Abstract
Background
No standard second‐line regimen exists for the treatment of advanced esophageal squamous cell carcinoma (ESCC). The aim of this study was to evaluate the efficacy and safety of irinotecan and fluorouracil‐based chemotherapy as a second or third‐line regimen for advanced ESCC patients.
Methods
We retrospectively reviewed a cohort of 27 consecutive patients with advanced ESCC in one institute, treated with a combination of irinotecan plus fluorouracil‐based regimens after the failure of first‐line platinum‐based therapy. Nine patients were treated with 150–160 mg/m2 irinotecan and 400 mg/m2 fluorouracil (5‐FU) on day 1, followed by 2000 mg/m2 5‐FU during a 48‐hour infusion every two weeks. Eighteen patients received 150–160 mg/m2 irinotecan on day 1 and 80–120 mg/day S‐1 on days 1–10 every two weeks. The S‐1 dose was based on the patients' body surface area.
Results
Twenty‐four of the 27 patients were assessable for response. One (3.7%) patient achieved complete response, seven (25.9%) achieved partial response, eight (29.6%) had stable disease, and eight (29.6%) had progressive disease. The median progression‐free and overall survival were 4.8 (95% confidence interval [CI]: 1.2–8.4) and 10.5 months (95% CI: 8.4–12.7), respectively. Grade 3 neutropenia and diarrhea were detected in four (15%) and one (4%) patient, respectively. No grade 4 toxicity was noted.
Conclusions
Our study indicates that an irinotecan plus 5‐FU‐based regimen is effective and well‐tolerated as a second or third‐line chemotherapy for patients with advanced ESCC.
Keywords: 5‐Fluorouracil, chemotherapy, esophageal squamous cell carcinoma, irinotecan
Introduction
Esophageal cancer is a common malignant tumor worldwide and the fourth leading cause of cancer‐related mortality in China.1 The predominant histological types of esophageal cancer are squamous cell carcinoma and adenocarcinoma. Despite the considerable variation of incidence in different countries, squamous cell carcinoma accounts for more than 90% of all esophageal carcinomas in China. Patients who present at diagnosis with metastatic disease are unsuitable for primary surgical resection. Cisplatin‐based regimens have been established as first‐line chemotherapy in such patients with advanced esophageal squamous cell carcinoma (ESCC), demonstrating response rates (RRs) of 30–60% and median overall survival ranging from five to 10 months.2, 3, 4 However, these patients could ultimately develop progression after the failure of first‐line treatment. Until recently, there has been no established standard regimen for the second or third‐line treatment of metastatic ESCC patients. Therefore, there is an increasing urgency to define effective chemotherapy after failure of first or second‐line chemotherapy.
A number of chemotherapy regimens have been investigated in ESCC patients and have shown generally low activity in the second or third‐line setting. The most utilized regimen in published studies has been a docetaxel combined with platinum analog.5, 6, 7 Irinotecan, a topoisomerase I inhibitor, has been shown to have an overall response rate of 15% as a single agent in the treatment of cisplatin‐refractory esophageal carcinoma.8 An irinotecan combined with a fluorouracil‐based (5‐fluorouracil or capecitabine) regimen has shown modest antitumor activity and acceptable toxicity for advanced esophageal and gastric cancer.9, 10 However, all of these studies were phase II clinical trials, in which the predominant histology type was adenocarcinoma; the proportion of patients enrolled with ESCC was low.
In this study, we retrospectively analyzed a series of 27 patients with recurrent/metastatic ESCC treated with an irinotecan and fluorouracil‐based regimen as second or third‐line chemotherapy. Efficacy and clinical safety were evaluated after observing treatment‐related toxicities.
Patients and methods
Patient eligibility
Data from 27 consecutive patients with recurrent or metastatic ESCC treated with irinotecan combined with fluorouracil‐based chemotherapy at the Cancer Hospital, Chinese Academy of Medical Sciences, between July 2010 and July 2014, were retrospectively reviewed. The eligibility criteria included: (i) histological diagnosis of ESCC; (ii) age > 18 years; (iii) Eastern Cooperative Oncology Group (ECOG) performance status 0–2; (iv) at least one measurable lesion; (v) failure of the initial or second‐line chemotherapy; (vi) no prior exposure to irinotecan or 5‐fluorouracil (5‐FU) as first‐line chemotherapy; and (vii) adequate hematologic, hepatic, and renal functions (absolute neutrophil count > 1.5 × 109/l, platelet count > 100 × 109/l, total bilirubin ≤ 1.5 × upper limit of normal, aspartate transaminase and alanine transaminase ≤ 2 × upper limit of normal, and creatinine ≤ 1.5 mg/dl).
