Abstract
Background
This study evaluates the feasibility and strategy of left tracheobronchial lymph node (LN) dissection in the surgical treatment of esophageal cancer, and its impact on surgical outcomes following thoracoscopic esophagectomy.
Methods
Data of 265 patients with thoracic esophageal cancer who underwent thoracoscopic and laparoscopic esophagectomy was retrospectively reviewed. In 80 cases, thoracoscopic esophagectomy was performed without left tracheobronchial LN dissection (group non‐4L), while 185 cases underwent thoracoscopic esophageal mobilization with routine left tracheobronchial node dissection (group 4L). We introduced a “mesoesophageal suspension” method in order to facilitate complete dissection of the left tracheobronchial nodes, along with left recurrent laryngeal nerve nodes. Both univariate and multivariate analyses were performed to evaluate risk factors correlated to left tracheobronchial node metastasis.
Results
The non‐4L group experienced less blood loss than the 4L group (P = 0.009). More mediastinal LNs were dissected in the 4L group (P < 0.001). There was no significant difference with regard to the incidence of major postoperative complications between the two groups. Compared with other LN metastases, the metastatic rate of the left tracheobronchial LNs was relatively lower. Based on multivariate analysis of six factors, lymphatic invasion and subcarinal node metastasis were shown to be strong independent predictors of left tracheobronchial metastasis.
Conclusion
Routine thoracoscopic extensive lymphadenectomy, including the left tracheobronchial LN, was technically feasible and safe in patients with esophageal cancer. Using a mesoesophagus suspension technique, we performed a meticulous LN dissection in the upper mediastinal space.
Keywords: Esophageal neoplasms, esophagectomy, laparoscopy, thoracoscopy, tracheobronchial lymph nodes
Introduction
The current surgical treatment for esophageal cancer includes radical esophagectomy with extended lymphadenectomy. According to Union for International Cancer Control (UICC) standards, the new threshold proposed for accurately defining the pN category in patients with esophageal cancer is the dissection of at least 15 nodes.1 A shortcoming of the 7th Tumor Node Metastasis (TNM) Classification of Malignant Tumors is that it only vaguely defines the mediastinal regional nodes as those that are “in the esophageal drainage area.” However, the rate of metastasis to each mediastinal LN station is vastly different. The effectiveness of dissection of different LN stations cannot be equally important.2, 3
In thoracoscopic esophagectomy, upper mediastinal LN dissection is a time‐consuming step because of the difficulty of operative exploration at the upper mediastinum, and a long training period is required before executing this procedure. Upper mediastinal lymphadenectomy includes a dissection of the upper thoracic paraesophageal nodes (No. 3p, assigned a station designation according to American Joint Committee on Cancer [AJCC] criteria, or No. 105, assigned a station designation according to Japan Esophageal Society [JES] criteria), recurrent nerve LNs (No. 2, or No. 106rec), and the tracheobronchial LNs (No. 4, or No. 106tb). Of these, No. 2 and No. 3p nodes show a high metastatic rate; complete dissection of No. 2 and No. 3p is a crucial step that is routinely recommended during thoracoscopic esophagectomy.4, 5 A currently controversial topic is whether routine prophylactic left No. 4 node (No. 106tb) dissection should be performed for all stages of esophageal cancer. The foremost reason given by opponents is that dissection of the left tracheobronchial LN, which is located deeply within the subaortic region, is a technically challenging and time‐consuming step. Additionally, the left tracheobronchial LN is defined N3, especially for the lower thoracic esophagus, according to the JES LN metastatic grading system, which means a low metastatic rate.6 In this study, we further clarify the feasibility and strategy for left tracheobronchial LN dissection during thoracolaparoscopic esophageal cancer surgery.
