Abstract
Background: As radioresistance of non‐small cell lung cancers (NSCLC) is one of the main causes of failure in radiotherapy, we examined whether micro ribonucleic acid (miR‐451) could function as a potential radiosensitizer of NSCLC and the related mechanism.
Methods: Radioresistant NSCLC cell line A549 was transfected with pre‐miR‐451 or a scrambled control. The miR‐451 messenger RNA level, colony‐forming ability, apoptosis, and phosphatase and tensin homolog (PTEN) protein level of 549 cells were examined by real‐time polymerase chain reaction, clonogenic assay, flow cytometry analysis, and Western blot.
Results: Upregulation of miR‐451 enhanced the suppressive effects of irradiation on the colony‐forming ability of A549 cells. The apoptosis and PTEN expression of A549 cells post‐irradiation were also enhanced by upregulation of miR‐451.
Conclusions: Upregulation of miR‐451 sensitized radioresistant NSCLC A549 cells to irradiation through the enhancement of apoptosis. The activation of PTEN post‐irradiation was possibly correlated with the radiosensitization of A549 cells induced by miR‐451 overexpression.
Keywords: Apoptosis, miR‐451, non‐small cell lung cancer, PTEN, radiosensitization
Introduction
As the leading cause of cancer death and the most commonly diagnosed cancer worldwide, lung cancer can be classified into two major groups: small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC). NSCLC represents the majority of lung cancer cases and the survival rate at five years is generally less than 15%.1 Radiotherapy is one of the important approaches and plays a critical role in locally advanced NSCLC treatment.2 However, therapeutic outcomes of radiotherapy are often not fully satisfactory, and development of radioresistance is a significant problem which limits the treatment efficiency of radiotherapy for NSCLC.3 Therefore, identification of the radiosensitizer candidates of NSCLC is urgent in order to make clinical strategy developments for the treatment of NSCLC.
Micro ribonucleic acids (miRNAs or miRs) are small endogenous non‐coding single‐stranded RNA molecules of about 18–25 nucleotides. MiRs play a key regulatory role in the expression of various oncogenes and tumor suppressor genes, which makes them one of the most relevant determinants of cancer biology.4, 5 In addition, more and more studies have reported that miRs are related to the radiotherapy response of various cancers, including NSCLC.6, 7, 8, 9 Among the miRs, miR‐451 was reported to possibly function as a tumor suppressor.10, 11 Moreover, miR‐451 was found to be downregulated in NSCLC and upregulated in radiotherapy sensitive NSCLC patients, compared with radiotherapy resistant counterparts.12, 13, 14 This indicates that the radioresistance of NSCLC might be related to the downregulation of miR‐451.
Phosphatase and tensin homolog (PTEN) is a phosphatase that antagonizes the phosphatidylinositol‐4,5‐bisphosphate 3‐kinase (PI3K)/protein kinase B (AKT) signaling pathway by dephosphorylating phosphatidylinositol‐3,4,5‐triphosphate. PTEN acts as a tumor suppressor, and its activation may mediate the apoptosis of cancer cells.15, 16 During tumor development, mutations and deletions of PTEN occur that inactivate its enzymatic activity, leading to increased cell proliferation and reduced cell death. Frequent genetic inactivation of PTEN occurs in glioblastoma and endometrial and prostate cancers; reduced expression of PTEN is found in many tumor types, such as lung cancer. Because of a lack of PTEN function, the PI3K/AKT signal pathway is usually activated to enhance tumor cell survival.17 In addition, PTEN has been reported to play a critical role in radiotherapy and is related to regulation of the radiosensitivity of cancers by some miRs.18, 19
To date, it is unknown whether the upregulation of miR‐451 could sensitize NSCLC to radiotherapy and whether there is a correlation between miR‐451 and PTEN. To address these points, we used an A549 cell line to examine the radiosensitivity of NSCLC by overexpressing miR‐451 to detect the underlying mechanism.