Treatment
Second‐line treatments commenced at least one month after receiving a prior chemotherapy regimen. Of the 27 patients, nine were administered a combined regimen of 150–160 mg/m2 irinotecan, followed by 200 mg leucovorin and 400 mg/m2 5‐FU intravenously, then followed immediately by continuous intravenous 5‐FU at a dose of 2000 mg/m2 in 48 hours every two weeks. Eighteen patients received a combined regimen of 150–160 mg/m2 irinotecan by intravenous infusion, and S‐1 orally on days 1–10 of every two week schedule. The dose of S‐1 was based on the patients' body surface area (BSA) as follows: 80 mg/day (BSA < 1.25 m2), 100 mg/day (BSA ≥ 1.25 m2, <1.5 m2), and 120 mg/day (BSA ≥ 1.5 m2). S‐1 was administered twice daily. Atropine 0.25 mg was given subcutaneously previous to irinotecan to prevent the cholinergic syndrome associated with irinotecan. There were no delays or dose reductions during chemotherapy.
Evaluation of the response and toxicity
Objective tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 after at least two cycles of treatment, and the diameter of the target lesion was measured by computed tomography scan. Complete response (CR) was defined by the disappearance of all measurable/evaluable disease, partial response (PR) by at least a 30% decrease in the target lesions, and progressive disease (PD) by at least a 20% increase in the target lesions or the appearance of one or more new lesions. Stable disease (SD) was defined as neither PR nor a sufficient increase to qualify for PD. The RR was defined as the percentage of cases with a best overall response of CR or PR. The tumor control rate was defined as the percentage of cases which achieved CR, PR or SD. Treatment‐related toxicities were graded according to Common Terminology Criteria Adverse Events (CTCAE) version 4.0.
Statistical analysis
Progression‐free survival (PFS) was calculated from the date of initial treatment with irinotecan and fluorouracil‐based chemotherapy to the date of progression or last follow‐up. Overall survival (OS) was calculated from the date of initial treatment with irinotecan and fluorouracil‐based chemotherapy to the date of death or last follow‐up. Both PFS and OS were analyzed using the Kaplan–Meier method. All analyses were performed by SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
The characteristics of the 27 patients are summarized in Table 1. All patients were male, with a median age of 56 years (range 44–63), and a histological diagnosis of squamous cell carcinoma. All patients had received prior systemic chemotherapy of paclitaxel combined with platinum, and 66% had undergone previous radical treatment with surgery (12/27) or radiotherapy (6/27). An irinotecan and fluorouracil‐based regimen was administered as second‐line chemotherapy in 23 cases (85%) and as third‐line chemotherapy in four cases (15%), including nine patients (33%) who were given irinotecan/5‐FU and 18 (67%) who were given irinotecan/S‐1.
Table 1.
Patient characteristics (n = 27)
| Characteristics | No. of patients |
|---|---|
| Gender | |
| Male | 27 (100%) |
| Age (years) | |
| Median (range) | 56 (44–63) |
| ECOG score | |
| 0 | 10 (37%) |
| 1 | 15 (56%) |
| 2 | 2 (7%) |
| Site of primary | |
| Upper thoracic esophageal | 3 (11%) |
| Middle thoracic esophageal | 9 (33%) |
| Lower thoracic esophageal | 15 (56%) |
| Differentiation | |
| Well differentiated | 2 (7%) |
| Moderately differentiated | 16 (59%) |
| Poorly differentiated | 9 (33%) |
| Disease extent | |
| Locally advanced | 5 (19%) |
| Metastatic | 22 (81%) |
| Sites of disease | |
| Local lymph nodes | 23 (85%) |
| Distant lymph nodes | 10 (37%) |
| Liver | 9 (33%) |
| Lung | 7 (26%) |
| Prior treatment | |
| Chemotherapy | 26 (96%) |
| Concurrent chemoradiotherapy | 1 (4%) |
| Surgery | 12 (44%) |
| Radiotherapy | 6 (22%) |
| Prior chemotherapy regimen | |
| Paclitaxel + Cisplatin | 24 (89%) |
| Paclitaxel + Nedaplatin | 2 (7%) |
| Paclitaxel + Lobaplatin | 1 (4%) |
| As second‐line chemotherapy | 23 (85%) |
| As third‐line chemotherapy | 4 (15%) |
ECOG, Eastern Cooperative Oncology Group.