Patients and methods
Patients
The institutional review board of the Affiliated Union Hospital of Fujian Medical University granted approval for this study. A database that had been prospectively created to record results from three‐stage thoracoscopic and laparoscopic esophagectomy (TLO) procedures was retrospectively reviewed. All patients were fully clinically evaluated and underwent disease staging. Clinical staging was based on esophagography, esophagoscopy, color ultrasound of the neck, and enhanced computed tomography (CT) of the chest and abdomen. Positron emission tomography or bronchofiberscopy were also performed if required for the determination of individual staging. The tumors were staged according to the 7th UICC TNM classification. All medically fit patients with resectable thoracic esophageal tumors (T1‐T3 tumors), with or without known N1 nodal status, were included in the study. Patients with T4 tumors were not included. Other exclusion criteria were as follows: patients with cervical esophageal carcinoma or gastroesophageal junction carcinoma, multiple primary cancers involving other organs, receiving neoadjuvant chemotherapy or radiotherapy, and patients with non‐squamous cell carcinoma.
A cohort of 265 patients with thoracic esophageal cancer who had undergone TLO between October 2009 to December 2013, were enrolled in the study. In 80 cases, thoracoscopic esophagectomy was performed without left tracheobronchial LN dissection between October 2009 to September 2011 (non‐4L group), and 185 cases underwent thoracoscopic esophageal mobilization with routine left tracheobronchial LN dissection from October 2011 to December 2013 (4L group). Data from all patients were recorded in our electronic database. The clinical and pathological factors and surgical information, including operation time, estimated blood loss, the number of mediastinal and abdominal LNs retrieved, and the incidence of major complications, were compared between the two groups. We expected to be able to clarify the feasibility and safety of left tracheobronchial node dissection following a TLO in patients with thoracic esophageal squamous cell carcinoma (ESCC). Major complications were considered in the final estimates if they required medical or surgical intervention, or if the patient had a prolonged recovery.
Surgical technique
The TLO was performed in three stages, beginning with thoracoscopic esophageal mobilization and mediastinal LN dissection. Our procedure for the lateral decubitus approach has been described in detail in a previous publication.7 With the patient in the supine position, laparoscopy was performed to mobilize and create the gastric conduit. We used a left cervical neck incision to create a cervical esophagogastric anastomosis. The abdominal and cervical surgical procedures were similar in both groups.
Left tracheobronchial lymph node (LN) dissection
During superior mediastinal LN dissection, the left tracheobronchial nodes are dissected in continuity with the left paratracheal nodes. The upper esophagus was pulled in the right posterior direction by gentle traction applied with tape. The trachea or the left main bronchus was simultaneously rotated using a smooth tip retractor in the opposite direction. The mesoesophagus of the proximal esophagus was then stretched tightly to allow meticulous dissection of LNs deep in the upper mediastinal space.
Dissection of the left tracheobronchial nodes (No. 4L) commenced along the upper rim of the left main bronchus. The tissue, including the No. 4L LNs, was dissected sharply with a narrow straight incision, just between the aortic arch and the left bronchus. Below the aortic arch, the recurrent portion of the left recurrent laryngeal nerve was identified. The left tracheobronchial nodes were completely dissected to expose the dorsal side of the left pulmonary artery (Fig 1). Upward dissection of the left recurrent laryngeal nerve node was continued. If necessary, the table was rotated 15° toward the assistant. On the left side, the dissection plane between the left paratracheal region and the left tracheal surface was separated to make the ventral border of the dissection. After dissection, the left paratracheal LNs (No. 2L) were easily exposed, as was the left recurrent nerve, by pulling the mesoesophagus in the right posterior direction (Fig 2). After the left recurrent laryngeal nerve was isolated from the explored tissue (without using an electric device, thereby avoiding injury by electricity or heat), the entire LN chain around the left recurrent laryngeal nerve, as well as the left tracheobronchial nodes, were removed in an en bloc fashion accompanied by the esophagus. Finally, excision of the proximal portions of the mesoesophagus is required to identify and preserve the recurrent laryngeal nerve. We have termed the abovementioned technique, which improves exposure to the left paratracheobronchial region with the use of the traction of the proximal mesoesophagus, “mesoesophagus suspension” (Video 1 Download link: http://c91.yunpan.360.cn/ ID: 13365910827, Password: 83481010).
