA as well as for bacterial transformation [26–28].
To generate a translational PrecA-gfpmut3a gene fusion, a 492-bp EcoRI/BamHI fragment containing the recA promoter (-345 to +147 relative to the recA start codon) was PCR amplified using chromosomal DNA from B. subtilis 168. The oligonucleotide primers used for this reaction were 5’-GCGAATTCCAGGACCTGATGCTCAAG-3’ (forward) and 5’-GCGGATCCCAGTGCTGTATCAAGAGC-3' (reverse). Restriction sites (underlined) were included in the primers for cloning the amplified product between the EcoRI-BamHI sites of plasmid pAD123 to generate plasmid pPERM1236. This latter plasmid was cut with EcoRI and SphI and the resulting PrecA-gfpmut3a DNA fragment was cloned between the same restriction sites of plasmid pDR111 (a generous gift from David Rudner), giving plasmid pPERM1237 (amyE::PrecA-gfpmut3a). This plasmid was linearized and independently used to transform B. subtilis 168 to generate B. subtilis strains PERM1238 and PERM1238a, respectively.
Chromosomal DNA was isolated from B. subtilis YB3001 (amyE::recA-lacZ) and used to transform competent cells of B. subtilis strains LAS600 and LAS523, generating B. subtilis PERM1486 and PERM1487, respectively.
Chromosomal DNA from B. subtilis SL7360 (ΔrecA::neo) was used to transform competent cells of B. subtilis strains168, PERM938 and PERM1233, generating strains PERM1030 (ΔrecA), PERM1033 (Δmfd ΔrecA) and PERM1280 (ΔrecA; spoVFA-lacZ), respectively. Proper single- and double-crossover events leading to the integration of vectors and/or to the inactivation of the appropriate genes were confirmed in all cases by PCR (data not shown).
ß-galactosidase assays
ß-galactosidase activities during B. subtilis sporulation were determined as described by [29]. In brief, samples harvested from sporulating cultures were disrupted with lysozyme, centrifuged and the supernatant fluid was assayed for ß-galactosidase using ortho-nitrophenyl-ß-D-galactopyranoside (ONPG) as the substrate. This activity was assigned as the mother cell fraction, and consisted of enzyme from mother cells as well as lysozyme-sensitive forespores. The pellet fraction that contains lysozyme-resistant forespores present from 4 to 8 h after T0 (the time when the slopes of the logarithmic and stationary phases of growth intersected and defined as the time of initiation of sporulation) was subjected to spore coat removal [29], and a second lysozyme treatment was used to allow assay of the ß-galactosidase activity in forespores. All ß-galactosidase activities were expressed in Miller units [24], and the basal specific activities of ß-galactosidase in the RecA strain without a lacZ fusion during growth and sporulation were determined in parallel and subtracted from the data obtained for the strain carrying the recA-lacZ fusion. These corrections were always ≤ 10%.
Microscopic analysis
B. subtilis cells were grown and induced to sporulate in DSM. 1 hour after T0, sporulating cell samples were supplemented with FM4-64 (Invitrogen) at a final concentration of 5μg/ml. Cell samples collected at the appropriate times were processed for microscopy essentially as described previously [11] and samples were observed under a ZEISS Axioscope A1 microscope equipped with an AxioCam ICc1. Image collection was carried out using AxioVision V 4.8.2 software and images were adjusted only for brightness and contrast. Excitation and emission wavelengths employed were 498 nm and 512 nm for GFP and 506 and 750 nm for FM4-64, respectively. More than 400 sporulating cells expressing the GFPmut3a protein (from ~600 cells analyzed) were counted in four different fields at 100☓ for each sporulation time point.
Treatment of sporulating cells with DNA-damaging factors
B. subtilis strains were induce to sporulate in DSM at 37°C. At 4.5h after T0, the cultures were challenged with M-C or UV-C radiation as described previously [10]. Briefly, different doses of M-C were added to 2 mL cell samples and the cultures were incubated for additional 1 h before plating. Prior to UV-C treatment, cells were collected by centrifugation and washed with cold phosphate-buffered saline (PBS; 0.7% Na2HPO4, 0.3% KH2PO4, 0.4% NaCl [pH 7.5]). Cell samples (8 mL at an OD600 = 0.5) in PBS were stirred continuously and UV-C irradiated at room temperature. Artificial UV-C light (monochromatic 254 nm UV irradiation) was provided by a commercial low-pressure mercury arc lamp (model UVG-11; UV Products, Upland, CA). Cell survival after these treatments was determined by plating serial dilutions on solid LB medium, and colony-forming units were counted after 16 h of incubation at 37°C.