Response and survival
A total of 87 cycles were administered to 27 patients, with a median of three cycles per patient (range 1–8). Twenty‐four of the 27 patients (89%) were assessable for response; treatment was discontinued in three patients after the first cycle as a result of grade 2 nausea and vomiting in two patients, and grade 3 diarrhea in one. Of the 24 patients, five were evaluated after two cycles of treatment, and 19 were evaluated after three cycles. All efficacy data are reported using the intention‐to‐treat population. The best overall response was evaluated in each case (Table 2). Objective responses to treatments were observed in eight cases (29.6%) with a disease‐control rate of 59.2%. One patient achieved CR (3.7%), seven patients achieved PR (25.9%), 29.6% of patients had SD, and 29.6% had PD. At a median follow‐up of 15.2 months, eight of the 24 patients were alive and 16 had died. Kaplan–Meier curves for PFS and OS are shown in Figure 1. Four patients who had SD received subsequent radiotherapy before tumor progression. The median PFS was 4.8 months (95% confidence interval [CI]: 1.2–8.4 months) and the median OS was 10.5 months (95% CI: 8.4–12.7 months).
Table 2.
Best overall response (RECIST version 1.1)
| Case (%) | |
|---|---|
| CR | 1 (3.7) |
| PR | 7 (25.9) |
| SD | 8 (29.6) |
| PD | 8 (29.6) |
CR, complete response; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Figure 1.
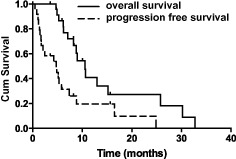
Progression‐free and overall survival.
Toxicity
Treatment‐related toxicities are shown in Table 3. Regarding hematological toxicity, neutropenia (8 cases, 30%) was the most frequently observed complication, followed by leucopenia (7 cases, 26%) and anemia (6 cases, 22%). Regarding grade 3 adverse events, four cases (15%) of neutropenia were observed; anorexia (9 cases, 34%) and nausea/vomiting (9 cases, 34%) were the most common nonhematological toxicities, followed by diarrhea (4 cases, 15%) and fatigue (3 cases, 11%). We observed one case (4%) of grade 3 diarrhea. No grade 4 adverse event was observed. The treatment was well tolerated and no toxic death occurred.
Table 3.
Hematological and non‐hematological toxicity (n = 27)
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematological toxicity | ||||
| Neutropenia | 2 (7%) | 2 (7%) | 4 (15%) | 0 |
| Leukopenia | 1 (4%) | 6 (22%) | 0 | 0 |
| Anaemia | 5 (19%) | 1 (4%) | 0 | 0 |
| Nonhematological toxicity | ||||
| Nausea/vomiting | 4 (15%) | 5 (19%) | 0 | 0 |
| Diarrhea | 1 (4%) | 2 (7%) | 1 (4%) | 0 |
| Anorexia | 6 (22%) | 3 (12%) | 0 | 0 |
| Fatigue | 1 (4%) | 2 (7%) | 0 | 0 |
Discussion
Our study provides evidence of the clinical utility of an irinotecan plus fluorouracil‐based regimen as salvage therapy in patients with ESCC. As a previous study demonstrated that irinotecan plus S‐1 was not inferior to 5‐FU and folinic acid in patients who received second‐line chemotherapy for metastatic colorectal cancer, our study analyzed a group of 27 patients treated with irinotecan and 5‐FU or S1.11 Our results showed an encouraging RR of 29.6% and SD in a further 29.6% of patients. Treatment was well‐tolerated with the most frequently observed adverse events being neutropenia and nausea/vomiting, while no grade 4 adverse events were noted. Median PFS was 4.8 months and median OS was 10.5 months.
