Abstract
Background
The clinical usefulness of flow cytometry (FCM) for the diagnosis of leptomeningeal diseases (LMD) in non-Hodgkin lymphomas has been suggested in previous studies but needs to be further validated. With this regards, we evaluated the use of FCM for LMD in a series of Korean patients with non-Hodgkin lymphoma.
Methods
FCM and cytomorphology were conducted using samples obtained from clinically suspected LMD patients, follow-up LMD patients, and those with high risk of developing tumorigenic diseases. We then compared results of FCM and cytomorphology. In total, 55 and 47 CSF samples were analyzed by FCM and cytomorphology, respectively.
Results
Of the samples analyzed, 25.5% (14/55) and 12.8% (6/47) were positive by FCM and cytomorphology, respectively. No samples were determined as negative by FCM but positive by cytomorphology. Seven patients were positive only by FCM and negative by cytomorphology, and six among them were clinically confirmed to have LMD either by follow-up cytomorphology or imaging study.
Conclusions
We observed a high detection rate of tumor cells by FCM compared with cytomorphology. FCM study can be useful in early sensitive detection of LMD.
Keywords: Flow cytometry, CSF, Leptomeningeal diseases
INTRODUCTION
Leptomeningeal disease (LMD) is a fatal complication of non-Hodgkin lymphoma (NHL); therefore, early and accurate diagnosis of LMD is important for the management of NHL. Although conventional cytomorphology is considered as the standard method for the diagnosis of LMD, its utility is limited owing to a low sensitivity with a false-negative rate of 20-60% [1,2]. Flow cytometry (FCM) has been used for rapid and sensitive diagnosis of various hematologic malignancies [3,4]. FCM analysis has also been demonstrated as an excellent diagnostic tool for detecting leukemia and lymphoma cells in various body fluids [5,6].
The utility of FCM for detecting LMD in cerebrospinal fluid (CSF) has also been evaluated in some previous studies, showing promising results [3,7]. However, the techniques are tricky owing to the low cell counts, high proportion of non-viable cells in CSF, and necessity for implementing multi-color FMC antibodies. Furthermore, clinical testing methods have not been standardized yet. Because the testing was validated only in a limited number of clinical cohorts, the method and its clinical implications should be further validated in various clinical settings.
In the present study, we explored the clinical usefulness of FCM for the diagnosis of LMD in a series of Korean NHL patients in comparison with the traditional cytology method. In addition, we analyzed the prognostic effect of LMD confirmed by FCM.
METHODS
1. Patient enrollment
CSF specimens were obtained in patients with clinically suspected LMD (headache, mental change, paralysis and/or other neurologic sign and symptoms) or high risk diseases (Burkitt lymphoma or multiple metastases of primary tumors) from March 2013 to May 2014 at Samsung Medical Center in Seoul, Korea. For each specimen, cytomorpholgical and FCM analyses were conducted. A total of 55 samples from 30 patients, including 26 males and 4 females (mean age: 51 yr) were analyzed by using FCM, and 47 of them were also analyzed by cytomorphology in parallel. Eight samples were collected at initial work-up, and the others were obtained during follow-up periods. Diffuse large B cell lymphoma (DLBCL) was the most common neoplasm analyzed, and cases of other rare lymphomas including extranodal NK/T cell lymphoma (ENKTL), anaplastic large cell lymphoma (ALCL) and Burkitt lymphoma (BL) were also included (Supplemental Data Table 1). The number of CD45-positive cells acquired by the FCM instrument ranged from 387 to 1,568,515.
The medical records were reviewed in detail for all cases of FCM and cytomorphology to analyze overall survival (OS). The current study was based on an analysis of routine clinical test results with patients' consent, and the approval of institutional review board was waived.