Results and Discussion
recA is induced by DNA damage during B. subtilis sporulation
Since RecA regulates the SOS response in B. subtilis [30], we generated a recombinant strain of B. subtilis to monitor in situ the transcriptional activation of the SOS response by DNA damage. To this end, the gfpmut3a reporter gene was fused to the recA promoter and the resulting PrecA-gfpmut3a fragment was recombined into the amyE locus of B. subtilis.
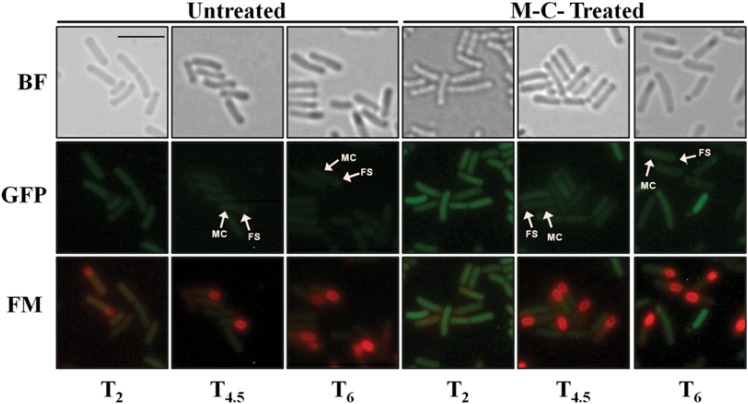
To demonstrate that the SOS response is active during sporulation [11], the B. subtilis PrecA-gfpmut3a strain was induced to sporulate, cell samples collected at 2, 4.5 and 6 hr after the onset of sporulation, were treated with M-C and then examined by fluorescence microscopy. The results of this analysis showed that the DNA damage promoted by M-C induced the synthesis of the GFPmut3a protein in the three-sporulation stages analyzed. Interestingly, in all cases, the GFP signal was detected in both the mother cell and the forespore compartments (Fig 1), indicating that DNA damage can induce the RecA-dependent SOS response in both compartments of the sporangium. In contrast, low fluorescence was detected in untreated sporangia of the PrecA-gfpmut3a strain that were collected at the same developmental stages (Fig 1).
Fig 1. Monitoring of activation of the SOS response induced by DNA damage using a PrecA-gfpmut3a during sporulation.
Sporulating cells of B. subtilis PERM1238 (PrecA-gfpmut3a) collected at different times after T0, in the sporulation program (T2, T4.5 and T6) were treated with M-C (125 ng/mL), incubated for additional 1 h and finally observed under fluorescence microscopy. BF, bright field; GFP, GFP channel; FM, FM4-64 staining; MC and FS, mother cell and forespore compartments. The scale bar is 2 μm and all images are at the same magnification.
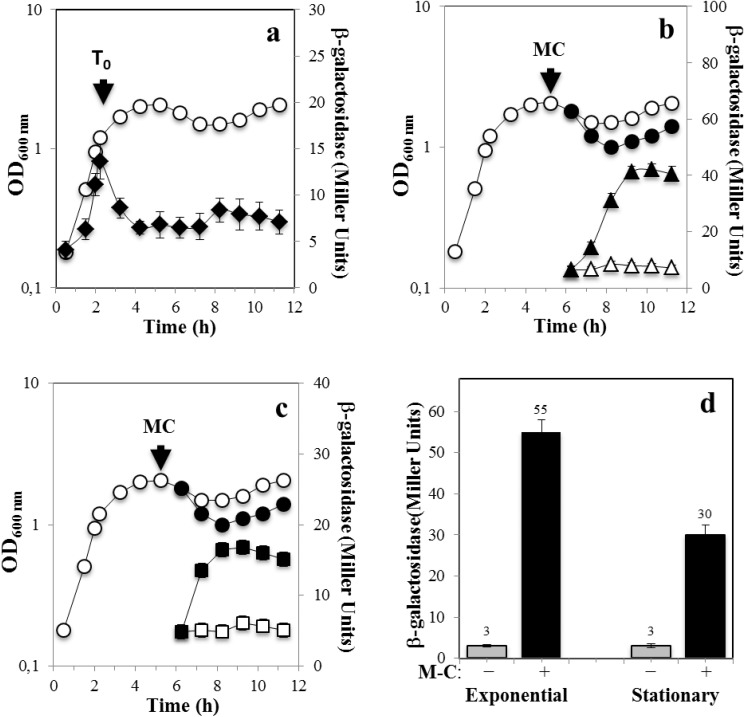
To better assess and quantify the recA expression during the life cycle of B. subtilis, we used a strain carrying a transcriptional recA-lacZ fusion and determined the levels of ß-galactosidase produced during exponential growth and sporulation and in both the mother cell and forespore compartments. The results of this analysis revealed that the recA-driven ß-galactosidase activity was present in exponentially growing cells as well as in developing sporangia (Fig 2a). However, the expression levels of the recA-lacZ fusion were significantly lower in sporulation than during logarithmic growth (Fig 2a). These results are in agreement with a previous study that detected a lower amount of RecA in dormant spores than in vegetative cells [16]. Moreover, as shown in Fig 2d, addition of M-C to exponentially growing cells increased ~18 fold the expression levels of the recA-lacZ fusion.