Figure 1.
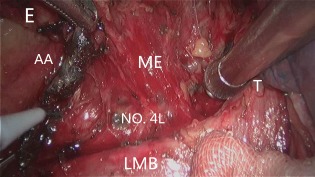
The mesoesophagus of proximal esophagus was stretched tightly to allow meticulous dissection of the left tracheobronchial nodes deep within the subaortic region. AA, aortic arch; E, esophagus; LMB, left main bronchus; ME, mesoesophagus, No. 4L, left tracheobronchial node; T, trachea.
Figure 2.
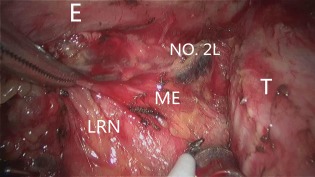
The left paratracheal lymph nodes (No. 2L) were exposed, along with the left recurrent nerve, by pulling the mesoesophagus in the right posterior direction. E, esophagus; LMB, left main bronchus; ME, mesoesophagus; LRN, left recurrent nerve; No. 2L, left paratracheal lymph node; T, trachea.
Statistics
Statistical analyses were performed using version 16.0 of the Statistical Package for Social Sciences (SPSS, Chicago, IL, USA). Continuous variables, including age, blood loss, operation time, number of dissected nodes, and the length of hospital stay were first tested for normal distribution. Continuous variables were expressed as means ± standard deviation if the assumptions of normality were met. The Student's t‐test was used for comparisons between groups. Blood loss, length of hospital stay and operation duration did not show normal distributions; they are expressed as medians (interquartile range). We used Mann‐Whitney U tests for comparisons between groups. Discrete variables, including gender, pathology type, tumor location, the incidence of complications, and accidental conversion, are described as numbers. We compared discrete variables between the groups using the χ2 test or Fisher's exact test. Multiple logistic regression analysis, using a backward Wald test, was conducted to evaluate the statistical significance of the differences in the effects of clinical factors. P values of <0.05 were considered statistically significant.
Results
Thoracoscopic esophagectomy was performed without left tracheobronchial LN dissection in the 80 patients in group non‐4L. In group 4L, thoracoscopic esophagectomy was performed with successful No. 4L node dissection in 170 patients. No. 4L node dissection failed in 15 patients as a result of: calcification of the subaortic LN and dense adhesions (n = 6); a narrow postmediastinum space because the patient was elderly or severely obese (n = 4); palliative resection for a bulky tumor (n = 3); and no bulky LN identified (n = 2). In group non‐4L, there were three conversions to open thoracotomy as a result of a bulk tumor (n = 2), and intraoperative bleeding from the azygos vein (n = 1). Conversion to open thoracotomy was required for six patients in group 4L; the specific indications for conversion were: bulk tumor (n = 4); dense pleural adhesions (n = 1); and intraoperative left main bronchial membranous injury (n = 1).
Patient characteristics
Patient and tumor characteristics are listed in Table 1. There were no differences in the clinical and pathological factors and characteristics of the tumors between the two groups (Table 1).
Table 1.
Clinicopathologic characteristics of the 4L and non‐4L groups
| Factor | 4L dissection | Non‐4L dissection | P value |
|---|---|---|---|
| Gender | |||
| Male | 130 | 61 | 0.969 |
| Female | 40 | 19 | |
| Age (years) | 58.45 ± 8.54 | 60.31 ± 9.10 | 0.117 |
| Tumor location | |||
| Upper | 22 | 8 | 0.381 |
| Middle | 114 | 50 | |
| Lower | 34 | 22 | |
| P stage | |||
| I | 43 | 19 | 0.665 |
| II | 54 | 30 | |
| III | 73 | 31 | |
| Tumor invasion degree | |||
| T1 | 39 | 26 | 0.263* |
| T2 | 33 | 16 | |
| T3 | 94 | 35 | |
| T4 | 4 | 3 | |
| Number of retrieved nodes | |||
| Mediastinal nodes | 21.06 ± 6.89 | 14.90 ± 5.65 | <0.001 |
| Abdominal nodes | 13.10 ± 6.71 | 11.73 ± 4.89 | 0.102 |
| Total operation time (minutes) | |||
| Chest | 130 (30) | 125 (30) | 0.675 |
| Abdomen and neck | 110 (22.5) | 120 (20) | 0.920 |
| Blood loss total (mL) | |||
| Thorax | 200 (112.5) | 150 (100) | 0.009 |
| Abdominal and neck | 30 (30) | 30 (10) | 0.124 |
| Conversion (cases) | |||
| Thoracotomy | 6 | 3 | 1.000* |
| Length of hospital stay | 13 (5) | 12 (5) | 0.261 |
*Fisher's exact test.
