Abstract
Conventional transbronchial needle aspiration (TBNA) using 19‐gauge needles can obtain larger histological specimens for hilar‐mediastinal diagnosis. A new 19‐gauge eXcelon needle was introduced in Taiwan in July 2012. We prospectively enrolled patients with hilar‐mediastinal lesions and pathology results of suspected benign origin or lymphoproliferative processes, to perform TBNA using a 19‐gauge eXcelon needle, between July 2012 and December 2012. The results were compared with historical control of TBNA using a WANG MW‐319 needle between January 2011 and June 2012. The procedure was performed by the same pulmonologist, and rapid on‐site cytologic evaluation was used. The 19‐gauge eXcelon needle was used in nine patients with 15 lymph nodes aspirated, with a mean diameter of 23.3 ± 10.7 mm. The mean number of needle passes was 2.7 ± 1.4, with a diagnostic accuracy of 77.8%. The MW‐319 needle was used in 12 patients with 18 lymph nodes aspirated, with a mean diameter of 21.3 ± 5.7 mm. The mean number of needle passes was 2.2 ± 0.4, with a diagnostic accuracy of 75.0%. Neither technical nor major clinical complications were noted in either group. We concluded that the 19‐gauge eXcelon needle was as safe and effective as the MW‐319 needle. A more adequate specimen could be obtained and fewer needle passes were required with the MW‐319 needle, although the difference did not reach significance.
Keywords: Bronchoscopy, diagnosis, histology, mediastinum, transbronchial needle aspiration
Introduction
Conventional transbronchial needle aspiration (TBNA) is a useful procedure for hilar‐mediastinal diagnosis and staging. Needle size varies from 19–22 gauge. The size of a needle can influence the result and larger gauge needles can obtain satisfactory sample volumes with higher diagnostic yield.1, 2 To obtain cytology specimens, 20–22 gauge needles are usually used, whereas 19‐gauge needles are needed to obtain a core of tissue for histologic diagnosis.2, 3, 4, 5, 6 They can also provide sufficient specimens for molecular testing, which is essential in the era of personalized therapy.7
The WANG MW‐319 TBNA needle (ConMed, Utica, NY, USA) is widely used and is the most commonly reported type of 19‐gauge TBNA needle (Fig 1a). We adopted the WANG MW‐319 TBNA needle for histologic diagnosis in June 2000. A new 19‐gauge eXcelon needle (Boston Scientific, Boston, MA, USA) was introduced in Taiwan in July 2012 (Fig 1b). The aim of this prospective study was to evaluate the performance of the 19‐gauge eXcelon needle and compare it with the MW‐319 needle.
Figure 1.

(a) The MW‐319 needle. (b) The 19‐gauge eXcelon needle. (c) Tips of the 21‐gauge NA‐2C‐1 needle, 19‐gauge eXcelon needle, and MW‐319 needle. (d) Longer needle bevel of the 19‐gauge eXcelon needle.
Materials and methods
Patient enrollment criteria for TBNA at the Sun Yat‐Sen Cancer Center, a 200 bed hospital, included the evaluation of unknown hilar and/or mediastinal lesions, the evaluation of lymph nodes (LNs) with a short axis diameter larger than 1 cm, or smaller positive LNs, on postitron emission tomography‐computed tomography (PET/CT), which have a clinically relevant impact on cancer management.8 The indications were evaluated by a multidisciplinary group including radiologists, nuclear medicine physicians, thoracic surgeons, medical oncologists, and pulmonologists, after review of the images. Nineteen gauge needles were used when the hilar and/or mediastinal lesions were the solitary intrathoracic abnormality and the pathology was suspected to be of benign origin, for example, sarcoidosis, tuberculosis, or lymphoproliferative processes (e.g. lymphoma). All examinations were performed under conscious sedation and topical anesthesia by the same pulmonologist. The needle was advanced and inspected before the procedure. The specimens in the TBNA needles were flushed by air, and rapid on‐site cytological evaluation (ROSE) was performed. For quality assessment of a pathological sample, the presence of numerous lymphocytes or a definite pathologic diagnosis was necessary for a specimen to be considered adequate. Blood contamination was defined as blood occupying more than half of the low‐power fields (40×) examined on the most bloody slide. If a histologic specimen could not be obtained using the 19‐gauge needle on the first aspiration, a second TBNA was attempted. The number of needle passes was limited to four adequate aspirations by ROSE if a histologic specimen could still not be obtained.9 The histologic specimens were fixed in formalin, and cytologic specimens were smeared on glass slides and fixed in alcohol before being sent to the Department of Pathology. Both International Association for the Study of Lung Cancer (IASLC) nodal station and Wang TBNA staging systems were used to denote LN aspiration.5, 10, 11 The 19‐gauge eXcelon transbronchial needle was used between July 2012 and December 2012, and the results were compared with the historical control of TBNA using the MW‐319 needle in the antecedent 18 months from January 2011 to June 2012.
