Abstract
Background: The endothelial progenitor cells (EPCs) dysfunction is a critical event in the initiation of atherosclerotic plaque development and the level of circulating EPCs can be considered a biomarker of cardiovascular events. The level and functional change in EPCs has been investigated in hemodialysis patients, but the effect of absolute number of EPCs on risk of death has not yet been explored. We hypothesized that the number of EPCs predicted death from cardiovascular and all-cause mortality in hemodialysis patients.
Methods: We evaluate the association between endothelial progenitor cells and clinical outcome in 154 patients on maintenance hemodialysis. The blood sample was drawn at the time of patient enrollment and EPCs were identified by flow cytometry using triple staining for CD34/CD133/KDR.
Results: The median duration of follow-up was 4.19 years. There were 79 (51.3%) deaths during the follow-up period, 41 of whom died due to a confirmed cardiovascular cause. The cumulative survival was greater in the high-EPC group than the low-EPC group for all-cause and cardiovascular mortality. Decreased EPCs levels were associated with a significant increase in the risk of cardiovascular and all-cause mortality after adjusting for age, gender, current smokers, diabetes mellitus, and hypertension.
Conclusions: The level of circulating EPCs independently predicts the clinical outcome in patients on maintenance hemodialysis. Thus, the EPCs levels may be a useful predictive tool for evaluating the risk of death in maintenance hemodialysis patients.
Keywords: endothelial progenitor cells, hemodialysis, mortality
Introduction
Circulating endothelial progenitor cells (EPCs) are bone-marrow derived CD34+ mononuclear cells (MNCs) capable of new vessel formation in ischemic injury, a process termed postnatal vasculogenesis. The EPCs induce proliferation, migration, and adhesion and further differentiate into fully functional endothelial cells to maintain vascular integrity. EPCs migrate from the bone marrow to the systemic circulation and damaged tissue, and then incorporate into the vascular endothelial cell monolayer after differentiating into mature endothelial cells. Numerous factors have been reported to be involved in EPC migration, including stromal-derived factor 1 (SDF1), vascular endothelial growth factor (VEGF), interleukin 8, and nitric oxide 1.
Cardiovascular disease is the leading cause of death in chronic kidney disease (CKD), particularly entry to hemodialysis. CKD shares many risk factors with cardiovascular disease, and one disease may lead to the other. Hypertension, diabetes mellitus, and hyperlipidemia are the major risk factors in the development of endothelial dysfunction and atherosclerotic plaque formation. Even minor renal functional impairment can trigger endothelial dysfunction or promote chronic inflammation, resulting in atherosclerosis and causing further cardiovascular morbidity and mortality 2. Elevated levels of circulating endothelial cells and a deficit of two angiogenic factors, VEGF and angiopoietin-1, indicate the destruction of vascular hemostasis and defective vascular repair as renal function deteriorates 3. Although the level and functional change in EPCs has been investigated in hemodialysis patients, the relationship between the absolute number of CD34+/CD133+/KDR+ (Kinase insert domain-conjugating receptor) EPCs and risk of death has not yet been explored.
Here, we proposed that circulating EPCs are associated with the risk of death and clarify the predictive values of circulating EPCs in long term mortality in hemodialysis patients.
Subjects and Methods
Patients
From May 2009 to September 2014, 154 patients undergoing maintenance hemodialysis for >3 months at Cardinal Tien Hospital (New Taipei City, Taiwan) were enrolled in our study (72 men, 82 women). The median duration of follow-up was 4.19 years (mean 3.78 ± 1.41 years, range 0.15-5.08 years). The duration of hemodialysis was 8.66 ± 4.3 years (range 4-24 years). We excluded subjects who switched to peritoneal dialysis, performed renal transplantation and presented with fever or other sign of acute infection and chronic inflammation. Causes of death related to cardiovascular complications included sudden death, heart failure, myocardial infarction, cerebral infarction, and cerebral hemorrhage. All patients provided written informed consent for participation and the study was performed in accordance with the Declaration of Helsinki. Data on survival status and causes of death were retrieved by a review of hospital records and rechecked by the Taiwan Society of Nephrology: Kidney Dialysis, Transplantation (TSN KiDiT) registration system. This study was approved by the Human Ethical Committees of Cardinal Tien Hospital.