Fig 2. Levels of ß-galactosidase from recA-lacZ in growth and sporulation and with and without DNA damage.
B. subtilis strain YB3001 containing a recA-lacZ fusion was grown and induced to sporulate in DSM (a-c) The optical densities of cultures were measured without (○) or after (●) DNA damaging treatment. Samples were also collected at different times during growth and sporulation and were processed and assayed for ß-galactosidase specific activity. In (a) ß-galactosidase from recA-lacZ was assayed throughout growth and sporulation (◆). In (b,c) 4 h after the onset of sporulation (T0), the culture was divided into two subcultures; one subculture was challenged with M-C (500 ng/mL) and the other one was untreated. Cells samples from untreated (open symbols) or treated (filled symbols) were collected at the indicated times and ß-galactosidase specific activity in the mother cell (b, triangles) and forespore (c, squares) fractions was determined, all as described in Materials and Methods. In (d), B. subtilis YB3001 was propagated in PAB medium, when the culture reached an OD600nm = 0.5 (Exponential) or 4 h after T0 (Stationary), vegetative cells were treated (black bars) or not (gray bars) with M-C (500 ng/mL) for 1.5 h and then the cultures were processed for determination of ß-galactosidase as described above. Results are the average of values from three independent experiments ± standard deviations (SD) of ß-galactosidase specific activity.
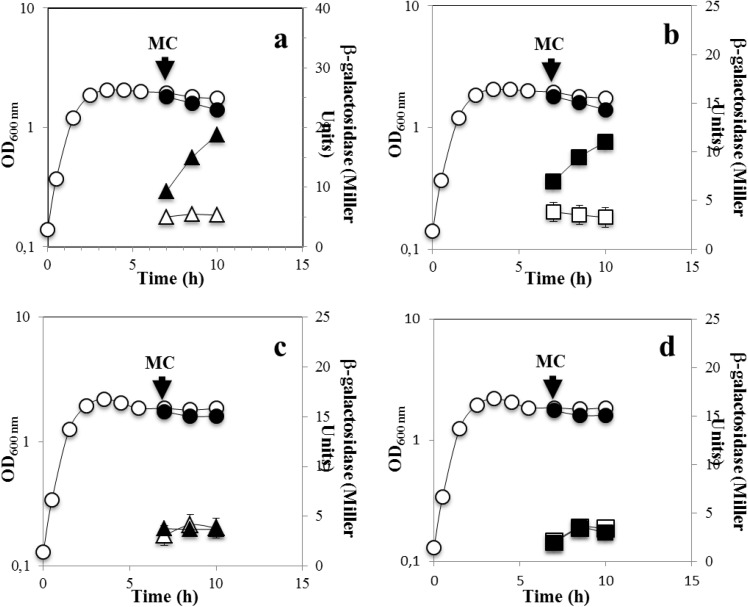
A recent report showed that M-C was able to induce the expression of a chromosomal uvrA-lacZ fusion during stage IV of sporulation suggesting that the SOS response may be triggered in this stage of sporulation [11]. To further examine this suggestion, the recA-lacZ strain was treated with M-C 4 h after the onset of sporulation. Notably, in comparison with an untreated sporulating culture, addition of this alkylating agent increased the expression of the recA-lacZ 6- and 3-fold in the mother cell and the forespore compartment fractions, respectively (Fig 2b and 2c). Taken together, these results support the notion that in B. subtilis cells committed to sporulate, the SOS response can be activated and is possibly required to induce the expression of DNA repair proteins necessary to eliminate genetic insults that may compromise spore formation. In support of this contention, expression of the recA-lacZ fusion in both sporangia compartments was abolished in a mutant strain of B. subtilis unable to trigger the SOS response as it carries a non-cleavable form of the SOS-repressor protein DinR [31] (Fig 3).
