Abstract
This study aimed to assess the efficacy and safety of a combination of paclitaxel and cisplatin/carboplatin for the treatment of advanced thymic carcinoma. Thirty‐seven patients (23 men and 14 women, median age 47 years, performance status score ≤2) with pathologically or cytologically diagnosed advanced thymic carcinoma were recruited. Patients received 175 mg/m2 paclitaxel on day 1 and 75 mg/m2 cisplatin or 300 mg/m2 carboplatin on day 2 of a 21 day cycle for at least two cycles to evaluate efficacy and adverse events. No complete response (CR) was observed; 11 patients had a partial response (PR), 16 patients had no change (NC), and 10 had progressive disease, resulting in an overall response rate of 29.7%, a stable rate of 43.2%, and a disease control rate (CR + PR + NC) of 72.9%. Grade I/II and III/IV neutropenia were observed in 21 (56.7%) and 13 (35.1%) patients, respectively. Four (10.8%) patients developed grade I/II thrombocytopenia. Grade I/II and III/IV nausea and vomiting were observed in 19 (51.2%) and five (13.5%) patients, respectively. Grade I/II liver dysfunction was observed in seven (18.9%) patients. Two patients with grade III liver dysfunction recovered after hepatoprotective treatment. The combination of paclitaxel and platinum was effective and well tolerated in patients with advanced thymic carcinoma.
Keywords: Carboplatin, cisplatin, paclitaxel, thymic carcinoma
Introduction
A thymic epithelial tumor is a rare type of thymus gland cancer, but is the most common anterior mediastinal tumor, constituting 20% of mediastinal tumors. Thymic epithelial tumors can be divided into thymoma and thymic carcinoma, according to pathological characteristics. Although both thymoma and thymic carcinomas originate from the epithelial cells of the thymus, the latter is characterized with a more aggressive biological behaviour, a higher metastasis rate, and a poor response to chemotherapy. Surgery is the main approach for thymoma, and the only option for radical treatment. Palliative chemotherapy is the main option for unresectable patients or patients who experience recurrence after radical resection.
A thymic carcinoma is a rare neoplasm originating from thymus epithelial cells and arising in the anterior mediastinum. Thymic carcinomas account for about 2.7% of mediastinal neoplasms and about 17.4% of thymus tumors. They exhibit malignant biological behavior, which is distinct from thymomas, and a five‐year survival rate of 20–30%.1 Because of the rarity of cases, optimal management of thymic carcinoma has not been defined. Herein, we present our review of a doublet regimen of paclitaxel combined with platinum in the treatment of 37 patients with pathologically or cytologically diagnosed advanced thymic carcinoma.
Patient and treatment characteristics
Study population and data collection
A prospective study was designed in which 37 patients with pathologically or cytologically documented stage IV thymic carcinoma were included. All of the patients underwent paclitaxel and platinum chemotherapy at the Cancer Institute and Hospital, Chinese Academy of Medical Sciences. After excluding patients who were deemed immeasurable as defined by Response Evaluation Criteria in Solid Tumors (RECIST) and those lost to follow‐up, 37 patients were included in the study. Demographics, clinicopathologic tumor characteristics, medical and surgical treatment, chemotherapy regimens, and courses were recorded.
Administration method
From day 1, paclitaxel was administered tri‐weekly at a dose of 175 mg/m2. From day 2, cisplatin (75 mg/m2) or carboplatin (300 mg/m2) was administered tri‐weekly. Tumor response was assessed every two cycles, with a final response assessment in the fourth week after the final cycle of chemotherapy. Patients were followed up every three months.
Curative effect and adverse reaction
Response to chemotherapy on cross‐sectional imaging was assessed by RECIST criteria version 1.1. Toxicity was evaluated according to National Cancer Institute Common Toxicity Criteria version 4.0. Long‐term outcomes were reviewed.
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 (SPSS, Chicago, IL, USA). A P value of less than 0.05 indicated statistical significance. Categorical variables were summarized with percentages and continuous variables with medians. Progression‐free survival (PFS) was calculated as the period from the date of initiation of paclitaxel and platinum chemotherapy to the first observation of disease progression. Overall survival (OS) was calculated as the period from the date of initiation of the paclitaxel and platinum regimen until death from any cause or until the date of last follow‐up. Both the time to progression and OS were estimated using the Kaplan–Meier method.
Results
Efficacy
Table 1 shows the clinicopathological characteristics of the 37 patients included in the study. Of the 37 treated individuals, 11 patients (29.73%) had a partial response (PR), 16 (35%) had stable disease (SD), and 10 (27.03%) had progressive disease. An overall response rate (ORR) of 29.7% and a disease control rate (DCR) of 72.9% ([11 + 16]/37) were achieved.