Materials and methods
Cell culture
The NSCLC cell line A549 was cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cell lines were cultured under an atmosphere of 5% CO2, with humidity at 37°C.
Cell transfection
Pre‐miR‐451 (50 pmol) and a scrambled control (50 pmol; Ambion, Austin, TX, USA) were transfected into NSCLC A549 cells grown in six‐well dishes (plated at 2 × 105 cells/well 24 hours before transfection). Transfection was performed with Lipofectamine 2000, according to the manufacturer's instructions (Invitrogen).
Micro ribonucleic acid (miR)‐451 detection by real‐time polymerase chain reaction
After transfection at different time points as indicated, cells were harvested and total RNA was extracted using TRIZOL reagent according to the manufacturer's protocol (Invitrogen). Polymerase chain reaction (PCR)‐based detection of mir‐451 level was performed by TaqMan miRNA assays (ABI, Forest City, CA, USA). Real‐time (RT)‐PCR results, recorded as threshold cycle numbers (Ct), were normalized against an internal control (U6 RNA), and then expressed as fold changes.20, 21
Irradiation treatment
Transfected cells (2 × 105 cells per well) were irradiated at room temperature with an RCR‐120 60Co γ‐ray therapeutic machine (Toshiba, Tokyo, Japan), at a dose rate of 1.6 Gy/min. Nonirradiated controls were handled identically to irradiated cells, with the exception of radiation treatment.
Assessment of clonogenicity
After exposure to various doses of irradiation (0, 2, 4 or 6 Gy), transfected cells were plated at low density (5 × 102 cells/10 cm plate) and incubated for 14 days at 37°C in a humidified atmosphere containing 5% CO2. Colony formation and growth were visualized with Giemsa staining. The number of colonies containing at least 50 cells was determined, and surviving fractions were calculated relative to the control nonirradiated cells.
Flow cytometry analysis of apoptosis
Forty‐eight hours after exposure (or sham exposure) to 6 Gy of irradiation, transfected cells were harvested following mild trypsinization, washed in phosphate‐buffered saline, and stained with fluorescein isothiocyanate‐labeled annexin V (Roche Applied Science, Basel, Switzerland) and propidium iodide. Apoptotic cells were measured using a fluorescence‐activated cell sorter apparatus (Becton Dickinson, Bellport, NY, USA).
Western blot analysis
After 6 Gy of irradiation, protein extraction from 1 × 106 transfected cells was performed with lysis buffer (20 mM Tris‐hydrochloride pH 7.4, 150 mM sodium chloride, 1% Triton X‐100, 0.1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 2 mM sodium orthovanadate, 2 mM sodium fluoride, and Complete Protease Inhibitor Mix [Roche Applied Science, Mannheim, Germany]). Protein concentration was measured by BCA protein assay (Pierce, Thermo Scientific, Rockford, IL, USA) and samples were resolved on a 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis gel, followed by transfer to nitrocellulose membranes. Membranes were blocked in 5% non‐fat dry milk in Tris Buffer saline Tween‐20 at room temperature and probed with primary antibodies (anti‐PTEN antibody [Santa Cruz Biotechnology, Paso Robles, CA, USA] and anti‐ß‐actin antibody [Cell Signaling, Danvers, MA, USA]) overnight at 4°C and with secondary antibody for two hours at room temperature. Antibody complexes were visualized using an enhanced chemiluminescence‐Western blotting detection system (Thermo Scientific).
Statistical analysis
All experimental data were shown as the mean ± standard deviation. Differences between samples were analyzed using the Student's t‐test. Statistical significance was accepted at P < 0.05. Statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
MiR‐451 was upregulated in A549 cells by pre‐miR‐451 transfection
We performed RT‐PCR analysis of miR‐451 expression in NSCLC A549 cells transfected with pre‐miR‐451 or a scrambled control to validate transfection efficiency. As shown in Figure 1, the relative expression level of miR‐451 was significantly higher in pre‐miR‐451‐transfected A549 cells compared with those transfected with the scrambled control (P < 0.05). Even at 72 hours after transfection, there was still about 30‐fold induction. This data confirmed the overexpression of miR‐451 by transfecting pre‐miR‐451 into A549 cells.