Fig 3. Levels of ß-galactosidase from recA-lacZ with and without DNA damage during sporulation in SOS-proficient (a and b) and –deficient (c and d) B. subtilis strains.
B. subtilis strains LAS600 (parental) and LAS523 (SOS-deficient) containing a recA-lacZ fusion were grown and induced to sporulate in DSM. The optical densities of cultures were measured without (○) or after (●) DNA damaging treatment. 4 h after the onset of sporulation (T0), the culture was divided into two subcultures; one subculture was challenged with M-C (500 ng/mL) and the other one was untreated. Cells samples from untreated (open symbols) or treated (filled symbols) were collected at the indicated times and ß-galactosidase specific activity in the mother cell (a and c, triangles) and forespore (b and d, squares) fractions was determined, all as described in Materials and Methods.
Of note, in comparison with exponentially growing cells (Fig 2d), the extent of induction of the recA-lacZ fusion by M-C in strain YB3001 was ~2 fold lower during sporulation (Fig 2b and 2c) as well as during the stationary phase in vegetative cells propagated in PAB, a rich medium unable to promote B. subtilis sporulation [32] (Fig 2d). Therefore, the SOS-response can be elicited in B. subtilis despite unfavorable metabolic conditions prevailing in cells committed to sporulation or facing nutritional stress.
Inactivation of recA delays sporulation
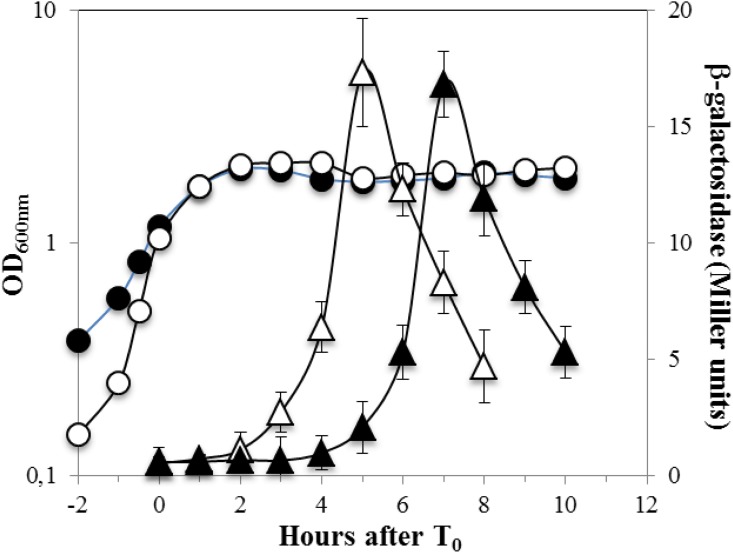
It has been reported that a RecA-deficient B. subtilis strain exhibits morphological irregularities and a delayed prespore nucleoid condensation during sporulation [15], suggesting that RecA is required for normal sporulation. Therefore, we investigated further whether the lack of RecA interferes with growth and sporulation in B. subtilis. Our results showed that the growth rate of a ΔrecA strain in DSM at 37°C had a significantly longer doubling time compared to the wild-type strain (33 ± 2 min and 24 ± 2 min, respectively; unpaired t-test, P < 0.05). Moreover, as previously described [15], the sporulation efficiency in the RecA-deficient strain was ~40% lower than that of the wild-type strain (data not shown). To directly examine effects of loss of RecA on sporulation-specific gene expression, we examined the expression of a spoVFA-lacZ fusion was used as a marker of gene expression late in sporulation of wild-type and ΔrecA strains. The spoVFA gene encodes for subunit A of the dipicolinic acid (DPA) synthetase [33], and is expressed exclusively in the mother cell compartment reaching its highest expression level at T5 of sporulation [33, 34]. Analysis of spoVFA-lacZ expression during sporulation of the wild-type and ΔrecA strains revealed a ~2 h delay in expression of spoVFA-lacZ fusion in the ΔrecA mutant compared to the wild-type parental strain (Fig 4). Therefore, the morphological abnormalities observed in RecA-deficient sporangia [15] seem to be accompanied and presumably caused by alterations in the program of gene expression that drives the process of sporulation in B. subtilis. Notably, a recent report showed that B. subtilis cells expressing a non-phosphorylatable RecA protein also exhibited slowed sporulation [20]. Taken together, these results highlight the importance of RecA in this developmental process, as a deficiency in this protein slows sporulation significantly, presumably as a consequence of the resulting transcriptional abnormalities.