2. Flow cytometric analysis of lymphoma cells
FCM analysis was done on the FACSCantoII flow cytometer (Becton Dickinson, San Jose, CA, USA) and the Kaluza software (Beckman Coulter, Brea, CA, USA). Compensation matrix was calculated by using BD CompBead particles (Becton Dickinson) and the compensation setup tool in BD FACSDiva software. The minimum white blood cell (WBC) count in CSF was 1×106/L (1-1,530×106/L).
For mature B-cell malignancy, 7-aminoactinomycin D (7-AAD) and a combination of antibodies including CD45-allophycocyanin-cyanine7 (APC-Cy7), CD19-APC, surface kappa-fluorescein isothiocyanate (FITC), and surface lambda-phycoerythrin (PE) were used. The criteria for determining the results were according to previous literatures [7,8,9] and as follows: (i) positive; ≥10 B-cells with a kappa/lambda (K/L) ratio of >4 or <0.5, (ii) negative: B-cells with a K/L ratio between 0.5 and 4, and (iii) equivocal; <10 B-cells with a K/L ratio of >4 or <0.5. For mature T- or NK-cell neoplasms, we selected surface markers according to the immunophenotypes of primary tumors characterized by immunohistochemistry or FCM. 7-AAD dye was used to select the viable cells [10]. A typical gating strategy is shown in Supplemental Data Fig. 1. All monoclonal antibodies were from Becton Dickinson.
3. Cytomorphology and CSF analysis
CSF samples were processed within several hours of collection and interpreted by a cytopathologist blinded to the FCM results. Liquid-based cytology (LBC) methods and Papanicolaou stain were used in the cytomorphological analysis as previously described [11]. Results were reported as negative, positive, and equivocal for lymphoma on the basis of cellular morphology. Lymphocytes or abnormal cells with abnormal nuclear-cytoplasmic ratios and irregular nuclear morphologies were categorized as "equivocal", which cannot be clearly categorized as "positive" [12]. WBC count was obtained by manual differential counting (100 WBCs), and protein and glucose levels were measured (COBAS Integra800, Roche Diagnostics, Mannheim, Germany).
4. Statistical analysis
The Mann-Whitney test was used to evaluate the difference of CSF protein, glucose, and WBC levels according to results of FCM or cytomorphology. A kappa value was calculated to investigate concordance rates. Kappa values over 0.8 were considered as good agreement. OS (Kaplan-Meier method) of DLBCL in FCM positive and FCM negative or equivocal cases was measured to analyze the prognostic effect of FCM-positive cases from the time of lymphoma diagnosis to death or last follow-up. A P value <0.05 was considered statistically significant. Statistical analysis was performed by using the PASW Statistics 20.0 software (IBM, Armonk, NY, USA).
RESULTS
Fourteen (25.5%) of the 55 samples analyzed by FCM were positive, whereas only six (12.8%) of the 47 samples examined by cytomorphology were positive. All six cytomorphologically positive cases had positive results by FCM, while eight samples from seven patients were determined as positive by FCM but negative or equivocal by cytomorphology (Table 1). FCM had lower rates of equivocal results than cytomorphology (2/55 vs. 7/47), and the overall concordance between FCM and cytomorphology was 70.2% (kappa value=0.377, P<0.001). The low concordance rate was due to the low positive rates in cytomorphology (positive by FCM and negative by cytomorphology). The types of lymphoma in the positive cases are shown in Table 2.
Table 1. Comparison of flow cytometry and cytomorphology in cerebrospinal fluid from suspected cases of leptomeningeal diseases.
| FCM | Cytomorphology | ||||
|---|---|---|---|---|---|
| Positive | Negative | Equivocal | NA | Total | |
| Positive | 6 | 5 | 3 | - | 14 |
| Negative | - | 27 | 4 | 8 | 39 |
| Equivocal | - | 2 | - | - | 2 |
| Total | 6 | 34 | 7 | 8 | 55 |
Abbreviations: FCM, flow cytometry; NA, not available.