The eXcelon transbronchial needle is available in 19, 20, and 21 gauges. All needles have a clear Teflon catheter with a length of 130 cm and an outer diameter of 1.8 mm, with a needle length of 15 mm. The catheter is designed to be kink resistant and the needle is designed with an increased volume for specimen collection. It has a button lock system to minimize the risk of unintentional needle deployment or retraction. There is a fused hub and needle configuration to help prevent needle detachment, a vacuum lock syringe to maintain the vacuum during puncture, and an ergonomic handle, designed for single‐hand needle manipulation. In Taiwan, the price of a 19‐gauge eXcelon needle is the same as a MW‐319 needle ($153 USD).
The institutional review board and the ethics committee of the Sun Yat‐Sen Cancer Center approved this study (No.20120522A). The study was conducted in accordance with the ethical principles stated in the 2000 Declaration of Helsinki. Written informed consent was obtained from all patients.
Statistical analysis
Data were expressed as numbers and percentages of cases or mean ± standard deviation, as appropriate. Diagnostic accuracy was calculated by the patient. Continuous variables were analyzed using the Student's t‐test, and categorical data were compared by Pearson χ2 test. All analyses were conducted with SAS version 8.02 (SAS Institute Inc., Cary, NC, USA), and a P value of less than 0.05 was considered to be statistically significant.
Results
The 19‐gauge eXcelon needle group included nine patients (4 women, 5 men), with a mean age of 48.3 years, and 15 LNs were aspirated. Sarcoidosis was diagnosed in four patients ( Fig 2a,b), lymphoma in one (Fig 2c,d), adenocarcinoma in one (Fig 3a,b), and Castleman's disease in one. A diagnosis could not be determined in two patients because of the lack of an adequate sample; one was diagnosed with spindle cell neoplasm by video‐assisted thoracic surgery (Fig 3c,d), and the other was diagnosed with benign lymphadenopathy which had been stable during a follow‐up of more than six months. The mean LN diameter was 23.3 ± 10.7 mm, and the mean number of needle passes was 2.7 ± 1.4. The diagnostic sensitivity and accuracy were 77.8% and 77.8%, respectively.
Figure 2.

(a) Enlarged sub‐subcarina lymph node from a 57‐year‐old woman. Transbronchial needle aspiration (TBNA) using the eXcelon needle was performed (inset). (b) Granulomatous inflammation was noted (hematoxylin and eosin stain; original ×100). (c) Enlarged posterior carina lymph node from a 74‐year‐old man. TBNA using the eXcelon needle was performed (inset). (d) Follicular lymphoma grade 2 was noted (hematoxylin and eosin stain; original ×100).
Figure 3.
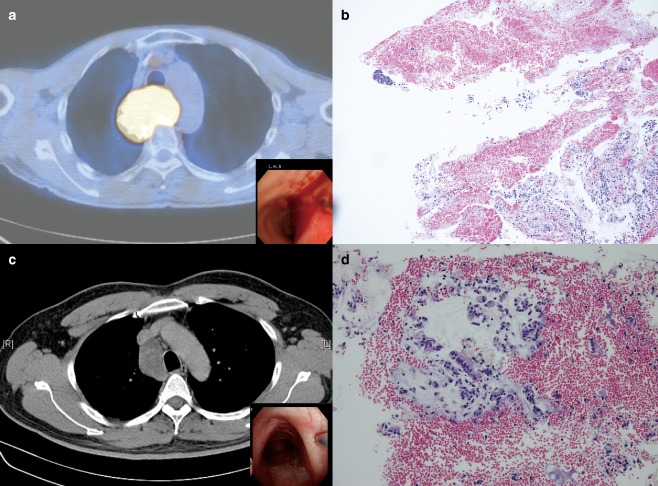
(a) Enlarged posterior carina lymph node from a 63‐year‐old man. Transbronchial needle aspiration (TBNA) using the eXcelon needle was performed (inset). (b) Carcinoma with significant blood and benign respiratory epithelia in the background were noted (hematoxylin and eosin stain; original ×100). (c) Enlarged right paratracheal lymph node from a 63‐year‐old man. TBNA using the eXcelon needle was performed (inset). (d) Significant blood contamination and ciliated epithelia were noted (hematoxylin and eosin stain; original ×400).