Fig 4. Expression of spoVFA-lacZ during sporulation of wild-type and ΔrecA B. subtilis strains.
B. subtilis strains PERM1233 (wild-type) (open symbols) and PERM1280 (ΔrecA) (closed symbols) containing the spoVFA-lacZ fusion were grown and induced to sporulate in DSM and the OD600nm (circles) was measured. Cells were collected during sporulation at the indicated times, treated with lysozyme and the extracts were assayed for ß–galactosidase in the mother cell fractions only (triangles). Results are the averages of values from three independent experiments ± SD of ß-galactosidase specific activity.
RecA protects B. subtilis sporulating cells from DNA damage
In stage T4.5 where sporangial cells are commited to sporulate, the mother cell is programmed to lyse whereas forespores will eventually give rise to a dormant spore [2]. Therefore surviving colonies arising after treatment of T4.5 sporulating cells with genotoxic agents are mostly generated from forespores [10]. Keeping these facts in mind, to investigate whether RecA contributes to the survival of sporulating cells treated with DNA damaging agents, we examined the resistance of wild-type and ΔrecA B. subtilis sporangia to M-C and UV-C radiation.
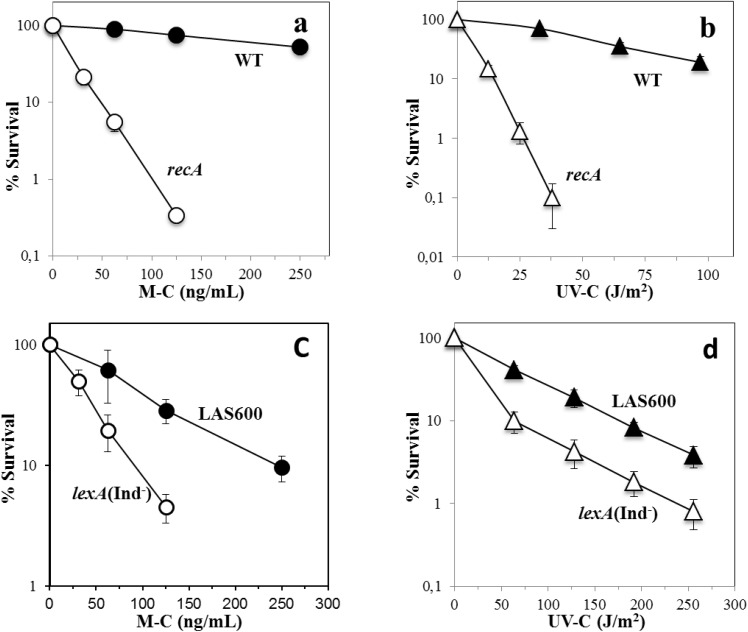
As noted above, the RecA-deficient strain is slowed ~2 h in sporulation. Therefore, in these experiments sporulating cells of the recA and parental strains were collected at equivalent morphological stages in sporulation (stages IV-V), as assessed by microscopic analysis. As shown in Fig 5a and 5b, in comparison with the wild-type strain the recA mutant exhibited an increased susceptibility to both M-C and UV-C radiation during sporulation. These two agents are known to produce lesions that block the progress of transcription elongation complexes [35, 36]. Notably, a previous report showed that the TCR factor Mfd plays a key role in protecting sporulating cells against M-C and UV-C [11], and the sporangia´s susceptibility to these genotoxic agents was greater in the ΔrecA mutant than in the Mfd-deficient strain (Table 2). Together, these results strongly suggest that RecA as well as Mfd are necessary during spore morphogenesis to process pyrimidine dimers as well as DNA crosslinks generated by UV-C and M-C, respectively.
Fig 5. Resistance of ΔrecA and SOS-deficient strains to M-C (a, c) and UV-C radiation (b, d) during sporulation.
Sporulating cells of strains 168 (wild-type), PERM1030 (ΔrecA), LAS600 (parental) and LAS523 (lexA[ind-]) were treated (open symbols) or not (filled symbols) with increasing doses of M-C (a and c, circles) or UV-C light (b and d, triangles) at 4.5 h (wild-type, LAS600 and LAS523) or 6.5 h (ΔrecA) after the onset of sporulation, and cell survival was determined as described in Materials and Methods. Data are expressed as the average ± SD of at least three independent experiments.