As a result of the limited number of studies published, no standard second or third‐line chemotherapy has emerged in the treatment of ESCC. Although there have been some reports on irinotecan combined with 5‐FU in patients with both esophageal and gastric cancer in the second‐line setting, only two phase II studies included a small number of patients with ESCC.9, 10 Both of these studies used irinotecan plus fluorouracil‐based (5‐FU or capecitabine) regimens as second‐line therapy, and yielded objective responses of 17% and 29%, and PFS of 3.1 and 3.7 months with corresponding OS of 6.4 and 6.5 months, respectively. Grade 3/4 neutropenia and diarrhea were observed in 31–36% and 8–15% of patients, respectively. However, both studies consisted of multiple histological subtypes, with only about 7% of squamous cell carcinomas. As there is increasing recognition that squamous cell carcinoma and adenocarcinoma should be studied as different entities, because optimal therapies may differ, it remains uncertain whether these efficacies can be acquired from patients with ESCC. To our knowledge, our study reported efficacy in the largest number of ESCC patients who received irinotecan plus fluorouracil‐based regimens as salvage therapy.
Several irinotecan‐based regimens have been tested as second‐line treatment for advanced gastroesophageal cancer. Burkart et al. evaluated weekly irinotecan at 100 mg/m2 every four weeks in 14 patients with relapsed esophageal cancer after cisplatin‐based chemotherapy; the RR was 15.4%, with PFS of two months and OS of five months.8 Grade 3 toxicities included diarrhea (n = 3), fever (n = 1), and pain (n = 1). Moreover, second‐line chemotherapy studies of irinotecan in combination with a docetaxel regimen in patients with advanced esophageal cancer resulted in RRs of 12.5% and 36%, PFS of 11 and 18.5 weeks, and OS of 24 and 26 weeks.12, 13 However, in these studies, 37 (90%) and 11 (45.8%) patients had adenocarcinoma histology. Grade 3 and 4 toxicities included neutropenia, diarrhea and nausea/emesis, and one of studies reported two deaths related to toxicity.12
Fluorouracil‐based regimens were also evaluated in second‐line treatment for patients with esophageal cancer. Akutsu et al. assessed the efficacy of S‐1 monotherapy as second or third line treatment in patients with unresectable and recurrent ESCC, with promising results: RR 25%, median PFS 3.3 months, and OS 10.8 months.14 Incidences of grade 3 leukopenia and diarrhea were 5% and 10%, respectively, and no grade 4 toxicity was observed. Another small phase II trial of second‐line treatment for patients with recurrent esophageal cancer, using docetaxel and S‐1, reported an RR of 21% and a median OS of 10 months.15
In our study, the regimen was well tolerated; the most common toxicities were grade 2 leukopenia (22%) and grade 1 anorexia (22%). The profiles and incidence of toxicities in our study were lower than those of other phase II studies, which may be attributed to the lower doses of irinotecan (150–160 mg/m2) that were used in our study.9, 10
Regarding molecular targeted agents, no significant survival benefit has been demonstrated in the treatment of metastatic ESCC patients. A phase II study evaluated the epidermal growth factor receptor inhibitor, cetuximab, in combination with irinotecan as second‐line treatment in 52 patients with platinum‐resistant gastroesophageal cancer. The RR was 14% and OS was 6.4 months.16 Grade 3/4 neutropenia was observed in 16% of patients, and grade 3 diarrhea in four patients (8%). In addition, the first prospective phase III trial to investigate gefitinib as second‐line treatment in patients with esophageal cancer demonstrated a PFS benefit of gefitinib compared with a placebo (1.57 vs. 1.17 months, P = 0.02), but no significant difference in overall survival (3.67 vs. 3.73 months, P = 0.29).17 To date, cytotoxic chemotherapy remains the mainstay of treatment for patients with metastatic ESCC.
In summary, our study indicated that irinotecan combined with a fluorouracil‐based regimen as second or third‐line chemotherapy was well‐tolerated and the RR of 29.6% was encouraging. Because of the limitations of our study – relatively small sample size and retrospective analysis – a definite conclusion cannot be drawn. A prospective randomized clinical trial detecting the merits of an irinotecan combined with fluorouracil‐based regimen should be conducted in the future.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This work was supported in part by grants from the Beijing Medical Award Foundation Grant and the Beijing Municipal Science & Technology Commission Grant (Z141100002114012).