Surgical outcomes after esophagectomy
The average duration of surgery in the 4L group was longer than in the non‐4L group (130 vs. 125 minutes); however, the difference was not statistically significant. The non‐4L group experienced significantly lower thoracic blood loss than the 4L group. One patient in each group required a blood transfusion. The number of dissected mediastinal LNs was larger in the 4L group (P < 0.05; Table 1). There was no significant difference in the length of hospital stay or incidence of accidental conversion between the two groups.
Table 2 shows the locoregional LN metastatic rates in both the 4L and the non‐4L node dissection groups. There was no significant difference in the incidence of metastasis to the LNs between the two groups. The pericardial LNs were the most frequently involved (23.5% in the 4L and 22.5% in the non‐4L group), followed by the right recurrent laryngeal nerve LNs (18.2% in the 4L and 15% in the non‐4L group). Only nine patients had left tracheobronchial LN metastasis, with a metastatic rate of 5.3% in the 4L group (Table 3).
Table 2.
The locoregional lymph node metastatic rates in the two groups
| Regional lymph nodes | JES | UICC/AJCC |
Dissection n = 170 No. (%) |
Non‐dissection n = 80 No. (%) |
P value |
|---|---|---|---|---|---|
| Thoracic lymph node | |||||
| Upper paraesophageal | 105 | 3P | 9 (5.3) | 6 (7.5) | 0.570* |
| Left recurrent nerve | 106recL | 2L | 22 (12.9) | 7 (8.8) | 0.334 |
| Right recurrent nerve | 106recR | 2R | 31 (18.2) | 12 (15) | 0.527 |
| Left tracheobronchial | 106tbl | 4L | 9 (5.3) | / | / |
| Left main bronchus | 109L | 10L | 4 (2.4) | 1 (1.3) | 1.000* |
| Subcarinal | 107 | 7 | 20 (11.8) | 6 (7.5) | 0.303 |
| Middle paraesophageal | 108 | 8M | 28 (16.5) | 12 (15) | 0.767 |
| Lower paraesophageal | 110 | 8L | 22 (12.9) | 9 (11.3) | 0.705 |
| Abdominal lymph node | |||||
| Pericardial | 1, 2 | 16 | 40 (23.5) | 18 (22.5) | 0.857 |
| Left gastric artery | 7 | 17 | 14 (8.2) | 5 (6.3) | 0.581 |
| Common hepatic artery | 8 | 18 | 4 (2.4) | 1 (1.3) | 1.000* |
| Splenic artery | 10 | 19 | 1 (0.6) | 0 (0) | 1.000* |
*Fisher's exact test. AJCC, American Joint Commission on Cancer; JES, Japan Esophageal Society; UICC, Union for International Cancer Control.
Table 3.
Complications after thoracolaparoscopic esophagectomy in the two groups
| Complication | Dissection (n = 170) | Non‐dissection (n = 80) | P value |
|---|---|---|---|
| Pneumonia and atelectasis | 22 (12.9%) | 10 (12.5%) | 0.922 |
| Vocal cord palsy | 16 (9.4%) | 8 (10%) | 0.883 |
| Anastomotic leaks | 11 (6.5%) | 4 (5%) | 0.780* |
| Arrhythmia | 15 (8.8%) | 6 (7.5%) | 0.725 |
| Chylothorax | 4 (2.4%) | 2 (2.5%) | 1.000* |
| Delayed gastric emptying | 5 (2.9%) | 2 (2.5%) | 1.000* |
| Overall | 62 (36.5%)† | 26 (32.5%)‡ | 0.540 |
*Fisher's exact test. †Seven patients had two complications; two patient had three complications. ‡Four patients had two complications; one patient had three complications.