Table 2. Killing of B. subtilis forespores of various strains by DNA-damaging treatments.
| Strain | LD90a | |
|---|---|---|
| M-C (ng/mL) | UV-C (J/m2) | |
| WT (168) | 1,347 ± 74.4 b | 119.2 ± 14.6 b |
| PERM938 (Δmfd) | 187.7 ± 13.2 c | 20.8 ± 3.9 c |
| PERM1030 (ΔrecA) | 53.1 ± 5.7 d | 16.1 ± 3.7 c |
| PERM1PP033 (Δmfd recA) | 12.7 ± 2.9 e | 3.9 ± 1.1 d |
a B. subtilis sporangia from stage T4.5 were exposed to different concentrations of M-C or different doses of UV-C irradiation in order to determine the lethal dose to kill 90% of initial colony forming units (LD90). Results are expressed as averages ± SD of at least three independent experiments.
Superscripts b, c, d and e indicate statistically significant differences between strains under the same treatment as determined by one-way ANOVA followed by a Tukey’s post hoc test; P < 0.05.
Since cells committed to sporulate no longer replicate the chromosomes in the mother cell and the forespore compartments [2, 23], a recombination-repair role for RecA in this developmental stage can largely be ruled out. Rather, as shown in this work, the function of RecA is most likely to activate the SOS response to provide B. subtilis sporangia with the repair machinery to process DNA lesions occurring during sporulation. The experiments in Fig 5c and 5d provided further support for this contention by showing that in comparison with its parental strain (B. subtilis LAS600), sporulating cells of the SOS-deficient lexA (Ind-) mutant exhibited a major susceptibility to UV-C and M-C. Consistent with a previous report in dormant spores treated with ionizing radiation [19], our results revealed that sporangia harboring the lexA (Ind-) allele were less susceptible to M-C and UV-C than RecA-deficient sporangia (Fig 5). These analyses, also found that the SOS-deficient sporangia exhibited a higher resistance to UV-C than to M-C. However, in addition to the NER pathway sporulating cells rely on Mfd as well as an alternative excision repair pathway to counteract the noxious effects of UV-C [10, 11].
Since spore formation in B. subtilis requires the activation of hundreds of genes, it seems possible that most if not all DNA repair activity during this developmental process will be dedicated to eliminate damage from actively transcribing genes. Thus DNA repair proteins induced in the SOS response may work not only alone, but also in concert with Mfd as indicated by the following evidence. First, uvrA, a component of the NER machinery and a member of the SOS regulon, was induced by DNA damage in both sporangial compartments [11]. In addition, the absence of this essential NER component sensitized sporulating cells to UV-C and M-C and the inactivation of both Mfd and NER made sporangia even more sensitive to these agents [11]. Second, a UV-endonuclease encoded by ywjD operates through an alternative excision repair mechanism to eliminate UV-induced DNA damage in sporulating cells and dormant spores of B. subtilis [10]. Of note, a previous report have revealed that in addition to the spatio-temporal program that drives ywjD expression during sporulation [10], this gene is induced during spore germination by UV light in a RecA-independent manner [37]. Third, a recent study suggested that under conditions that overwhelm the repair capacity of the NER system, YwjD may repair UV-C-promoted lesions in an error-prone manner employing the polymerase activity of the low-fidelity enzymes YqjH and/or YqjW [11]. Indeed, YqjW also belongs to the SOS regulon [38], and is operative during B. subtilis sporulation and protects the forespore genome against mutagenic effects of UV-C irradiation [9].
Interestingly, as shown in Table 2, in comparison with sporangia lacking either RecA or Mfd, the absence of both functions increased the sporangia´s susceptibility to M-C and UV-C irradiation even more. These results strongly suggest that RecA and Mfd work independently to eliminate MC and UV-C promoted lesions; although it is also possible that by triggering the SOS response, RecA collaborates in the Mfd-mediated TCR pathway to eliminate genetic insults that may interfere with spore development.
Finally, as demonstrated in this work, RecA by triggering the SOS response provides the sporulating cells with the repair machinery necessary to eliminate genetic DNA damage. However, DNA lesions that are left unrepaired in the forespore compartment of sporangia or accumulated during dormancy are also eliminated during spore germination/outgrowth with participation of RecA in order to guarantee an ‘appropriate return’ to life of the dormant spores [16, 19, 39].