Table 2. Positive cases according to types of lymphoma.
| Positive | ||
|---|---|---|
| Flow cytometry | Cytomorphology | |
| Anaplastic large B-cell lymphoma | 1 | 1 |
| Burkitt lymphoma | ||
| Diffuse large B cell lymphoma | 9 | 4 |
| Extranodal marginal zone lymphoma | 1 | |
| Extranodal NK/T cell lymphoma | 2 | 1 |
| Mature T cell neoplasm, not otherwise specified | 1 | |
| 14 (11 patients) | 6 (5 patients) | |
The clinical characteristics of discrepant cases that were positive by FCM and negative/equivocal by cytomorphology are summarized in Table 3. Among these seven patients, four (patient no. 1, 5, 9, and 11) had history of LMD, and their CSF samples were obtained during treatment of LMD. Patients 1 and 9 were initially positive by FCM and equivocal/negative by cytomorphology but were found to be positive by cytomorphology later in the follow-up study. The CD45-positive cells ranged from 636 to 94,258 in these discrepant cases.
Table 3. Summary of discrepant cases that were positive by FCM and negative/equivocal by cytomorphology.
| Patient-sample | Age (yr) |
Sex | Histology | WBC count (×106/L) |
FCM | CM | Reason for request | IT-CTx | Positive in previous study | Positive in f/u study | Imaging study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 45 | F | DLBCL (neck LN) | 40 | P | E | f/u of LMD, brain, breast metastasis mental change | Yes | Yes (by CM) | Yes (by CM) | 1 month f/u PET: not evident LMD |
| 1-2 | 2 | P | N | 3 days after | Yes | Yes (by CM) | Yes (by CM) | ||||
| 3-3 | 46 | M | DLBCL (brain) | 1 | P | N | Lower extremity weakness, dysuria | Yes | Yes (by CM) | No | |
| 5 | 53 | M | DLBCL (neck LN) | 18 | P | N | f/u of recurred DLBCL (nasal cavity), diplopia | Yes | No | No | Brain MRI: skull base enhancement |
| 6* | 51 | M | EMZL (lung) | 5 | P | E | Brain imaging work-up – brain involvement | Yes | No | No | Brain MRI: both cavernous |
| 7* | 66 | M | Mature T cell neoplasm (small intestine) | 1 | P | N | HLH, BM involvement | No (expired) | No | No | Brain CT: not evident |
| 9 | 48 | M | ENKTL (nasal cavity) | 5 | P | E | f/u of recurred ENKTL (liver, BM) visual disturbance | Yes | No | Yes (by CM) | Orbit MRI: LMD |
| 11 | 34 | M | DLBCL (brain) | 1 | P | N | f/u of recurred (nasal cavity, brain) DLBCL, f/u of LMD | Yes | Yes (by CM) | No | Brain MRI: periventricular enhancement |
*Initial work-up of primary hematologic malignancy.
Abbreviations; BM, bone marrow; CT, computed tomography; CM, cytomorphology; DLBCL, diffuse large B cell lymphoma; E, equivocal; EMZL, extranodal marginal zone lymphoma; ENKTL, extranodal NK/T cell lymphoma; FCM, flow cytometry; F, female; f/u, follow up; HLH, hemophagocytic lymphohistiocytosis; IT-CTx, intrathecal chemotherapy; LMD, leptomeningeal disease; LN, lymph node; M, male; N, negative; NA, not available; P, positive; PET, positron emission tomography; MRI, magnetic resonance imaging.
The clinical characteristics of other discrepant cases are summarized in Table 4. Patients 3, 23, and 26 were negative by FCM and equivocal by cytomorphology, while patients 13 and 20 were equivocal by FCM and negative by cytomorphology. All patients except patient 13 had histories of LMD (samples were obtained during treatment). The CD45-positive cells ranged from 437 to 12,349 in these discrepant cases. CSF protein and WBC levels were significantly higher in positive samples (determined by FCM) compared with negative samples, while CSF glucose levels were not significantly different (Table 5).