The MW‐319 needle was used in 12 patients, (4 women, 8 men) with a mean age of 53.1 years, and 18 LNs were aspirated. Sarcoidosis was diagnosed in three patients, non‐small cell lung cancer in three, colorectal cancer metastasis in one, and mediastinal tuberculosis in one. One patient was regarded as having a benign lymphadenopathy, with the LN remaining stable for more than six months. The specimens were inadequate for diagnosis in three patients with sarcoidosis, thymic carcinoma, and lung adenocarcinoma metastasis. The mean LN diameter was 21.3 ± 5.7 mm, and the mean number of needle passes was 2.2 ± 0.4. The diagnostic sensitivity and accuracy were 72.7% and 75.0%, respectively.
The characteristics of the patients and lesions were similar between the two groups (Table 1). There was no significant difference in the diagnostic yield; however, the MW‐319 needle group had fewer needle passes for smaller LNs, although the difference did not reach statistical significance. No technical complication occurred. There were neither major clinical complications leading to premature termination of the procedure nor post‐procedural pneumothorax or infection in either group. Although blood loss was less than 50 mL in both groups, there was brisk bleeding on withdrawal of the needle, more blood mixed with secretion suctioned during TBNA (mean, 10.8 vs. 7.2 mL; P value < 0.001) according to the nursing procedural records, and more frequent blood contamination (4/9, 44.4% vs. 2/12, 16.7%; P value = 0.331) in the eXcelon needle group (Fig 3b,d).
Table 1.
Comparison between TBNA using 19‐gauge eXcelon and MW‐319 needles
| Variable | eXcelon (n = 9) | MW‐319 (n = 12) | P value |
|---|---|---|---|
| Age, years | 48.3 ± 18.7 | 53.1 ± 20.1 | 0.806 |
| Gender F/M | 4/5 | 4/8 | 0.690 |
| Final diagnosis | 0.714 | ||
| Malignant | 4 | 5 | |
| Benign disease | 5 | 7 | |
| Lymph node location | 15 | 18 | 0.431 |
| 4R | |||
| anterior carina | 2 | 5 | |
| right paratracheal | 4 | 6 | |
| right main bronchus | 0 | 2 | |
| 4L | |||
| left paratracheal | 0 | 1 | |
| 7 | |||
| posterior carina | 2 | 2 | |
| subcarina | 1 | 1 | |
| sub‐subcarina | 1 | 0 | |
| 11R | |||
| right upper hilar | 3 | 1 | |
| 11L | |||
| left hilar | 2 | 0 | |
| Lymph node size, mm | 23.3 ± 10.7 | 21.3 ± 5.7 | 0.458 |
| Needle passes | 2.7 ± 1.4 | 2.2 ± 0.4 | 0.254 |
| TBNA results | 0.700 | ||
| True positive | 7 | 8 | |
| True negative | 0 | 1 | |
| False negative* | 2 | 3 | |
| Sensitivity | 7/9 (77.8%) | 8/11 (72.7%) | |
| Accuracy | 7/9 (77.8%) | 9/12 (75.0%) |
*Including inadequate for diagnosis. TBNA, transbronchial needle aspiration.
Discussion
Using a 19‐gauge TBNA needle to obtain larger specimens is essential in certain circumstances requiring histologic diagnosis or molecular testing. Both 19‐gauge and 21‐gauge eXcelon needles have been evaluated in a few studies.12, 13, 14, 15 However, to the best of our knowledge, this is the first study to make a direct comparison between the 19‐gauge eXcelon and MW‐319 needles.