There were no operative deaths in either of the two groups. Postoperative major complications developed in 62 of the 170 patients in the 4L group (36.5%) and in 26 of the 80 patients in the non‐4L group (32.5%). Pneumonia was the most common complication in both groups. Of these cases, one patient in each group developed respiratory failure and required mechanical ventilation. A water‐soluble swallow was administered to assess for leak on day eight. Eleven patients in the 4L group experienced an anastomotic leak (6.5%), compared to only four patients in the non‐4L group (5%). All of these patients were treated conservatively with drainage and nutritional support. Postoperative hoarseness was observed in 11 patients in the 4L group (9.4%) versus eight patients in the non‐4L group (10%). If the hoarseness persisted, patients underwent direct laryngoscopy for postoperative assessment of vocal cords. All patients had recovered from hoarseness at their six month follow‐up. There was no statistically significant difference in the incidence of respiratory complications, vocal cord palsy, anastomotic leaks, chylothorax, delayed gastric emptying, intestinal obstruction, or arrhythmia between the two groups (Table 3). One patient from each group died from respiratory failure caused by pneumonia.
Risk factors correlated to left tracheobronchial LN metastasis
Single logistic regression analysis revealed a significant difference between the groups in: the depth of tumor invasion; pathological stage; pathological N stage; left recurrent LN metastasis; subcarinal LN metastasis; and lymphovascular invasion (Table 4). In multiple logistic regression analysis using the six factors that showed a significant difference, the odds ratio for lymph vascular invasion was 15.66, while the odds ratio for subcarinal LN metastasis was 5.37 (Table 5). Among the predictors of LN metastases analyzed in this study, lymphatic invasion and subcarinal node metastasis were shown to be strong independent predictors of left tracheobronchial node metastasis.
Table 4.
Risk factors for left tracheobronchial lymph node metastasis
| No. 4L metastasis | No. 4L non‐metastasis | P | |
|---|---|---|---|
| Gender | 1.000* | ||
| Male | 7 | 123 | |
| Female | 2 | 38 | |
| Tumor length (cm) | 0.447* | ||
| <3 cm | 1 | 46 | |
| ≥3 cm | 8 | 115 | |
| Tumor location | 0.163* | ||
| Upper thoracic | 1 | 21 | |
| Middle thoracic | 4 | 110 | |
| Lower thoracic | 4 | 30 | |
| Histological grade | 0.480* | ||
| G1 | 4 | 47 | |
| G2 | 4 | 99 | |
| G3 | 1 | 15 | |
| Tumor invasion degree | 0.014* | ||
| T1 | 2 | 37 | |
| T2 | 0 | 33 | |
| T3 | 5 | 89 | |
| T4 | 2 | 2 | |
| Pathological stage | 0.002* | ||
| I | 0 | 43 | |
| II | 0 | 54 | |
| III | 9 | 64 | |
| Lymph vascular invasion | <0.001* | ||
| Negative | 2 | 136 | |
| Positive | 7 | 25 | |
| Nerve invasion | 1.000* | ||
| Negative | 8 | 144 | |
| Positive | 1 | 17 | |
| Pathological N stage | <0.001* | ||
| N0 | 0 | 75 | |
| N1 | 1 | 53 | |
| N2 | 3 | 21 | |
| N3 | 5 | 12 | |
| LRLN metastasis | 0.017* | ||
| Negative | 5 | 143 | |
| Positive | 4 | 18 | |
| Subcarinal LN metastasis | 0.012* | ||
| Negative | 5 | 145 | |
| Positive | 4 | 16 |
*Fisher's exact test. LN, lymph node; LRLN, left recurrent nerve lymph node.