Clinical and laboratory parameters
The clinical characteristics of the patients, including age, gender, and duration of hemodialysis, were obtained from medical records. Each patient was interviewed face to face at the time of enrollment regarding cigarette smoking status and alcohol consumption. Individuals who had not smoked more than 100 cigarettes in their lifetime were classified as never-smokers based on common conventions in epidemiological research. The pattern of drinking, including frequency of drinking days and number of drinks consumed in a day, was recorded. Patients who drank a bottle of alcoholic beverage (including beer, rice beer, and sorghum liquor) or more per month for at least 1 year were defined as ever drinkers. Current and former smokers were grouped together in the smoker's group and compared to individuals in the never-smoker's group. The ever drinker's group was compared to individuals in the non-drinkers group. Body weight was used to calculate the body mass index (BMI). Systolic blood pressure and diastolic blood pressure were measured in the supine position after a 10-15 minute rest. The definition of hypertension was based on the Seventh Joint National Committee: systolic blood pressure before dialysis ≥ 140/90 mmHg or antihypertensive treatment. All of the participants met the diagnostic criteria for diabetes mellitus set forth by the American Diabetes Association: fasting glucose ≥ 126 mg/dL (7.0 mmol/L) or 2-h plasma glucose ≥ 200 mg/dL (11.1 mmol/L) after 75 g oral glucose loading test, or HbA1C ≥ 6.5%.
Blood samples were collected after overnight fasting and stored at -20°C until analysis. The concentrations of plasma glucose, serum albumin, blood urea nitrogen (BUN), creatinine, total cholesterol, and hemoglobin (Hb) were measured using an automatic chemistry analyzer (Synchron LXi-725; Beckman Coulter Inc., Brea, CA, USA). The Kt/V value was calculated using Daugirdas' formula: -ln(Ratio-(0.03)) + [(4-(3.5 * Ratio)) * (Ultrafiltrate Volume/Weight)], where ratio is the post-/pre-dialysis BUN ratio.
Isolation of EPCs
The blood sample was drawn at the time of patient enrollment and circulating EPCs were assayed within 1 h of blood withdrawal. Briefly, 10 mL venous blood was drawn from the antecubital vein and collected in a 4 mL tube containing heparin. Mononuclear cells (MNCs) were separated by Ficoll-Hypaque density gradient centrifugation (Ficoll-PlaqueTM plus, Amersham Biosciences, Sweden).After MNCs washing with phosphate buffer solution (PBS), cell were resuspended with 300 μL PBS. Cell viability > 95.0% was required in each group. EPC identity was determined by the co-expression of different stem cell markers (i.e., CD34, CD133) and endothelial cell (EC) lineage markers [i.e., kinase insert domain-conjugating receptor (KDR)]. Using immunofluorescent cell staining, the EPCs were identified by performing fluorescent conjugated monoclonal antibodies against fluorescein isothiocyanate (FITC)-conjugated CD34, APC-conjugated CD133, and phycoerythrin (PE)-conjugated KDR. The IgG2a-PE-FITC was used as a background control to eliminate any non-specific glycoprotein binding. Stain with PE-conjugated goat anti-mouse antibody was used to identify KDR positive MNCs. For fluorescence-activated cell-sorting analysis, quantitative three-color flow cytometric analysis was chosen (Beckman Coulter Cytomics FC500 Flow Cytometry). Calculation the number of EPC per 100,000 cells was performed and duplicate record reports the mean levels. To quantify the blood sample more precisely, intra-assay variability of the same sample was tested that mean coefficient of variation < 4.0% in necessary.
Statistical analysis
Continuous variables were represented as mean and standard deviation (SD) if normally distributed and compared by parametric test such as Student's t-test; variable that don't have normal distribution is compared with non-parametric test like Mann-Whitney U-test. The Kolmogorov-Smirnov test and Shapiro-Wilk test were commonly used to check if the variable has normal distribution. Chi-square test and Fisher's exact test were used to analyze categorical data.
The Kaplan-Meier estimation method was computed to assess the probability of survival and compared statistically using log rank test. Cox proportional hazard regression assumes the association between various clinical data and time of death. Confounding factors were included in multivariate models if they had significant associations in the univariate analysis or clinical evidence indicated a relationship with the risk of mortality. A two-tailed p value <0.05 was considered significant. All analyses were performed with IBM SPSS Statistics version 20.0 (SPSS Inc., Chicago, Ill., USA) and Stata/SE 10.0 (StataCorp LP, College Station TX) for Windows.
Results
The clinical characteristics of the participants are summarized in Table 1 based on the level of circulating EPCs. The number of circulating EPCs ranged from 2/μL to 10/μL (median 5.0/μL), with a mean (±SD) of 5.29 ± 2.06/μL. No significantly different variable factor was found between patients with high and low circulating EPC levels (Table 1). The cause of death was reviewed in 79 (51.3%) hemodialysis patients during the follow-up period, 41 of whom died due to a confirmed cardiovascular cause. The cause of death is shown in detail in Table 2.
Table 1.