Between September 1999 and March 2013, TBNA using a 21‐gauge NA‐2C‐1 (Olympus, Tokyo, Japan) (Fig 1c) or 19‐gauge needle was performed in 275 patients with 440 LNs aspirated at our hospital.16 The mean short‐axis diameter of the LNs aspirated was 14.4 ± 3.0 mm, and the mean number of needle passes to successfully aspirate the LNs or to make a diagnosis of cancer was 2.4 ± 0.6. The diagnostic sensitivity and accuracy were 73.2% and 76.7%, respectively. The plateau of TBNA diagnostic accuracy was reached after the first two years of a learning curve.17 Based on the same proficiency level, we made a head‐to‐head comparison between the 19‐gauge eXcelon and MW‐319 needle groups. The diagnostic sensitivity or accuracy of 19‐gauge eXcelon was comparable to the MW‐319 needle, despite the fact that the LN stations targeted were different, with more 4R and 7 stations in the MW‐319 needle group (88.9% vs. 66.7%), which assumed higher yields as more prevalent and easily accessible from prior experience.11, 16 The cost of the 19‐gauge eXcelon needle was also comparable to MW‐319 needle. The MW‐319 needle has an inner 21‐gauge needle (Fig 1c) to prevent plugging by a piece of normal bronchial mucosa, which possibly contributed to a more adequate specimen and required fewer needle passes, albeit non‐significantly. The 19‐gauge eXcelon needle had the advantage of easy penetration; however, brisk bleeding on withdrawal of the needle and more blood contamination on the slides were also noted, which could be attributed to the longer needle bevel of the 19‐gauge eXcelon needle (Fig 1d). A pathologist's opinion should be sought if contamination by blood leads to an inability to interpret slides.
On the other hand, the MW‐319 needle requires continuous application of negative suction by an assistant, whereas the eXcelon needle has a vacuum syringe attached. Doctors and assistants views upon the ease of application should be sought. Compared with the simplicity of the eXcelon needle with the safety lock, core expulsion of the MW‐319 needle could occur if the assistant is not familiar with the process.
Through education and experience, conventional TBNA has become a safe and accurate procedure for hilar‐mediastinal diagnosis and staging at our institute.8, 16, 17 Endobronchial ultrasonography‐guided (EBUS)‐TBNA using real‐time imaging is helpful to confirm that the needle is within the lesion, and it has been reported to result in a higher diagnostic yield.18, 19, 20 At present, the maximum needle size for EBUS‐TBNA is 21‐gauge (Olympus, NA‐201SX‐4021).21, 22 Nakajima et al. reported histological evaluation using “core material” was even possible using the 22‐gauge needle for EBUS‐TBNA.23 Further studies are needed to examine whether there are differences in obtaining adequate tissue for histology, immunohistochemistry, molecular analysis, and gene typing between 19, 21, and 22 gauge TBNA needles, and the continuous development of a 19‐gauge or larger EBUS‐TBNA needle is necessary.9
Procedures by the same operator at the same institution exclude bias as a result of technical differences in LN sampling and specimen processing. However, the control group is a historical comparison group. The procedure was not performed on the same patient using both needles or in two parallel groups to assure random distribution of the two different needles. Unfamiliarity of the 19 gauge eXcelon needle may bias the results. However, operator and assistant skill, and the pathologist's comfort with needle specimens all may have improved over the course of the study period, which would bias the results in favor of the eXcelon needle. The small sample size and different disease demographics between the two groups are also limitations of this study. In addition to needle size, the ability to obtain a suitable histologic specimen also depends on the characteristics of the lesion.24 Taking into account the study limitations, the 19‐gauge eXcelon needle is as safe and effective as the MW‐319 needle, according to preliminary results. Larger comparative studies are required.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The authors would like to thank Miss Yun‐Ying Chen and Miss Shiao‐Chiu Huang for their assistance with preparation of the figures and references.
Part of the data from this study was presented at the 18th World Congress for Bronchology and Interventional Pulmonology, Kyoto, Japan, April 13–17, 2014.