Acknowledgments
The authors wish to acknowledge Victor M. Ayala-Garcia for the excellent assistance with microscopic analysis and are deeply grateful to Patrick Piggot (RIP), Lyle A. Simmons and Ralf Moeller for kindly donating strains SL7360, LAS600 and LAS523, respectively. We also thank to anonymous referees for pertinent suggestions.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT; Grants 205744 and 221231) and Universidad de Guanajuato (UGTO; Grant 602-215). F.H.R-G., R.C.B-O. and S.U.C-B. were supported by scholarships from CONACYT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996; 30: 297–341. 10.1146/annurev.genet.30.1.297 [DOI] [PubMed] [Google Scholar]
- 2.Errington J. Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol. 2003; 1: 117–126. 10.1038/nrmicro750 [DOI] [PubMed] [Google Scholar]
- 3.Setlow P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. Soc Appl Bacteriol Symp Ser. 1994; 23: 49S–60S. . [DOI] [PubMed] [Google Scholar]
- 4.Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000; 64: 548–572. 10.1128/MMBR.64.3.548-572.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy MJ, Reader SL, Swierczynski LM. Preservation records of micro-organisms: evidence of the tenacity of life. Microbiol. 1994; 140: 2513–2529. . [DOI] [PubMed] [Google Scholar]
- 6.Pedraza-Reyes M, Ramírez-Ramírez N, Vidales-Rodríguez LE, Robleto EA. Mechanisms of bacterial spores survival In: Abel-Santos E, editor. Bacterial Spores: Current Research and Applications. Caister Academic Press; Norfolk, UK; 2012. pp. 73–84. [Google Scholar]
- 7.Setlow P. Spore germination. Curr Opin Microbiol. 2003; 6 (6): 550–556. . [DOI] [PubMed] [Google Scholar]
- 8.Bejerano-Sagie M, Oppenheimer-Shaanan Y, Berlatzky I, Rouvinski A, Meyerovich M, Ben-Yehuda S. A checkpoint protein that scans the chromosome for damage at the start of sporulation in Bacillus subtilis. Cell. 2006; 125: 679–690. [DOI] [PubMed] [Google Scholar]
- 9.Rivas-Castillo AM, Yasbin RE, Robleto E, Nicholson WL, Pedraza-Reyes M. Role of the Y-family DNA polymerases YqjH and YqjW in protecting sporulating Bacillus subtilis cells from DNA damage. Curr Microbiol. 2010; 60: 263–267. 10.1007/s00284-009-9535-3 [DOI] [PubMed] [Google Scholar]
- 10.Ramírez-Guadiana FH, Barraza-Salas M, Ramírez-Ramírez N, Ortiz-Cortes M, Setlow P, Pedraza-Reyes M. Alternative excision repair of ultraviolet B- and C-induced DNA damage in dormant and developing spores of Bacillus subtilis. J Bacteriol. 2012; 194: 6069–6104. 10.1128/JB.01340-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramírez-Guadiana FH, Barajas-Ornelas RC, Ayala-García VM, Yasbin RE, Robleto EA, Pedraza-Reyes M. Transcriptional coupling of DNA repair in sporulating Bacillus subtilis cells. Mol Microbiol. 2013; 90: 1088–1099. 10.1111/mmi.12417 [DOI] [PubMed] [Google Scholar]
- 12.Yasbin RE, Cheo D, Bayles KW. The SOB system of Bacillus subtilis: A global regulon involved in DNA repair and differentiation. Res Microbiol. 1991; 142: 885–892. [DOI] [PubMed] [Google Scholar]
- 13.Goranov AI, Kuester-Shoeck E, Wang JD, Grossman AD. Characterization of the global transcriptional responses to different types of DNA damage and disruption of replication in Bacillus subtilis. J Bacteriol. 2006; 188: 5595–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez H, Kidane D, Castillo Cozar M, Graumann PL, Alonso JC. Recruitment of Bacillus subtilis RecN to DNA doublé-strand breaks in the absence of DNA end processing. J Bacteriol. 2006;188: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sciochetti SA, Blakely GW, Piggot P. Growth phase variation in cell and nucleoid morphology in a Bacillus subtilis recA mutant. J Bacteriol. 2001; 183: 2963–2968. 10.1128/JB.183.9.2963-2968.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setlow B, Setlow P. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol. 1996; 178: 3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanlin JH, Lombardi SJ, Slepecky RA. Heat and UV light resistance of vegetative cells and spores of Bacillus subtilis Rec-mutants. J Bacteriol. 1985; 163: 774–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibarra JR, Orozco AD, Rojas JA, López K, Setlow P, Yasbin RE, et al. Role of the Nfo and ExoA apurinic/apyrimidinic endonucleases in repair of DNA damage during outgrowth of Bacillus subtilis spores. J Bacteriol. 