Table 5.
Logistic regression analysis of factors correlated to left tracheobronchial lymph node metastasis
| Factors | Regression coefficient | Standard error | Wald value | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Lymphatic invasion | 2.751 | 0.847 | 10.563 | 0.001 | 15.664 | 2.981–82.318 |
| Subcarinal LN metastasis | 1.682 | 0.800 | 4.420 | 0.036 | 5.374 | 1.121–25.775 |
CI, confidence interval; LN, lymph node; OR, odds ratio.
Discussion
In order to avoid misunderstanding, left tracheobronchial LNs should not be confused with aortopulmonary LNs. According to the definition of thoracic LNs proposed by both the AJCC and JSE in 2009, the superior border of left tracheobronchial LN (No. 4L or No. 106tbL) is the inferior wall of the aortic arch. The LNs are located in the area surrounded by the medial wall of the aortic arch, with the lower border being the upper rim of the left main bronchus.1, 6 From the traditional view, left tracheobronchial LNs are the regional LNs in esophageal cancer, which can be removed when the right transthoracic procedure is used. However, aortopulmonary LNs (ligamentum arteriosum nodes, No. 5 or No. 113) are located on the left side of the arterial ligament. Therefore, the aortopulmonary LNs were removed when the left transthoracic procedure was used. Aortopulmonary LNs (No. 113) are defined as N4 for thoracic esophageal cancer according to the JES LN metastatic grading system.6
Currently, there are two major staging systems used for esophageal cancer: the UICC/AJCC TNM classification (7th edition) and the Japanese Classification of Esophageal Cancer (10th edition). The updated 7th edition TNM staging of esophageal cancer reflects the importance of metastatic spread to the LNs for prognosis and it reclassifies N stage into three categories according to the number of involved regional LNs. It is reasonable that in patients with esophageal cancer, the left tracheobronchial LNs, which belong to the regional LNs, should be routinely dissected during radical surgery. In contrast, the latest edition of the Japanese Classification of Esophageal Cancer divided the location of metastatic LNs into four categories in relation to the primary esophageal lesion. Therefore, the left tracheobronchial LN is defined as N2 (tumor location in the upper and middle thoracic) or N3 (tumor location in the lower thoracic). Although the number of metastatic LNs might be a better prognostic indicator than the Japanese N grading, the Japanese N grading seems more informative for balancing the aggressiveness and safety of the operation. For this reason, some experts have considered whether left tracheobronchial LN dissection can be omitted from the standard LN dissection in high risk patients.
According to our pathological examinations, the pericardial LNs, the bilateral recurrent nerve LNs, and the paraesophageal LNs were high frequency metastatic sites, followed by the subcarinal and left gastric artery LNs. However, only nine patients had one No. 4L node metastasis, with a metastatic rate of 5.3% in the 4L group (No. 106tb), which is comparable to a previous report (Table 3).2 Compared with other LN metastases, the metastatic rate to the left tracheobronchial LNs was lower. In addition, all left tracheobronchial LN metastases were accompanied by other locoregional LN metastases. These results correlated closely with the current Japanese N grading. Accordingly, we advise that left tracheobronchial LN metastasis is a delayed event in the lymphatic metastases of esophageal carcinoma.