Clinical characteristics and laboratory finding of study patients.
| Characteristics | High-EPCs, EPC ≥ 5/ul (n = 56) |
Low-EPCs, EPC < 5/ul (n = 98) |
p value |
|---|---|---|---|
| Age (years) | 67.82 ± 14.73 | 70.29 ± 15.98 | 0.20† |
| Male (%) | 41 (41.8%) | 31 (55.4%) | 0.10# |
| Body mass index(kg/m2) | 22.10 ± 3.77 | 22.08 ± 3.90 | 0.98† |
| Hemodialysis duration (years) | 8.83 ± 4.36 | 8.38 ± 4.28 | 0.41† |
| Current smoker | 17 (17.3%) | 8 (14.3%) | 0.62# |
| Ever drinking | 3 (3.1%) | 1 (1.8%) | 1.00! |
| Diabetes mellitus | 58 (59.2%) | 28 (50.0%) | 0.27# |
| Hypertension | 66 (67.3%) | 40 (71.4%) | 0.59# |
| Pre-dialysis SBP (mmHg) | 143.73 ± 24.59 | 146.63 ± 28.23 | 0.52† |
| Endothelial progenitor cells (/ul) | 6.00 ± 3.00 | 3.00 ± 1.00 | <0.01‡ |
| Albumin(g/dL) | 3.65 ± 0.56 | 3.93 ± 2.00 | 0.55† |
| Blood urea nitrogen (mg/dL) | 65.08 ± 18.76 | 66.93 ± 14.11 | 0.42† |
| Creatinine(mg/dL) | 9.88 ± 2.33 | 9.71 ± 2.55 | 0.46† |
| Calcium(mg/dL) | 9.00 ± 0.79 | 8.90 ± 0.83 | 0.73‡ |
| Phosphate(mg/dL) | 5.10 ± 1.58 | 4.90 ± 1.55 | 0.24‡ |
| Intact parathyroid hormone (pg/mL) | 422.7 ± 414.2 | 440.6 ± 434.7 | 0.64‡ |
| Dialysis efficiency (Kt/Vurea) | 1.48 ± 0.42 | 1.49 ± 0.33 | 0.48† |
| Total cholesterol(mg/dL) | 181.23 ± 91.88 | 159.21 ± 39.19 | 0.053† |
| Low-density lipoprotein(mg/dL) | 131.0 ± 26.23 | 132.0 ± 16.89 | 0.82‡ |
| High-density lipoprotein(mg/dL) | 29.0 ± 6.3 | 33.0 ± 6.5 | 0.24‡ |
| Blood glucose (mg/dL) | 130.51 ± 65.76 | 142.78 ± 63.41 | 0.18† |
| Hemoglobin (g/dL) | 10.00 ± 1.60 | 9.55 ± 1.60 | 0.08‡ |
Values are mean ± standard deviation if normally distributed.
Value are medians ± interquartile ranges if not normally distributed.
† Student's t-test, ‡ Mann-Whitney U test, # Chi-square test, ! Fisher's exact test
*p<0.05
Table 2.
Cause of death in hemodialysis patients.
| Total | High-EPCs group (n=46 ) | Low-EPCs group (n=33 ) | P value |
|---|---|---|---|
| Cardiovascular disease | 18 (39.1%) | 23 (69.7 %) | <0.01 |
| Infectious disease | 11 (23.9%) | 7 (21.2%) | 0.78 |
| Malignant disease | 8 (17.4%) | 1 (3%) | 0.07 |
| Other disease | 9 (20.0%) | 2 (6%) | 0.11 |
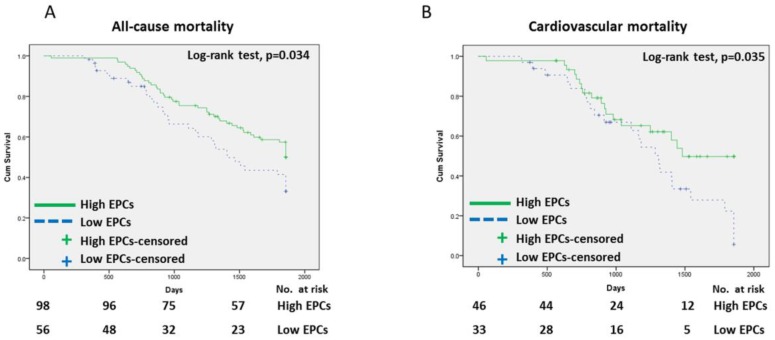