Figure 1.
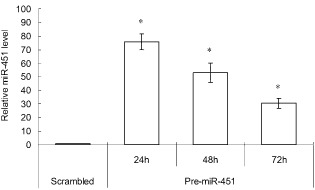
Micro ribonucleic acid (miR)‐451 was successfully upregulated in A549 cells by pre‐miR‐451 transfection. Relative expression levels of miR‐451 in A549 cells were measured at 24, 48, and 72 hours after transient transfection with pre‐miR‐451 by real‐time quantitative polymerase chain reaction. RNU6 served as an endogenous control. Each value represents the mean ± standard deviation of three independent experiments. *P < 0.05 versus cells transfected with scrambled control.
Upregulation of miR‐451 sensitized A549 cells to irradiation
To examine whether miR‐451 upregulation could sensitize radioresistant NSCLC A549 cells to irradiation, clonogenic assay was performed 14 days after pre‐miR‐451‐transfected A549 cells and scrambled control‐transfected A549 cells were irradiated at a dose of 0, 2, 4 or 6 Gy. The survival fraction in pre‐miR‐451‐transfected A549 cells was suppressed compared with the scrambled control group following irradiation, implying that upregulation of miR‐451 could enhance the suppressive effects of irradiation on the colony‐forming ability of A549 cells and sensitize radioresistant NSCLC A549 cells to irradiation (Fig 2).
Figure 2.
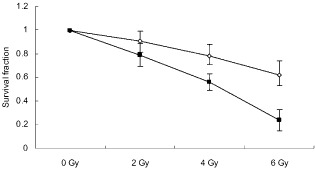
Survival fraction of A549 cells was suppressed following irradiation by micro ribonucleic acid (miR)‐451 overexpression. Clonogenicity was evaluated by Giemsa staining and presented as the percentage of colonies formed in A549 cells treated with irradiation and transfected with pre‐miR‐451 or scrambled control relative to untreated cells. Each value represents the mean ± standard deviation of three independent experiments.  , scrambled;
, scrambled;  , pre‐miR‐451.
, pre‐miR‐451.
Irradiation‐induced apoptosis of A549 cells was enhanced by upregulation of miR‐451
Cell apoptosis was analyzed with flow cytometry after A549 cells transfected with pre‐miR‐451 or a scrambled control were exposed (or sham exposed) to 6 Gy of irradiation. As shown in Figure 3, cells overexpressing miR‐451 exhibited a higher level of apoptosis compared to scrambled control cells without irradiation. Moreover, when cells were exposed to 6 Gy of irradiation, although more apoptotic cells were found both in miR‐451‐overexpressing and scrambled control cells, the amount of apoptosis induced by irradiation in miR‐451‐overexpressing cells was significantly greater than in the scrambled control cells (P < 0.05). This suggested that upregulation of miR‐451 could promote radiosensitivity of NSCLC A549 cells by enhancing cell apoptosis.
Figure 3.
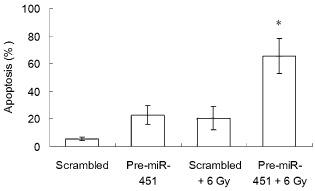
Irradiation‐induced apoptosis of A549 cells was enhanced by by micro ribonucleic acid (miR)‐451 overexpression. The apoptotic A549 cells were stained with annexin V‐ fluorescein isothiocyanate and propidium iodide and examined by flow cytometry. The percentage of apoptotic cells is presented and each value represents the mean ± standard deviation of three independent experiments. *P < 0.05 versus 6 Gy irradiation group.