Table 1.
Patient characteristics (n = 37)
| Characteristic | No. of patients | % |
|---|---|---|
| Gender | ||
| Male | 23 | 62.2 |
| Female | 14 | 37.8 |
| Age (years) | ||
| Median (range) | 47 (26–75) | |
| ECOG performance status score | ||
| Median (range) | 1 (0–1) | |
| Metastatic site(s) | ||
| Single | 11 | 29.7 |
| Multiple | 26 | 70.3 |
| Prior treatment | ||
| Surgery | 21 | 56.7 |
| Radiotherapy | 10 | 27.0 |
ECOG, Eastern Cooperative Oncology Group.
Survival
The median PFS was six months (Fig 1), and the median OS was 43 months (Fig 2).
Figure 1.
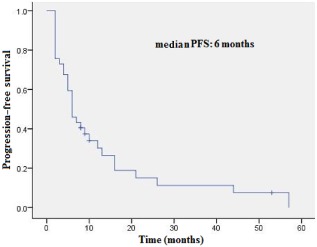
Progression‐free survival.
Figure 2.
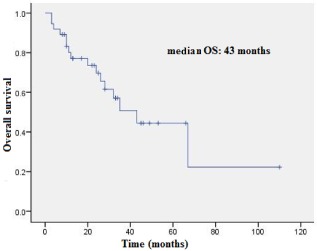
Overall survival.
Adverse events
The most common adverse events were hematologic toxicity and gastrointestinal disturbances. Grade I/II and III/IV neutropenia occurred in 56.7% and 35.1% of patients, respectively. Grade I/II thrombocytopenia occurred in four patients (10.8%). Grade I/II and III/IV grade nausea/emesis occurred in 51.2% and 13.5% of patients, respectively. Grade I/II grade liver function impairment was observed in seven patients (18.9%). Two patients had grade III hepatic function impairment and recovered after treatment. Overall, the combination of paclitaxel and platinum was well tolerated by the patients.
Discussion
Surgical resection is the main approach for the treatment of thymic epithelial neoplasms, although whether these diseases are chemosensitive remains unclear.2 Thymic carcinoma is a rare type of cancer, and its nonspecific manifestations pose a great challenge to early diagnosis. At the time of diagnosis, invasion of the mediastinum, pericardium, and pleura are often present. Thymic carcinoma was categorized as a type C thymoma by the World Health Organization (WHO) in 1999.3 Microscopically, thymic carcinoma is composed of highly atypical cells with cytoarchitectural features of carcinoma similar to those seen in other organs, and mature T/B cell type lymphocytes and plasma cells can be seen in its stroma. The five‐year survival rate is only 15–20% in patients with aggressive types of thymic carcinoma (e.g. multi‐lobed, poorly differentiated, extensive necrosis, and highly mitotic). Surgery and radiotherapy are the main treatment options, and the role of chemotherapy has not been extensively investigated.4 Thymic carcinoma was considered to be a malignancy insensitive to chemotherapy; however, with the development of novel cytotoxic agents, several regimens had been explored in clinical trials and have shown encouraging results. Cisplatin and/or adriamycin regimens have been recommended by some published studies. Response rates of 32–75% have been achieved with various chemotherapy regimens, including cisplatin, vincristine, adriamycin, and etoposide (CORE: DD, VCR, ADM, and VP16), cisplatin, doxorubin, vincristine, and cyclophosphamide (ADOC: AMD, DDP, VCR, and CTX), and etoposide, ifosfamide, and cisplatin (VIP: VP16, IFO, and DDP).5, 6, 7
Most published studies have reported evidence of the first‐line use of cisplatin‐based regimens in patients with metastatic thymic carcinomas. A multi‐center, randomized clinical trial initiated by the Eastern Cooperative Oncology Group using single cisplatin produced poor results, with an ORR of 10% and a median OS rate of 76 weeks; however, cisplatin‐based regimens, as demonstrated in this study, usually confer a satisfying outcome.8 The addition of cisplatin increases the ORR from 58–84%, in which the dose of cisplatin is proportional to the efficacy; however, a dose of cisplatin lower than 50 mg/m2 fails to exhibit additional benefits.