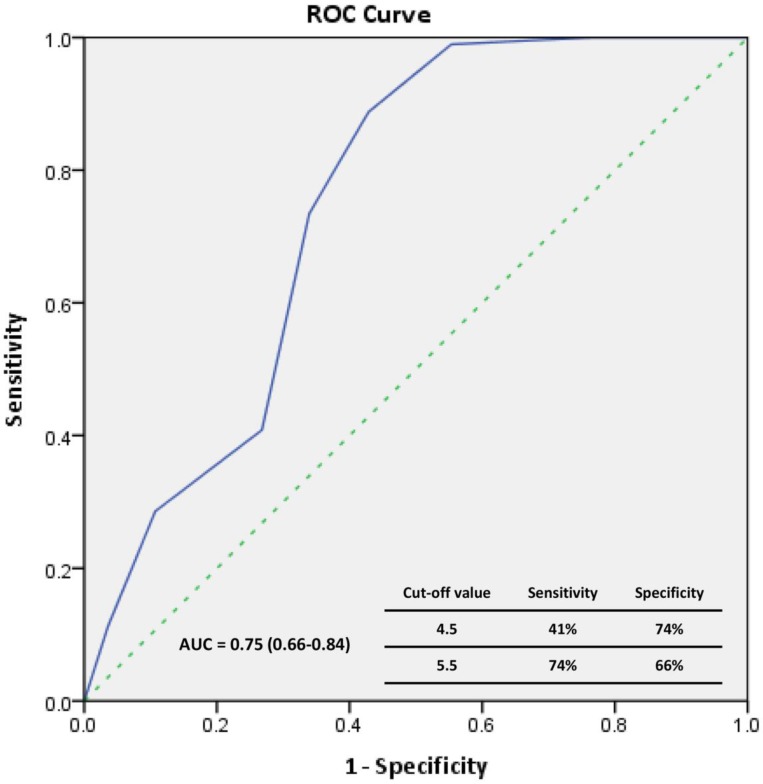
The Kaplan-Meier survival data for all patients were divided into two groups according to the median EPC level (Figure 1) at the time of enrollment: high-EPC group (EPCs ≥ 5 cells/μL) or low-EPC group (EPCs < 5 cells/μL). The cumulative survival was greater in the high-EPC group than the low-EPC group for all-cause mortality (p = 0.034, log-rank test; Figure 1A) and cardiovascular mortality (p = 0.035, log-rank test; Figure 1B). For all-cause mortality, the 1-, 3-, and 5-year cumulative survival rates for the high-EPC group were 98.0%, 73.5%, and 46.9%, and in the low-EPC group 94.6%, 55.4%, and 35.7%, respectively. The Receiver-operating characteristic curve analysis identify the significant predictive power of EPC level in all-cause mortality (area under the curve = 0.75, p<0.01)(Figure 2).
Figure 1.
Cumulative survival curves for hemodiallysis patients. (A) All-cause mortality, (B) Cardiovascular mortality.
Figure 2.
The receiver operating characteristic (ROC) curve for the EPCs to predict patient's all-cause mortality.
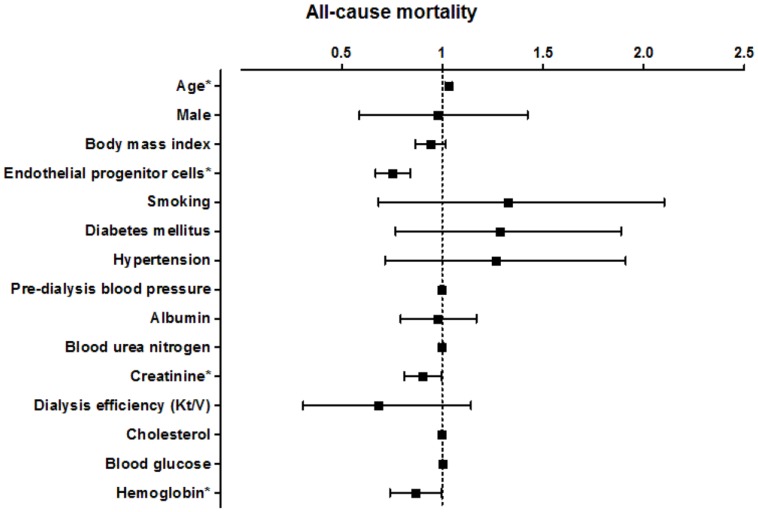
The association between the level of circulating EPCs and patient survival according to the univariate Cox regression model is presented in Figure 3 and Figure 4. In a model using the forced-entry method, decreased EPC levels were associated with a significant increase in the risk of all-cause mortality (HR 0.750, p < 0.01; Figure 3). The incidence of all-cause death was also significantly influenced by age (HR 1.031 [95% CI, 1.014-1.049], p<0.01; Figure 3). In addition, the variable serum creatinine and Hb levels were also significant prognostic factors associated with survival in all hemodialysis patients (serum creatinine: HR 0.898 [95% CI, 0.811-0.994], p=0.04; Hb: HR 0.858 [95% CI, 0.710-0.995], p=0.04; Figure 3).After adjusting for age, gender, current smokers, diabetes mellitus, and hypertension, the association between decreased EPC levels and increased risk of all-cause death remained significant (HR 0.737 [95% CI, 0.653-0.832], p<0.01; Table 3, Model 2, All-cause mortality). In other words, every 1/uL increase of EPC might reduce 26% risk of all-cause mortality.