Among the predictors of left tracheobronchial node metastasis analyzed in this study, lymphatic invasion was shown to be the strongest independent predictor of metastasis. This result was confirmed by the fact that one patient with a pT1b tumor with lymphatic vessel invasion, revealed by postoperative pathological examination, was found with positive No. 4L node metastasis. Knowedge of the anatomy of lymphatic drainage of the esophagus is crucial to understanding the pattern of LN metastasis in patients with esophageal carcinoma. The abundant lymphatic channels in the mucosa of the lamina propria and submucosa of the esophagus are well known from classic descriptions.8 The process of metastasis formation is based on the ability of tumor cells to loosen from their primary tumor cell mass and invade to lymphovascular structures. The presence of lymphatic vessel invasion demonstrates a state of advanced and more aggressive tumor behavior. For the same reason, the histologic identification of lymphatic vessel invasion by tumor cells has been recognized as a potential prognostic indicator in various other malignancies.9, 10, 11, 12 Tumor dissemination to subcarinal LN metastasis was shown to be another strong independent predictor of left tracheobronchial metastasis. Fu et al. reported that metastatic disease in the subcarinal node indicates a worse prognosis for patients with thoracic ESCC compared with patients having paraesophageal node metastases.13 Shimada et al. also reported that the presence of subcarinal node metastasis was an independent risk factor for poorer survival.14 Based on the results of multivariate analysis, patients with left tracheobronchial node metastasis tend to present with multiple or multigroup LN metastases and clinically defined advanced disease.
In thoracoscopic esophagectomy, dissection of the No. 4L node, which is located deep in the right lateral of the subaortic region, is thought to be a technically challenging step because of the difficulty in operative exploration at the upper mediastinum. We introduce a mesoesophageal suspension technique in order to facilitate complete dissection of the No. 4L node, along with the No. 2L node. The structure of the mesoesophagus is seldom discussed in the literature because it is not easily identified; however, the embryology of the mesentery can provide information that is useful for identification of the mesoesophagus. The vessels, regional LNs, and the nerves of the esophagus are located adjacent to each other in the same anatomical compartment; therefore, we defined the mesoesophagus as this anatomical compartment.15 Total excision of the regional portion of the mesentery with the primary lesion might be a promising radical treatment strategy for patients with esophageal carcinoma.16 As mentioned above, with the suspension of the proximal portion of the mesoesophagus, we greatly improved infra‐aortic operative exposure, even around the left recurrent laryngeal nerve. We perform a precise and accurate LN dissection in this area, and harvest more LNs along the left recurrent laryngeal nerve below the aortic arch as far as the thoracic inlet with an en‐bloc excised specimen. Additionally, other measures can be taken to shorten the learning curve and help surgeons become technically proficient with this procedure. Our technique routinely uses a right‐sided double‐lumen tube for the distal trachea in order to make the left mainstem bronchus more flexible, thus, allowing deep dissection into the left tracheoesophageal groove and the subaortic region. Recently, we even successfully applied a single‐lumen tube combined with an artificial pneumothorax during a procedure for the thoracoscopic resection of early stage esophageal cancer.
Our initial experience confirmed that dissection of the left tracheobronchial LN can be achieved safely during a thoracoscopic esophagectomy when performed in correctly selected patients and by a surgeon with vast experience in this field. The surgical outcomes in the 4L group were compared with those in the non‐4L group. Although No. 4L node dissection increased thoracic blood loss, there were no significant differences in blood transfusion and postoperative major complications between the two groups. Because LN pathology cannot be accurately determined before or during surgery, we believe that No. 4L node dissection is essential for both accurate staging and local control in thoracic esophageal carcinoma patients, even with stage T1b tumors. On the other hand, our clinical observation is that the metastatic rate of the No. 4L node is relatively low. In a patient with considerable risk factors, such as dense adhesions, calcification below the aortic region, or a narrow subaortic space found in elderly or severely obese patients, omission of the left tracheobronchial LN clearance from the standard LN dissection is a more logical therapeutic strategy in order to strike a balance between the surgical arm of the procedure and the safety of the patient.
Conclusion
In conclusion, routine thoracoscopic extensive lymphadenectomy, including the left tracheobronchial LN, is technically feasible and safe when performed by a specialist in thoracoscopic surgery. Lymphatic invasion and subcarinal LN metastasis were shown to be strong independent predictors of No. 4L metastasis in this study. With the application of the mesoesophagus suspension technique, we performed not only a meticulous LN dissection in the upper mediastinal space, but also harvested the No. 4L LNs along the left recurrent laryngeal nerve node in an en bloc fashion. However, the best indicator for determining whether No. 4L dissection is necessary in the surgical resection of esophageal cancer is the impact of resection on survival. Further studies including long‐term follow‐up and prospective trials should be performed.