Table 4. Summary of other cases showing discrepant results.
| Patient-sample | Age (yr) |
Sex | Histology | WBC count (×106/L) | FCM | CM | Reason for request | IT-CTx | Positive in previous study | Positive in f/u study | Imaging study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-2 | 46 | M | DLBCL (brain) | 1 | N | E | f/u of LMD | Yes | Yes (by CM, FCM) | No | MRI: persistent diffuse leptomeningeal abnormal enhancement |
| 3-4 | 1 | N | E | 10 days after | Yes | Yes (by CM, FCM) | No | ||||
| 13 | 58 | M | Burkitt lymphoma (terminal ilium) | 1 | E | N | Initial work up | Yes | No | No | No evidence (CT, MRI) |
| 20 | 25 | F | DLBCL (stomach) | 1 | E | N | f/u of LMD seizure, altered mentality | Yes | Yes (by CM) | No | MRI: multifocal brain involvement |
| 23 | 53 | M | DLBCL (brain) | 4 | N | E | f/u of multiple metastasis gait/visual disturbance | No | Yes (by CM) | No | MRI: brain involvement |
| 26 | 76 | M | DLBCL (brain) | 48 | N | E | f/u of recurred CNS DLBCL neck pain | No | Yes (by CM) | No | MRI: diffuse leptomeningeal enhancement |
Abbreviations; CNS, central nervous system; CT, computed tomography; CM, cytomorphology; DLBCL, diffuse large B cell lymphoma; E, equivocal; FCM, flow cytometry; F, female; f/u, follow up; IT-CTx, intrathecal chemotherapy; LMD, leptomeningeal disease; M, male; MRI, magnetic resonance imaging.
Table 5. Comparison of CSF glucose, protein, and white blood cell levels between positive and negative samples.
| Flow cytometry | |||
|---|---|---|---|
| Positive | Negative | P* | |
| Glucose (mg/dL) | 89.2±31.7 (n=13) | 89.8±28.2 (n=31) | 0.827 |
| Protein (mg/dL) | 69.1±84.0 (n=13) | 23.4±21.8 (n=31) | 0.037 |
| White blood cell (×106/L) | 139.4±420.0 (n=13) | 3.36±9.3 (n=28) | 0.002 |
Data are expressed as mean±SD.
*Mann-Whitney test.
Abbreviation: CSF, cerebrospinal fluid.
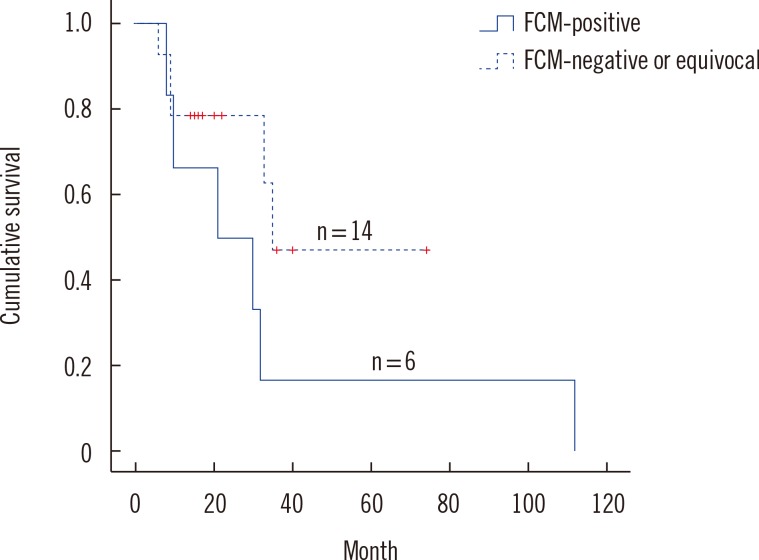
There was a trend that DLBCL cases positive by FCM have worse prognosis than DLBCL cases negative by FCM, but it did not reach statistical significance (P=0.139) probably owing to the small number of cases (Fig. 1).