Figure 3.
Hazard ratio for various factors for all-cause mortality in all hemodialysis patients.
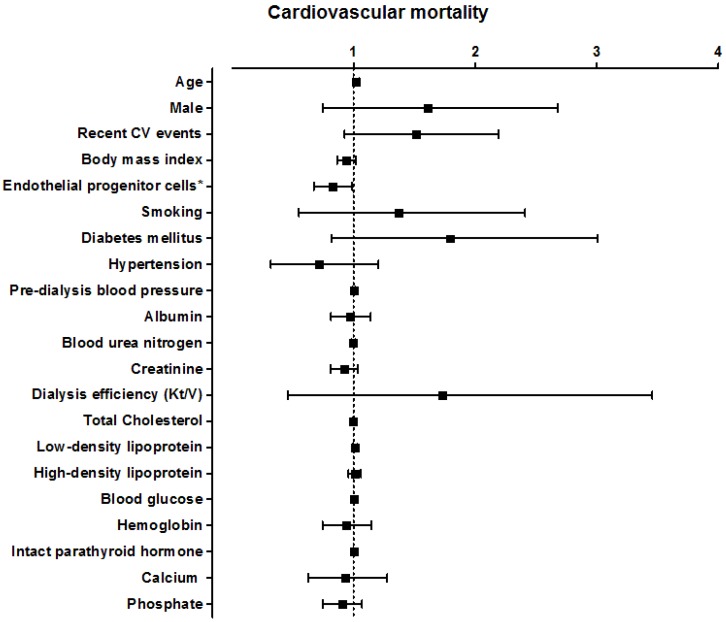
Figure 4.
Hazard ratio for various factors for cardiovascular mortality in all hemodialysis patients.
Table 3.
Hazard ratio (95%CI) of risk factors in all hemodialysis patients, as determined by multivariate Cox's proportional regression hazard models.
| All-cause mortality | Cardiovascular mortality | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
|
Harrell's Concordance |
0.7193 | 0.7232 | 0.7258 | 0.7275 | 0.7433 | 0.7492 |
| Endothelial progenitor cells | 0.742* (0.658 - 0.837) |
0.737* (0.653 - 0.832) |
0.745* (0.658 - 0.844) |
0.790* (0.651-0.959) |
0.783* (0.641-0.955) |
0.787* (0.645-0.959) |
| Age | 1.032* (1.014 - 1.049) |
1.034* (1.016 - 1.052) |
1.038* (1.019 - 1.057) |
1.022 (0.998-1.046) |
1.020 (0.995-1.046) |
1.019 (0.994-1.046) |
| Male | 0.846 (0.539 - 1.327) |
0.911 (0.560 - 1.484) |
1.029 (0.609 - 1.739) |
1.234 (0.623-2.444) |
1.323 (0.651-2.687) |
1.278 (0.614-2.661) |
| Current smoker | 1.477 (0.795- 2.746) |
1.459 (0.780- 2.729) |
1.591 (0.705-3.589) |
1.648 (0.725-3.750) |
||
| Diabtes mellitus | 1.119 (0.709- 1.766) |
1.459 (0.780- 2.729) |
1.490 (0.771-2.880) |
1.472 (0.754-2.876) |
||
| Hypertension | 0.861 (0.514 - 1.444) |
0.821 (0.485 - 1.390) |
0.625 (0.298-1.309) |
0.609 (0.287-1.290) |
||
| Dialysis efficiency (Kt/V) | 0.422* (0.190- 0.937) |
1.039 (0.291-3.708) |
||||
| Hemoglobulin | 0.909 (0.770- 1.073) |
0.935 (0.736-1.187) |
||||
*p<0.05
The higher circulating level of EPCs had significantly positive benefits of reducing death from cardiovascular cause (HR 0.816 [95% CI, 0.674-0.988], p=0. 04; Figure 4). Multivariate analysis adjusted for age, gender, current smokers, diabetes mellitus, and hypertension confirmed an independent significant association between EPC level and cardiovascular mortality (HR 0.783 [95% CI, 0.641-0.955], p=0.015; Table 3, Model 2, Cardiovascular mortality). In addition, EPC level was also independently predicted cardiovascular mortality after adjusting for known important confounding factors in hemodialysis patients, such as HDL-C, LDL-C, calcium, phosphate, intact parathyroid hormone and history of cardiovascular disease (HR 0.712 [95% CI, 0.572-0.888], p <0.01) . Using univariate logistic regression, no variable other than circulating EPCs had enough significance to predict cardiovascular mortality in our study.