References
- 1. Chen W, Zheng R, Zhang S et al Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res 2013; 25: 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hayashi K, Ando N, Watanabe H et al Phase II evaluation of protracted infusion of cisplatin and 5‐fluorouracil in advanced squamous cell carcinoma of the esophagus: A Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol 2001; 31: 419–423. [DOI] [PubMed] [Google Scholar]
- 3. Bleiberg H, Conroy T, Paillot B et al Randomised phase II study of cisplatin and 5‐fluorouracil (5‐FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer 1997; 33: 1216–1220. [DOI] [PubMed] [Google Scholar]
- 4. Huang J, Zhou Y, Zhang H et al A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Med Oncol 2013; 30: 343. [DOI] [PubMed] [Google Scholar]
- 5. Minamide J, Aoyama N, Takada K, Oota Y. [Evaluation of docetaxel, CDDP and 5‐FU combined therapy as second‐line chemotherapy for esophagus cancer.] Gan to Kagaku Ryoho 2007; 34: 49–52. (In Japanese.) [PubMed] [Google Scholar]
- 6. Jin J, Xu X, Wang F et al Second‐line combination chemotherapy with docetaxel and nedaplatin for Cisplatin‐pretreated refractory metastatic/recurrent esophageal squamous cell carcinoma. J Thorac Oncol 2009; 4: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 7. Nakajima Y, Suzuki T, Haruki S et al A pilot trial of docetaxel and nedaplatin in cisplatin‐pretreated relapsed or refractory esophageal squamous cell cancer. Hepatogastroenterology 2008; 55: 1631–1635. [PubMed] [Google Scholar]
- 8. Burkart C, Bokemeyer C, Klump B, Pereira P, Teichmann R, Hartmann JT. A phase II trial of weekly irinotecan in cisplatin‐refractory esophageal cancer. Anticancer Res 2007; 27: 2845–2848. [PubMed] [Google Scholar]
- 9. Leary A, Assersohn L, Cunningham D et al A phase II trial evaluating capecitabine and irinotecan as second line treatment in patients with oesophago‐gastric cancer who have progressed on, or within 3 months of platinum‐based chemotherapy. Cancer Chemother Pharmacol 2009; 64: 455–462. [DOI] [PubMed] [Google Scholar]
- 10. Assersohn L, Brown G, Cunningham D et al Phase II study of irinotecan and 5‐fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol 2004; 15: 64–69. [DOI] [PubMed] [Google Scholar]
- 11. Muro K, Boku N, Shimada Y et al Irinotecan plus S‐1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second‐line chemotherapy for metastatic colorectal cancer: A randomized phase 2/3 noninferiority study (FIRIS study). Lancet Oncol 2010; 11: 853–860. [DOI] [PubMed] [Google Scholar]
- 12. Hawkes E, Okines AF, Papamichael D et al Docetaxel and irinotecan as second‐line therapy for advanced oesophagogastric cancer. Eur J Cancer 2011; 47: 1146–1151. [DOI] [PubMed] [Google Scholar]
- 13. Lordick F, von Schilling C, Bernhard H, Hennig M, Bredenkamp R, Peschel C. Phase II trial of irinotecan plus docetaxel in cisplatin‐pretreated relapsed or refractory oesophageal cancer. Br J Cancer 2003; 89: 630–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akutsu Y, Kono T, Uesato M et al S‐1 monotherapy as second‐ or third‐line chemotherapy for unresectable and recurrent esophageal squamous cell carcinoma. Oncology 2013; 84: 305–310. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura T, Ota M, Narumiya K et al [Docetaxel plus S‐1 as a second‐line chemotherapy for metastasis or recurrence of esophageal cancer.] Gan to Kagaku Ryoho 2012; 39: 227–230. (In Japanese.) [PubMed] [Google Scholar]
- 16. Schoennemann KR, Bjerregaard JK, Hansen TP et al Biweekly cetuximab and irinotecan as second‐line therapy in patients with gastro‐esophageal cancer previously treated with platinum. Gastric Cancer 2011; 14: 219–225. [DOI] [PubMed] [Google Scholar]
- 17. Dutton SJ, Ferry DR, Blazeby JM et al Gefitinib for oesophageal cancer progressing after chemotherapy (COG): A phase 3, multicentre, double‐blind, placebo‐controlled randomised trial. Lancet Oncol 2014; 15: 894–904. [DOI] [PubMed] [Google Scholar]