Disclosure
No authors report any conflict of interest.
Supporting information
Video S1 With the application of the mesoesophagus suspension technique, we harvested the No. 4L lymph nodes along the left recurrent laryngeal nerve node in an en bloc fashion.
References
- 1. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers, V.2. 2013. [Cited 14 July 2013] Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
- 2. Udagawa H, Ueno M, Shinohara H et al The importance of grouping of lymph node stations and rationale of three‐field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012; 106: 742–747. [DOI] [PubMed] [Google Scholar]
- 3. Shimada H, Okazumi S, Matsubara H et al Impact of the number and extent of positive lymph nodes in 200 patients with thoracic esophageal squamous cell carcinoma after three‐field lymph node dissection. World J Surg 2006; 30: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 4. Noshiro H, Iwasaki H, Kobayashi K et al Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg Endosc 2010; 24: 2965–2973. [DOI] [PubMed] [Google Scholar]
- 5. Puntambekar SP, Agarwal GA, Joshi SN, Rayate NV, Sathe RM, Patil AM. Thoracolaparoscopy in the lateral position for esophageal cancer: The experience of a single institution with 112 consecutive patients. Surg Endosc 2010; 24: 2407–2414. [DOI] [PubMed] [Google Scholar]
- 6. Japan Esophageal Society . Japanese Classification of Esophageal Cancer, tenth edition: Part I. Esophagus 2009; 6: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin J, Kang M, Chen C et al Thoracolaparoscopy esophagectomy and extensive two‐field lymphadenectomy for oesophageal cancer: Introduction and teaching of a new technique in a high‐volume centre. Eur J Cardiothorac Surg 2013; 43: 115–121. [DOI] [PubMed] [Google Scholar]
- 8. Weinberg JA. Lymphatics of the esophagus In: Haagensen CD, Feind CR, Herter FP, Slanetz CA, Jr, Weinberg JA. (eds). The Lymphatics in Cancer. Saunders, Philadelphia: 1972; 245–249. [Google Scholar]
- 9. Nentwich MF, Bohn BA, Uzunoglu FG et al Lymphatic invasion predicts survival in patients with early node‐negative non‐small cell lung cancer. J Thorac Cardiovasc Surg 2013; 146: 781–787. [DOI] [PubMed] [Google Scholar]
- 10. Dicken BJ, Graham K, Hamilton SM et al Lymphovascular invasion is associated with poor survival in gastric cancer: An application of gene‐expression and tissue array techniques. Ann Surg 2006; 243: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishii M, Ota M, Saito S, Kinugasa Y, Akamoto S, Ito I. Lymphatic vessel invasion detected by monoclonal antibody D2‐40 as a predictor of lymph node metastasis in T1 colorectal cancer. Int J Colorectal Dis 2009; 24: 1069–1074. [DOI] [PubMed] [Google Scholar]
- 12. Arnaout‐Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2‐40 in node negative breast cancer. Mod Pathol 2007; 20: 183–191. [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Hu Y, Xie X, Fu J. Subcarinal node metastasis in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 2012; 93: 423–427. [DOI] [PubMed] [Google Scholar]
- 14. Shimada H, Okazumi S, Matsubara H et al Long‐term results after dissection of positive thoracic lymph nodes in patients with esophageal squamous cell carcinoma. World J Surg 2008; 32: 255–261. [DOI] [PubMed] [Google Scholar]
- 15. Yuji T. Total mesoesophageal esophagectomy. Chin Med J 2014; 127: 574–579. [PubMed] [Google Scholar]
- 16. Matsubara T, Ueda M, Nagao N, Takahashi T, Nakajima T, Nishi M. Cervicothoracic approach for total mesoesophageal dissection in cancer of the thoracic esophagus. J Am Coll Surg 1998; 187: 238–245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 With the application of the mesoesophagus suspension technique, we harvested the No. 4L lymph nodes along the left recurrent laryngeal nerve node in an en bloc fashion.