The Harrell's C indexes of concordance statistics indicated good predictability in each model (0.7193-0.7492; Table 3). The increments in concordance statistics for EPCs in Model 1 were 0.0037 and 0.0083 for all-cause mortality and cardiovascular mortality, respectively. Age and level of circulating EPCs increased the concordance statistics in all models.
Discussion
To the best of our knowledge, this is the first study to demonstrate that the number of circulating EPCs predicts all-cause and cardiovascular mortality in patients undergoing hemodialysis for at least 3 months. This study had a longer follow-up period, which allowed us to clarify the association between circulating EPC levels and clinical outcome.
In the last decade, EPCs have been the focus of investigation for vascular injury, cardiovascular events, and response to therapy. The level of circulating EPCs has been inversely correlated with atherosclerotic risk factors in coronary artery disease 4 and advanced heart failure 5. The level of EPCs has also been shown to be a surrogate biological marker of vascular function 6 or cumulative cardiovascular risk in the general population 7, 8. Statins can induce the proliferative capacity of EPCs in vitro by preventing senescence 9. The angiotensin-converting enzyme inhibitor, ramipril, can enhance the functional activity of EPCs in patients with coronary artery disease 10, and rosiglitazone can increase the level and improve the migratory function of circulating EPCs in type 2 diabetic patients via up-regulation of the Akt/eNOS pathways 11, 12. These phenomena indicate the therapeutic potential of EPC transplantation in patients with cardiovascular diseases.
In CKD, the circulating number 13, 14, colony formation, migratory ability, adhesion 15, and angiogenic function16 of EPCs are decreased 16, 17; thus, EPC dysfunction is a critical event in the initiation of atherosclerotic plaque development and the level of circulating EPCs can be considered a biomarker of cardiovascular events in patient on maintenance hemodialysis 18, 19. The uremic toxin can inhibit EPC activity and differentiation in vitro; therefore, imitating the dialysis modality may ameliorate the EPC level and restore angiogenesis 20. Compared to conventional hemodialysis, nocturnal hemodialysis is associated with significant improvement in the ability of EPCs to promote tissue perfusion in ischemic insult 21.
The aim of our study was to investigate the level of circulating CD34+/CD133+/KDR+ EPCs based on all-cause and cardiovascular mortality in patients on maintenance hemodialysis. No variable significantly differed between the high-EPC and low-EPC group. Notably, the cumulative survival curve in the low-EPC group (EPC < 5/μL) predicted poor survival for all-cause mortality and cardiovascular mortality. Lee et al. concluded that a decreased level of circulating EPCs is associated with cardiovascular events but not all-cause mortality18. The proportion of deaths from all-cause mortality in this study was 51.3% (79/154), whereas Lee et al. reported that 7% (5/70) of hemodialysis patients in their study died, all from cardiovascular causes. With a large sample size and longer follow-up, this study confirmed the significance association between the level of circulating EPCs and patient survival. Moreover, Maruyama et al. demonstrated that circulating CD34+ hematopoietic stem cells are significantly associated with vascular risk and all-cause mortality in chronic hemodialysis patients 22. However, screening for CD34/CD133/KDR triple-positive cells rather than only CD34-positive cells can more accurately characterize EPCs, as some circulating mature endothelial cells also express CD34, stem cell marker CD133 acts as a more suitable marker of EPCs 23, and CD133/KDR dual-positive cells exhibit a greater capacity to migrate and differentiate into mature endothelial cells in postnatal vasculogenesis.
In univariate analysis, the level of circulating EPCs was significantly associated with all-cause and cardiovascular mortality. Even after adjusting for covariates, a low level of circulating EPCs was an independent predictor of poor prognosis. Limited studies have shown that the level of circulating EPCs has an influence on prognosis. Among patients with acute lung injury, decreased levels of circulating EPC colonies were associated with an increased risk of death after multivariate adjustment 24. Among patients with coronary artery disease confirmed on angiography, decreased levels of circulating EPCs were associated with an increased risk of death from cardiovascular disease, but not death from all-cause mortality after multivariate adjustment 25. Among CKD and hemodialysis patients, the EPC level has been shown to be an independent predictor of cardiovascular events 18, 19. In the present study, we further clarified the role of the level of circulating EPCs in predicting all-cause and cardiovascular mortality in patients on maintenance hemodialysis.