Phosphatase and tensin homolog expression was promoted in miR‐451 upregulated A549 cells after irradiation
To detect the association between miR‐451 and PTEN, the protein level of PTEN in NSCLC A549 cells following overexpression of miR‐451 was detected by Western blot analysis. Without irradiation, PTEN protein was expressed at low levels in NSCLC A549 cells. After treatment with either pre‐miR‐451‐transfection or 6 Gy irradiation, the PTEN protein level in the A549 cells slightly increased. Moreover, the combination of miR‐451 overexpression and irradiation notably promoted PTEN expression compared to the corresponding control group (Fig 4). This result indicated that miR‐451 overexpression‐induced apoptosis enhancement after irradiation might be associated with the upregulation of the PTEN protein level in A549 cells.
Figure 4.
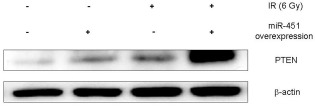
Phosphatase and tensin homolog (PTEN) expression was promoted in micro ribonucleic acid (miR)‐451‐overexpressed A549 cells after irradiation (IR). The PTEN protein level in A549 cells with (or without) miR‐451 overexpression after exposure (or sham exposure) to irradiation was measured by Western blot. Results shown are representative of three independent experiments.
Discussion
Conventionally, it is believed that radioresistance is a major obstacle in locally advanced NSCLC treatment. To overcome this problem, radiosensitizers have been pursued for a long time. In recent years, miRs have been regarded as potential candidates to regulate the radiosensitivity of NSCLC. Some were reported to induce radiosensitivity,14, 22, 23 while others were reported to confer radioresistance.6, 9, 24 To date, there is little information about the role of miR‐451 in NSCLC response to radiotherapy.
Because clonogenic assay is frequently used in cancer research laboratories to determine the effectiveness of irradiation on the survival and proliferation of tumor cells, changes in the colony‐forming ability of radioresistant NSCLC A549 cells caused by miR‐451 overexpression after irradiation were examined in this study.25 As expected, by increasing the inhibition of the survival fraction of A549 cells caused by various doses of irradiation, we found that upregulation of miR‐451 sensitized A549 cells to irradiation. Previously, Bandres et al. found that overexpression of miR‐451 in gastric and colorectal cancer cells reduced cell proliferation and increased sensitivity to radiotherapy.26 However, almost no information is available regarding the effects of miR‐451 overexpression on the radiosensitivity of NSCLC. Wang et al. compared the expression profile of miRNA in radiotherapy sensitive and resistant groups of NSCLC patients and noted that the miR‐451 expression level in the radiotherapy sensitive NSCLC group was much higher than in the radiotherapy resistant group. However, they did not further explore the function of miR‐451 in radiotherapy for NSCLC.14 Our data showed that upregulation of miR‐451 could possibly enhance the sensitivity of NSCLC to radiotherapy and miR‐451 might be considered a potential radiosensitizer of NSCLC.
Because cell apoptosis is one of the most critical reasons underlying tumor growth inhibition caused by irradiation, we examined whether miR‐451 overexpression could increase the apoptosis of NSCLC A549 cells induced by irradiation, which might account for the higher sensitivity to irradiation of A549 cells resulting from miR‐451 overexpression. It has been reported that overexpression of miR‐451 could induce NSCLC cell apoptosis.27, 28 Accordingly, our results were consistent with previous studies. Moreover, we further demonstrated that upregulation of miR‐451 enhanced irradiation‐induced apoptosis of A549 cells, suggesting that increased radiosensitivity of NSCLC A549 cells by miR‐451 overexpression might be attributed, at least in part, to the enhancement of cell apoptosis. This similar function of miR‐451 was also found by Zhang et al. in nasopharyngeal carcinoma cells by showing that the high levels of miR‐451 expression enhanced radiosensitivity by increasing apoptosis.29
Phosphatase and tensin homolog is one of the most important proteins associated with the regulation of cancer cell apoptosis, and the activation of PTEN plays a crucial role in the mediation of cell apoptosis induced by irradiation. Zhuang et al. reported that the irradiation‐induced apoptosis of lung cancer cells was increased by the upregulation of PTEN.30 In addition, Qu et al. noted that knocking down miR‐205 in nasopharyngeal carcinoma cells compromises the inhibition of PTEN and increases cell apoptosis post‐irradiation.31 It is unclear whether upregulation of miR‐451 could affect PTEN expression; therefore our study detected PTEN protein levels in NSCLC A549 cells. Our results showed that PTEN expression after irradiation was remarkably increased in miR‐451 overexpressed A549 cells, indicating that there might be an association between upregulation of PTEN protein level and apoptosis enhancement post‐irradiation, induced by miR‐451 overexpression in A549 cells.