Fig. 1. Overall survival in cases with diffuse large B cell lymphoma according to the results of flow cytometry (FCM).
DISCUSSION
The clinical significance of FCM for the detection of LMD has been evaluated in several studies thus far. In general, FCM has been found to be more sensitive compared with cytomorphology [6,7,13,14]. For example, Di Noto et al. [14] reported positive rates of 26% and 9.5% by FCM and cytomorphology, respectively, which are comparable to the present findings.
The clinical implication of patients who were positive by FCM and negative by cytomorphology has been evaluated in many studies [13,14,15,16]. Benevolo et al. [16] reported a higher risk of central nervous system (CNS) progression in such FCM-positive cases compared with those negative by both FCM and cytomorphology (hazard ratio=8.16). Likewise, Sancho et al. [17] reported a higher CNS relapse or progression rate in such FCM-positive cases compared with those negative by both methods (13% vs. 2.4%, P=0.04). Although our study did not show the adverse OS in cases which were positive by FCM, Hegde et al. [12] showed a high incidence of occult LMD (11 of 55 patients) using FCM in newly diagnosed non-aggressive B-cell lymphomas and an association with multiple metastasis of lymphoma. Bernstein et al. [18] suggested that methods can be used to detect subclinical CNS at diagnosis, such as using flow cytometry, and to develop better treatment strategies for patients with subclinical CNS. Thus, the use of FCM for high-risk patients at diagnosis and close follow-up or reevaluation is recommended in cases of occult LMD [13,14,15,16,19].
Although the proportion is low, cases that are positive by cytomorphology and negative by FCM may also deserve special attention from clinicians. Bromberg et al. [15] reported that these patients had higher frequency of clinical symptoms and pleocytosis and poorer progression-free survival compared with patients who were positive by FCM only. Since false-negative results may occur during specimen processing (inadequate storage temperature or prolonged storage) or owing to the presence of heterogeneous population (high proportion of normal lymphocyte with minor lymphoma cells) [20], the implementation of cytomorphology in conjunction with FCM could be recommended [13,15].
A major problem with the clinical use of FCM is the lack of standardized guidelines for sample preparation, antibody panel, interpretation criteria, and others. Moreover, sufficient amounts of CSF samples cannot be obtained easily, and the low volume and paucity of cells makes it difficult to interpret the results of FCM. Some efforts have been made to overcome these problems [19]. For example, multi-color FCM methods using three or more antibodies and the acquisition of sufficient number of CD45-positive cells in combination with viability selection have proven to increase sensitivity and reliability [7,21,22,23,24]. Cesana et al. [7] reported that the diagnostic accuracy of FCM could be significantly improved by acquiring more than 220 CD45-positive cells.
In conclusion, we have evaluated the use of FCM in CSF samples from patients clinically suspected of having LMD. We observed the higher prevalence of LMD by FCM than by cytomorphology. Although we did not observe an adverse prognostic effect of LMD determined by FCM, further studies would be needed to evaluate the diagnostic usefulness of FCM.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A2043879).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Supplementary Material
Histology and primary sites of 30 patients
Gating strategy of flow cytometry. (A) A case of extranodal marginal zone lymphoma (Patient 6). Viable lymphocytes with negative 7-AAD (7-Aminoactinomycin D) staining and positive CD45 expression were selected. CD19-positive populations with light chain restriction were considered as malignant B-cells (red color). (B) A case of extranodal NK/T cell lymphoma (Patient 9). After gating on the population with negative 7-AAD and positive CD45 staining, surface CD3-negative and CD56-positive cells were identified (red color). (C) A case of CD30-positive anaplastic large cell lymphoma (ALCL) (Patient 10). Malignant cells with CD30-positive, CD3-negative and CD14-negative immunophenotype.