Vitamin D has the positive effect on the number of circulating EPC and optimal level of 25-vitamind D can reduce the risk of cardiovascular event and diabetes26. Calcitriol, active form vitamin D3, stimulate endothelial colony-forming cells (ECFCs) proliferation through increasing VEGF expression and pro-MMP-2 activity in vitro, which is very important in angiogenesis27. Deficiency of vitamin D might decrease the EPC number and function in hemodialysis patients 28. HMG-CoA reductase inhibitors (statins) increase the number of EPC by inducing bone marrow hematopoietic precursor cell differentiation through PI3K/Akt pathway 29 and to improve the vascular wall pathology in balloon induced injury 30. This offers another explanation of clinical benefit of statin therapy in coronary artery disease patient 31. Erythropoietin had been reported to improve cardiac function after myocardial infarction by the proangiogenic properties of EPC 32, 33. Administration of erythropoietin can increase the number of functionally active EPC by Akt protein kinase pathway and then repair the vascular injury caused by ischemia or inflammation 34, 35. Also, angiotensin II-related blocker affects the number of EPC 36. In vitro study, uremic toxins such as β2-microglobulin and indole-3 acetic acid may also induce EPC apoptosis during early differentiation 37. Taken together, numerous factors had been shown to be associated with the number of EPC in hemodialysis patients and each of those possibly influence the role of EPC level as a prognostic marker.
Our study has several limitations. First, we did not take into account EPC colonies to determine the functional change in hemodialysis patients. Second, a limited number of confounding factors were recorded and adjusted for in the Cox regression model. To confirm the utility of EPC levels in predicting mortality, a multicenter interventional study with a larger number of patients and multiple dialysis centers is needed.
Conclusion
The level of circulating EPCs in hemodialysis patients is worthy of consideration, as low EPC levels were associated with death from all-cause and cardiovascular mortality. These findings suggest that circulating EPC levels independently predict the clinical outcome in patients on maintenance hemodialysis. Thus, the EPCs levels may be a useful predictive tool for evaluating the risk of death in maintenance hemodialysis patients.
Acknowledgments
This work is supported by a grant from the Cardinal Tien Hospital (CTH-NC-100-03) and Tri-Service General Hospital (TSGH-C105-006-008-S03).
Abbreviations
- EPC
endothelial progenitor cells
- KDR
Kinase insert domain-conjugating receptor
- MNCs
mononuclear cells
- VEGF
vascular endothelial growth factor
- CKD
chronic kidney disease
- SBP
systolic blood pressure
- BUN
Blood urea nitrogen
- Kt/Vurea
Dialysis efficiency
- Hb
Hemoglobulin.
References
- 1.Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: mechanisms of mobilization of endothelial progenitor cells. British journal of clinical pharmacology. 2009;68:484–92. doi: 10.1111/j.1365-2125.2009.03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stam F, van Guldener C, Becker A. et al. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. Journal of the American Society of Nephrology: JASN. 2006;17:537–45. doi: 10.1681/ASN.2005080834. [DOI] [PubMed] [Google Scholar]
- 3.Futrakul N, Butthep P, Futrakul P. Altered vascular homeostasis in chronic kidney disease. Clinical hemorheology and microcirculation. 2008;38:201–7. [PubMed] [Google Scholar]
- 4.Vasa M, Fichtlscherer S, Aicher A. et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 5.Valgimigli M, Rigolin GM, Fucili A. et al. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–12. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 6.Glowinska-Olszewska B, Luczynski W, Bossowski A. [Endothelial progenitor cells as a new marker of endothelial function with respect to risk of cardiovascular disorders] Postepy higieny i medycyny doswiadczalnej. 2011;65:8–15. doi: 10.5604/17322693.931086. [DOI] [PubMed] [Google Scholar]
- 7.Hill JM, Zalos G, Halcox JP. et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. The New England journal of medicine. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 8.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. Journal of the American College of Cardiology. 2007;49:741–52. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 9.Assmus B, Urbich C, Aicher A. et al. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res. 2003;92:1049–55. doi: 10.1161/01.RES.0000070067.64040.7C. [DOI] [PubMed] [Google Scholar]
- 10.Min TQ, Zhu CJ, Xiang WX. et al. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2004;18:203–9. doi: 10.1023/B:CARD.0000033641.33503.bd. [DOI] [PubMed] [Google Scholar]
- 11.Pistrosch F, Herbrig K, Oelschlaegel U. et al. PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183:163–7. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Liang C, Ren Y, Tan H. et al. Rosiglitazone via upregulation of Akt/eNOS pathways attenuates dysfunction of endothelial progenitor cells, induced by advanced glycation end products. British journal of pharmacology. 2009;158:1865–73. doi: 10.1111/j.1476-5381.2009.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eizawa T, Murakami Y, Matsui K. et al. Circulating endothelial progenitor cells are reduced in hemodialysis patients. Current medical research and opinion. 2003;19:627–33. doi: 10.1185/030079903125002379. [DOI] [PubMed] [Google Scholar]