Although ORRs ranging from 55–90% were observed in patients who received cisplatin‐based chemotherapy, a standard regimen for patients who experience disease progression has not been established.9 In addition, targeted agents also fail to improve a patient's outcome. The poor prognosis of thymic carcinoma emphasizes the dilemma of the lack of effective systemic treatment options.10, 11
Paclitaxel is a cytoskeletal drug that targets tubulin. Its mechanism of action involves interference with the normal breakdown of microtubules during cell division. Paclitaxel shows anti‐tumor activity in a series of preclinical studies and is used to treat various types of cancer, including ovarian, breast, lung, and pancreatic cancers. Published clinical trials have shown that paclitaxel is effective and well tolerated.
In the present study, PR was observed in 11 patients and SD in 16 patients, while no complete response was observed. The ORR was 29.7%, and the DCR 43.2%. Grade I/II and III/IV grade neutropenia occurred in 56.7% and 35.1% of patients, respectively. Grade I/II thrombocytopenia occurred in four patients (10.8%). Grade I/II and III/IV grade nausea/emesis occurred in 51.2% and 13.5% of patients, respectively. Grade I/II grade liver function impairment was observed in seven patients (18.9%). No therapy was discontinued as a result of severe adverse events.
Published studies have shown that regimens such as cytoxan, adriamycin and platinum (CAP), platinum and etoposide (PE), ADOC, and VIP are effective, and cisplatin‐based chemotherapy is recommended for patients with metastatic or advanced thymic carcinoma. However, because of the rarity of cases, the role of chemotherapy in the management of thymic carcinoma is unclear. Paclitaxel is an effective and well‐tolerated cytotoxic agent, and has been used to treat a variety of cancers, such as non‐small lung cancer, small cell lung cancer, and breast and ovarian cancers. A study that included 23 patients with thymic carcinoma and 21 patients with unresectable thymoma (among which only 13 were grade IV or greater) showed that carboplatin plus paclitaxel had moderate clinical activity in patients with thymic malignancies; in addition, PFS and OS rates were poorer in thymic carcinoma than in thymoma patients.12
With the development of both cytotoxic and targeted agents, treatment for thymic carcinoma will be individualized and more effective; thus, beneficial to patients' quality of life and survival. However, the role of paclitaxel in the treatment of thymic carcinoma needs further investigation.
References
- 1. Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: Tumors of the anterior mediastinum. Chest 1997; 112: 511–522. [DOI] [PubMed] [Google Scholar]
- 2. Wheatley‐Price P, Jonker H, Jonker D, Shamji F, Gomes MM. Thymic epithelial neoplasms: A 12‐year Canadian regional cancer program experience. Clin Lung Cancer 2014; 15: 231–236. [DOI] [PubMed] [Google Scholar]
- 3. Valente M, Schinzari G, Ricciotti A, Barone C. Role of chemotherapy in malignant thymoma. Ann Ital Chir 2007; 78: 377–380. [PubMed] [Google Scholar]
- 4. Rzyman W, Skokowski J, Marjański T, Walczak A, Szymanowska A, Bilinska M. [Thymectomy in myasthenia gravis.] Pol Merkur Lekarski 2005; 18: 41–44. (In Polish.) [PubMed] [Google Scholar]
- 5. Loehrer PJ Sr, Jiroutek M, Aisner S et al Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: An intergroup trial. Cancer 2001; 91: 2010–2015. [PubMed] [Google Scholar]
- 6. Yoh K, Goto K, Ishii G et al Weekly chemotherapy with cisplatin, vincristine, doxorubicin, and etoposide is an effective treatment for advanced thymic carcinoma. Cancer 2003; 98: 926–931. [DOI] [PubMed] [Google Scholar]
- 7. Yokoi K, Matsuguma H, Nakahara R et al Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. J Thorac Oncol 2007; 2: 73–78. [DOI] [PubMed] [Google Scholar]
- 8. Okuma Y, Saito M, Hosomi Y, Sakuyama T, Okamura T. Key components of chemotherapy for thymic malignancies: A systematic review and pooled analysis for anthracycline‐, carboplatin‐ or cisplatin‐based chemotherapy. J Cancer Res Clin Oncol 2014; 141: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okuma Y, Hosomi Y, Takagi Y et al Clinical outcomes with chemotherapy for advanced thymic carcinoma. Lung Cancer 2013; 80: 75–80. [DOI] [PubMed] [Google Scholar]
- 10. Litvak AM, Woo K, Hayes S et al Clinical characteristics and outcomes for patients with thymic carcinoma: Evaluation of Masaoka staging. J Thorac Oncol 2014; 9: 1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajan A, Carter CA, Berman A et al Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: A multicentre, open‐label, phase 2 trial. Lancet Oncol 2014; 15: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lemma GL, Lee JW, Aisner SC et al Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011; 29: 2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]