2008; 190: 2031–2038. 10.1128/JB.01625-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlašić I, Mertens R, Seco EM, Carrasco B, Ayora S, Reitz G, et al. Bacillus subtilis RecA and its accessory factors, RecF, RecO, RecR and RecX, are required for spore resistance to DNA double-strand break. Nucleic Acids Res. 2014; 42: 2295–2307. 10.1093/nar/gkt1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bidnenko V, Shi L, Kobir A, Ventroux M, Pigeonneau N, Henry C, et al. Bacillus subtilis serine/threonine protein kinase YabT is involved in spore development via phosphorylation of a bacterial recombinase. Mol Microbiol. 2013; 88: 921–935. 10.1111/mmi.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons LA, Grossman AD, Walker GC. Replication is required for the RecA localization response to DNA damage in Bacillus subtilis. Proc Natl Acad Sci USA. 2007; 104: 1360–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilbert DW, Piggot PJ. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol Mol Biol Rev. 2004; 68: 234–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veening JW, Murray H, Errington J. A mechanism for cell cycle regulation of sporulation initiation in Bacillus subtilis. Genes Dev. 2009; 23: 1959–1970. 10.1101/gad.528209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 25.Schaeffer P, Millet J, Aubert JP. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965; 54: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: 2001. [Google Scholar]
- 27.Cutting SM, Vander Horn PB. Genetic analysis In Harwood CR C.R. and SM Cutting, editors. Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, England: 1990; p. 27–74. [Google Scholar]
- 28.Boylan RJ, Mendelson NH, Brooks D, Young FE. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972; 110: 281–290. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson W, Setlow P. Sporulation germination and outgrowth In Harwood CR and Cutting SM, editors. Molecular Biological Methods for Bacillus. John Wiley & Sons Ltd, Chichester, UK: 1990; pp. 391–450. [Google Scholar]
- 30.Cheo DL, Bayles KW, Yasbin RE. Cloning and characterization of DNA damage-inducible promoter regions from Bacillus subtilis. J Bacteriol. 1991; 173: 1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons LA, Goranov AI, Kobayashi H, Davies BW, Yuan DS, Grossman AD, et al. Comparison of responses to double-strand breaks between Escherichia coli and Bacillus subtilis reveals different requirements for SOS induction. J. Bacteriol. 2009; 91: 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung HM and Yasbin RE. Adapative, or stationary-phase, mutagenesis, a component of bacterial differentiation in Bacillus subtilis J. Bacteriol. 2002; 184: 5641–5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniel RA, Errington J. Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipicolinic acid synthetase required for sporulation in Bacillus subtilis. J Mol Biol. 1993; 232: 468–483. . [DOI] [PubMed] [Google Scholar]
- 34.Setlow B, McGinnis KA, Ragkousi K, Setlow P. Effects of major spore-specific DNA binding proteins on Bacillus subtilis sporulation and spore properties. J Bacteriol. 2000; 182: 6902–6912. 10.1128/JB.182.24.6906-6912.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tornaletti S, Hanawalt PC. Effect of DNA lesions on transcription elongation. Biochimie. 1999; 81: 139–46. . [DOI] [PubMed] [Google Scholar]
- 36.Dronkert ML, Kanaar R. Repair of DNA interstrand cross-links. Mutat Res. 2001; 486: 217–247. 10.1016/S0921-8777(01)00092-1 [DOI] [PubMed] [Google Scholar]
- 37.Moeller R. Characterization of different types of radiation- and pressure-induced DNA damage in Bacillus subtilis spores and their global transcriptional response during spore germination. PhD Thesis, Technical University of Braunschweig, Germany. 2008.
- 38.Duigou S, Ehrlich SD, Noirot P, Noirot-Gros MF. Distinctive genetic features exhibited by the Y-family DNA polymerases in Bacillus subtilis. Mol Microbiol. 2004; 54: 439–451. . [DOI] [PubMed] [Google Scholar]
- 39.Ibarra JR, Orozco AD, Rojas JA, López K, Setlow P, Yasbin RE, et al. Role of the Nfo and ExoA apurinic/apyrimidinic endonucleases in repair of DNA damage during outgrowth of Bacillus subtilis spores. J. Bacteriol. 2008; 190: 2031–2038. 10.1128/JB.01625-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.