- 14.Chen YT, Cheng BC, Ko SF. et al. Value and level of circulating endothelial progenitor cells, angiogenesis factors and mononuclear cell apoptosis in patients with chronic kidney disease. Clinical and experimental nephrology. 2013;17:83–91. doi: 10.1007/s10157-012-0664-9. [DOI] [PubMed] [Google Scholar]
- 15.Herbrig K, Pistrosch F, Oelschlaegel U. et al. Increased total number but impaired migratory activity and adhesion of endothelial progenitor cells in patients on long-term hemodialysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2004;44:840–9. [PubMed] [Google Scholar]
- 16.Choi JH, Kim KL, Huh W. et al. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1246–52. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Ayala E, Yao Q, Holmen C. et al. Imbalance between detached circulating endothelial cells and endothelial progenitor cells in chronic kidney disease. Blood purification. 2006;24:196–202. doi: 10.1159/000090519. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Kim W, Kim WS. et al. Circulating Endothelial Progenitor Cell Levels Predict Cardiovascular Events in End-Stage Renal Disease Patients on Maintenance Hemodialysis. Nephron. 2015;130:151–8. doi: 10.1159/000430471. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzen J, David S, Bahlmann FH. et al. Endothelial progenitor cells and cardiovascular events in patients with chronic kidney disease-a prospective follow-up study. PloS one. 2010;5:e11477. doi: 10.1371/journal.pone.0011477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groot K, Bahlmann FH, Sowa J. et al. Uremia causes endothelial progenitor cell deficiency. Kidney international. 2004;66:641–6. doi: 10.1111/j.1523-1755.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 21.Yuen DA, Kuliszewski MA, Liao C. et al. Nocturnal hemodialysis is associated with restoration of early-outgrowth endothelial progenitor-like cell function. Clinical journal of the American Society of Nephrology: CJASN. 2011;6:1345–53. doi: 10.2215/CJN.10911210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama S, Taguchi A, Iwashima S. et al. Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney international. 2008;74:1603–9. doi: 10.1038/ki.2008.495. [DOI] [PubMed] [Google Scholar]
- 23.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 24.Burnham EL, Taylor WR, Quyyumi AA. et al. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–60. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 25.Werner N, Kosiol S, Schiegl T. et al. Circulating endothelial progenitor cells and cardiovascular outcomes. The New England journal of medicine. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 26.Mikirova NA, Belcaro G, Jackson JA. et al. Vitamin D concentrations, endothelial progenitor cells, and cardiovascular risk factors. Panminerva medica. 2010;52:81–7. [PubMed] [Google Scholar]
- 27.Grundmann M, Haidar M, Placzko S. et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. American journal of physiology Cell physiology. 2012;303:C954–62. doi: 10.1152/ajpcell.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cianciolo G, La Manna G, Cappuccilli ML. et al. VDR expression on circulating endothelial progenitor cells in dialysis patients is modulated by 25(OH)D serum levels and calcitriol therapy. Blood purification. 2011;32:161–73. doi: 10.1159/000325459. [DOI] [PubMed] [Google Scholar]
- 29.Dimmeler S, Aicher A, Vasa M. et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. The Journal of clinical investigation. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walter DH, Rittig K, Bahlmann FH. et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 31.Vasa M, Fichtlscherer S, Adler K. et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 32.Westenbrink BD, Lipsic E, van der Meer P. et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. European heart journal. 2007;28:2018–27. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 33.van der Meer P, Lipsic E, Henning RH. et al. Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infarction. Journal of the American College of Cardiology. 2005;46:125–33. doi: 10.1016/j.jacc.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 34.Satoh K, Kagaya Y, Nakano M. et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation. 2006;113:1442–50. doi: 10.1161/CIRCULATIONAHA.105.583732. [DOI] [PubMed] [Google Scholar]
- 35.Heeschen C, Aicher A, Lehmann R. et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–6. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 36.Bahlmann FH, de Groot K, Mueller O. et al. Stimulation of endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension. 2005;45:526–9. doi: 10.1161/01.HYP.0000159191.98140.89. [DOI] [PubMed] [Google Scholar]
- 37.Jourde-Chiche N, Dou L, Sabatier F. et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. Journal of thrombosis and haemostasis: JTH. 2009;7:1576–84. doi: 10.1111/j.1538-7836.2009.03540.x. [DOI] [PubMed] [Google Scholar]