References
- 1.Wilson WH, Bromberg JE, Stetler-Stevenson M, Steinberg SM, Martin-Martin L, Muniz C, et al. Detection and outcome of occult leptomeningeal disease in diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2014;99:1228–1235. doi: 10.3324/haematol.2013.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill QA, Owen RG. CNS prophylaxis in lymphoma: who to target and what therapy to use. Blood Rev. 2006;20:319–332. doi: 10.1016/j.blre.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Woo J, Baumann A, Arguello V. Recent advancements of flow cytometry: new applications in hematology and oncology. Expert Rev Mol Diagn. 2014;14:67–81. doi: 10.1586/14737159.2014.862153. [DOI] [PubMed] [Google Scholar]
- 4.Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111:3941–3967. doi: 10.1182/blood-2007-11-120535. [DOI] [PubMed] [Google Scholar]
- 5.de Graaf MT, de Jongste AH, Kraan J, Boonstra JG, Sillevis Smitt PA, Gratama JW. Flow cytometric characterization of cerebrospinal fluid cells. Cytometry B Clin Cytom. 2011;80:271–281. doi: 10.1002/cyto.b.20603. [DOI] [PubMed] [Google Scholar]
- 6.Kentrou NA, Tsagarakis NJ, Tzanetou K, Damala M, Papadimitriou KA, Skoumi D, et al. An improved flow cytometric assay for detection and discrimination between malignant cells and atypical mesothelial cells, in serous cavity effusions. Cytometry B Clin Cytom. 2011;80:324–334. doi: 10.1002/cyto.b.20608. [DOI] [PubMed] [Google Scholar]
- 7.Cesana C, Klersy C, Scarpati B, Brando B, Faleri M, Bertani G, et al. Flow cytometry and cytomorphology evaluation of hematologic malignancy in cerebrospinal fluids: comparison with retrospective clinical outcome. Ann Hematol. 2011;90:827–835. doi: 10.1007/s00277-010-1145-4. [DOI] [PubMed] [Google Scholar]
- 8.Chizuka A, Kanda Y, Nannya Y, Oshima K, Kaneko M, Yamamoto R, et al. The diagnostic value of kappa/lambda ratios determined by flow cytometric analysis of biopsy specimens in B-cell lymphoma. Clin Lab Haematol. 2002;24:33–36. doi: 10.1046/j.1365-2257.2002.00175.x. [DOI] [PubMed] [Google Scholar]
- 9.Ståhlberg A, Aman P, Strömbom L, Zoric N, Diez A, Nilsson O, et al. Comparison of reverse transcription quantitative real-time PCR, flow cytometry, and immunohistochemistry for detection of monoclonality in lymphomas. ISRN Oncol. 2014;2014:796210. doi: 10.1155/2014/796210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zembruski NC, Stache V, Haefeli WE, Weiss J. 7-Aminoactinomycin D for apoptosis staining in flow cytometry. Anal Biochem. 2012;429:79–81. doi: 10.1016/j.ab.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Argon A, Uyaroğlu MA, Nart D, Veral A, Kitapcioğlu G. The effectiveness of the liquid-based preparation method in cerebrospinal fluid cytology. Acta Cytol. 2013;57:266–270. doi: 10.1159/000346716. [DOI] [PubMed] [Google Scholar]
- 12.Hegde U, Filie A, Little RF, Janik JE, Grant N, Steinberg SM, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105:496–502. doi: 10.1182/blood-2004-05-1982. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez R, Dupuis J, Plonquet A, Christov C, Copie-Bergman C, Hemery F, et al. Clinical relevance of flow cytometric immunophenotyping of the cerebrospinal fluid in patients with diffuse large B-cell lymphoma. Ann Oncol. 2012;23:1274–1279. doi: 10.1093/annonc/mdr436. [DOI] [PubMed] [Google Scholar]