Conclusion
In conclusion, the present study demonstrated that miR‐451 was a regulator of NCSLC radiosensitivity. Upregulation of miR‐451 sensitized radioresistant NSCLC A549 cells to irradiation through the enhancement of apoptosis. The activation of PTEN post‐irradiation might be correlated with the radiosensitization of A549 cells induced by miR‐451 overexpression. Our results will assist in overcoming NSCLC radioresistance and developing new therapeutic strategies for NSCLC. Further studies on the targets of miR‐451 in radiosensitization and the mechanism underlying PTEN activation after irradiation, accompanied by the upregulation of miR‐451 in NSCLC, are still needed.
Disclosure
No authors report any conflict of interest.
References
- 1. Jemal A, Siegel R, Ward E et al Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. [DOI] [PubMed] [Google Scholar]
- 2. Laine AM, Westover KD, Choy H. Radiation therapy as a backbone of treatment of locally advanced non‐small cell lung cancer. Semin Oncol 2014; 41: 57–68. [DOI] [PubMed] [Google Scholar]
- 3. Kang J, Kim E, Kim W et al Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial‐mesenchymal transition (EMT) by miR‐34a‐mediated suppression of Notch‐1 expression in non‐small cell lung cancer cell lines. J Biol Chem 2013; 288: 27343–27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 2010; 79: 351–379. [DOI] [PubMed] [Google Scholar]
- 5. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6: 857–866. [DOI] [PubMed] [Google Scholar]
- 6. Grosso S, Doyen J, Parks SK et al MiR‐210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis 2013; 4: e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quintavalle C, Mangani D, Roscigno G et al MiR‐221/222 target the DNA methyltransferase MGMT in glioma cells. PLoS ONE 2013; 8(9): e74466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gasparini P, Lovat F, Fassan M et al Protective role of miR‐155 in breast cancer through RAD51 targeting impairs homologous recombination after irradiation. Proc Natl Acad Sci U S A 2014; 111: 4536–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA‐21 (miR‐21) expression promotes growth, metastasis, and chemo‐ or radioresistance in non‐small cell lung cancer cells by targeting PTEN. Mol Cell Biochem 2013; 372: 35–45. [DOI] [PubMed] [Google Scholar]
- 10. Gits CM, van Kuijk PF, Jonkers MB et al MicroRNA expression profiles distinguish liposarcoma subtypes and implicate miR‐145 and miR‐451 as tumor suppressors. Int J Cancer 2013; 135: 348–361. [DOI] [PubMed] [Google Scholar]
- 11. Zhu H, Wu H, Liu X et al Role of MicroRNA miR‐27a and miR‐451 in the regulation of MDR1/P‐glycoprotein expression in human cancer cells. Biochem Pharmacol 2008; 76: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Markou A, Sourvinou I, Vorkas PA, Yousef GM, Lianidou E. Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer 2013; 81: 388–396. [DOI] [PubMed] [Google Scholar]
- 13. Gao W, Shen H, Liu L, Xu J, Xu J, Shu Y. MiR‐21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol 2011; 137: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang XC, Du LQ, Tian LL et al Expression and function of miRNA in postoperative radiotherapy sensitive and resistant patients of non‐small cell lung cancer. Lung Cancer 2011; 72: 92–99. [DOI] [PubMed] [Google Scholar]
- 15. Steck PA, Pershouse MA, Jasser SA et al Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362. [DOI] [PubMed] [Google Scholar]
- 16. Blanco‐Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis 2007; 28: 1379–1386. [DOI] [PubMed] [Google Scholar]
- 17. Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN: Mutation, marker and potential target for therapeutic intervention. Expert Opin Ther Targets 2004; 8: 537–550. [DOI] [PubMed] [Google Scholar]
- 18. Chun‐Zhi Z, Lei H, An‐Ling Z et al MicroRNA‐221 and microRNA‐222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer 2010; 10: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang P, Rao EY, Meng N, Zhao Y, Wang JJ. MicroRNA‐17‐92 significantly enhances radioresistance in human mantle cell lymphoma cells. Radiat Oncol 2010; 5: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dmitriev P, Barat A, Polesskaya A et al Simultaneous miRNA and mRNA transcriptome profiling of human myoblasts reveals a novel set of myogenic differentiation‐associated miRNAs and their target genes. BMC Genomics 2013; 14: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen C, Ridzon DA, Broomer AJ et al Real‐time quantification of microRNAs by stem‐loop RT‐PCR. Nucleic Acids Res 2005; 33: e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balca‐Silva J, Sousa Neves S, Goncalves AC et al Effect of miR‐34b overexpression on the radiosensitivity of non‐small cell lung cancer cell lines. Anticancer Res 2012; 32: 1603–1609. [PubMed] [Google Scholar]
- 23. Chen S, Wang H, Ng WL, Curran WJ, Wang Y. Radiosensitizing effects of ectopic miR‐101 on non‐small‐cell lung cancer cells depend on the endogenous miR‐101 level. Int J Radiat Oncol Biol Phys 2011; 81: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 24. Salim H, Akbar NS, Zong D et al miRNA‐214 modulates radiotherapy response of non‐small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence. Br J Cancer 2012; 107: 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffman RM. In vitro sensitivity assays in cancer: A review, analysis, and prognosis. J Clin Lab Anal 1991; 5: 133–143. [DOI] [PubMed] [Google Scholar]
- 26. Bandres E, Bitarte N, Arias F et al microRNA‐451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res 2009; 15: 2281–2290. [DOI] [PubMed] [Google Scholar]
- 27. Wang R, Wang ZX, Yang JS, Pan X, De W, Chen LB. MicroRNA‐451 functions as a tumor suppressor in human non‐small cell lung cancer by targeting ras‐related protein 14 (RAB14). Oncogene 2011; 30: 2644–2658. [DOI] [PubMed] [Google Scholar]
- 28. Bian HB, Pan X, Yang JS, Wang ZX, De W. Upregulation of microRNA‐451 increases cisplatin sensitivity of non‐small cell lung cancer cell line (A549). J Exp Clin Cancer Res 2011; 30: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang T, Sun Q, Liu T et al MiR‐451 increases radiosensitivity of nasopharyngeal carcinoma cells by targeting ras‐related protein 14 (RAB14). Tumour Biol 2014; 35: 12593–12599. [DOI] [PubMed] [Google Scholar]
- 30. Zhuang HQ, Wang J, Yuan ZY, Zhao LJ, Wang P, Wang CL. The drug‐resistance to gefitinib in PTEN low expression cancer cells is reversed by irradiation in vitro. J Exp Clin Cancer Res 2009; 28: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qu C, Liang Z, Huang J et al MiR‐205 determines the radioresistance of human nasopharyngeal carcinoma by directly targeting PTEN. Cell Cycle 2012; 11: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]