- 14.Di Noto R, Scalia G, Abate G, Gorrese M, Pascariello C, Raia M, et al. Critical role of multidimensional flow cytometry in detecting occult leptomeningeal disease in newly diagnosed aggressive B-cell lymphomas. Leuk Res. 2008;32:1196–1199. doi: 10.1016/j.leukres.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Bromberg JE, Breems DA, Kraan J, Bikker G, van der Holt B, Smitt PS, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68:1674–1679. doi: 10.1212/01.wnl.0000261909.28915.83. [DOI] [PubMed] [Google Scholar]
- 16.Benevolo G, Stacchini A, Spina M, Ferreri AJ, Arras M, Bellio L, et al. Final results of a multicenter trial addressing role of CSF flow cytometric analysis in NHL patients at high risk for CNS dissemination. Blood. 2012;120:3222–3228. doi: 10.1182/blood-2012-04-423095. [DOI] [PubMed] [Google Scholar]
- 17.Sancho JM, Orfao A, Quijano S, Garcia O, Panizo C, Pérez-Ceballos E, et al. Clinical significance of occult cerebrospinal fluid involvement assessed by flow cytometry in non-Hodgkin's lymphoma patients at high risk of central nervous system disease in the rituximab era. Eur J Haematol. 2010;85:321–328. doi: 10.1111/j.1600-0609.2010.01478.x. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin's lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol. 2009;27:114–119. doi: 10.1200/JCO.2008.16.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galati D, Di Noto R, Del Vecchio L. Diagnostic strategies to investigate cerebrospinal fluid involvement in haematological malignancies. Leuk Res. 2013;37:231–237. doi: 10.1016/j.leukres.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 20.El-Sayed AM, El-Borai MH, Bahnassy AA, El-Gerzawi SM. Flow cytometric immunophenotyping (FCI) of lymphoma: correlation with histopathology and immunohistochemistry. Diagn Pathol. 2008;3:43. doi: 10.1186/1746-1596-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stacchini A, Aliberti S, Demurtas A, Benevolo G, Godio L. Ten antibodies, six colors, twelve parameters: a multiparameter flow cytometric approach to evaluate leptomeningeal disease in B-cell non-Hodgkin's lymphomas. Cytometry B Clin Cytom. 2012;82:139–144. doi: 10.1002/cyto.b.21001. [DOI] [PubMed] [Google Scholar]
- 22.Cesana C, Klersy C, Scarpati B, Brando B, Volpato E, Bertani G, et al. Flow cytometry vs cytomorphology for the detection of hematologic malignancy in body cavity fluids. Leuk Res. 2010;34:1027–1034. doi: 10.1016/j.leukres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Finn WG, Peterson LC, James C, Goolsby CL. Enhanced detection of malignant lymphoma in cerebrospinal fluid by multiparameter flow cytometry. Am J Clin Pathol. 1998;110:341–346. doi: 10.1093/ajcp/110.3.341. [DOI] [PubMed] [Google Scholar]
- 24.Kraan J, Gratama JW, Haioun C, Orfao A, Plonquet A, Porwit A, et al. Flow cytometric immunophenotyping of cerebrospinal fluid. Curr Protoc Cytom. 2008;Chapter 6:Unit 6.25. doi: 10.1002/0471142956.cy0625s45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histology and primary sites of 30 patients
Gating strategy of flow cytometry. (A) A case of extranodal marginal zone lymphoma (Patient 6). Viable lymphocytes with negative 7-AAD (7-Aminoactinomycin D) staining and positive CD45 expression were selected. CD19-positive populations with light chain restriction were considered as malignant B-cells (red color). (B) A case of extranodal NK/T cell lymphoma (Patient 9). After gating on the population with negative 7-AAD and positive CD45 staining, surface CD3-negative and CD56-positive cells were identified (red color). (C) A case of CD30-positive anaplastic large cell lymphoma (ALCL) (Patient 10). Malignant cells with CD30-positive, CD3-negative and CD14-negative immunophenotype.