References
- 1. Wang KP, Marsh BR, Summer WR, Terry PB, Erozan YS, Baker RR. Transbronchial needle aspiration for diagnosis of lung cancer. Chest 1981; 80: 48–50. [PubMed] [Google Scholar]
- 2. Shenk DA, Chambers SL, Derdak S et al Comparison of the Wang 19‐gauge and 22‐gauge needles in the mediastinal staging of lung cancer. Am Rev Respir Dis 1993; 147: 1251–1258. [DOI] [PubMed] [Google Scholar]
- 3. Wang KP, Brower R, Haponik EF, Siegelman S. Flexible transbronchial needle aspiration for the staging of bronchogenic carcinoma. Chest 1983; 84: 571–576. [DOI] [PubMed] [Google Scholar]
- 4. Wang KP. Flexible transbronchial needle aspiration biopsy for histology specimens. Chest 1985; 88: 860–863. [DOI] [PubMed] [Google Scholar]
- 5. Wang KP. Staging of bronchogenic carcinoma by bronchoscopy. Chest 1994; 106: 588–593. [DOI] [PubMed] [Google Scholar]
- 6. Stratakos G, Porfyridis I, Papas V et al Exclusive diagnostic contribution of the histology specimens obtained by 19‐gauge transbronchial aspiration needle in suspected malignant intrathoracic lymphadenopathy. Chest 2008; 133: 131–136. [DOI] [PubMed] [Google Scholar]
- 7. Czarnecka‐Kujawa K, Yasufuku K. Molecular alterations in non‐small cell lung cancer: Perspective for targeted therapy and specimen management for the bronchoscopist. Respirology 2014; 19: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 8. Hsu LH, Ko JS, You DL, Liu CC, Chu NM. Transbronchial needle aspiration accurately diagnoses subcentimetre mediastinal and hilar lymph nodes detected by integrated positron emission tomography and computed tomography. Respirology 2007; 12: 848–855. [DOI] [PubMed] [Google Scholar]
- 9. van der Heijden EH, Casal RF, Trisolini R et al Guideline for the acquisition and preparation of conventional and endobronchial ultrasound‐guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014; 88: 500–517. [DOI] [PubMed] [Google Scholar]
- 10. Rusch VW, Asamura H, Watanabe H et al The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009; 4: 568–577. [DOI] [PubMed] [Google Scholar]
- 11. Wang KP, Browning R. Transbronchial needle aspiration with or without endobronchial ultrasound. Thorac Cancer 2010; 1: 87–93. [DOI] [PubMed] [Google Scholar]
- 12. Yung RC. Efficacy and safety of the new eXcelon transbronchial needle: Experience with the first 50 cases. Chest 2004; 126 (4 Meeting Abstracts): Abstract 820s. [Google Scholar]
- 13. Hermens FHW, Thunnissen E, Janssen JP. Diagnostic yield of eXelon, a new transbronchial histology aspiration needle in patients with mediastinal lymph node enlargement. J Bronchol 2007; 14: 86–89. [DOI] [PubMed] [Google Scholar]
- 14. Fernández‐Villar A, Leiro V, Blanco M et al Efficacy and safety of the eXcelon transbronchial aspiration needle in mediastinal lymph node enlargement: A case‐control study. Respiration 2007; 74: 208–213. [DOI] [PubMed] [Google Scholar]
- 15. Fernández‐Villar A, Botana M, Leiro V, González A, Represas C, Ruano‐Raviña A. Validity and reliability of transbronchial needle aspiration for diagnosing mediastinal adenopathies. BMC Pulm Med 2010; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu LH, Liu CC, Ko JS, Chen CC, Feng AC. Safety of interventional bronchoscopy through complication review at a cancer center. Clin Respir J 2014. doi: 10.1111/crj.12225 [DOI] [PubMed] [Google Scholar]
- 17. Hsu LH, Liu CC, Ko JS,. Education and experience improve the performance of transbronchial needle aspiration: A learning curve at a cancer center. Chest 2004; 125: 532–540. [DOI] [PubMed] [Google Scholar]
- 18. Yasufuku K, Chiyo M, Sekine Y et al Real‐time endobronchial ultrasound‐guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004; 126: 122–128. [DOI] [PubMed] [Google Scholar]
- 19. Yasufuku K, Nakajima T, Motoori K et al Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006; 130: 710–718. [DOI] [PubMed] [Google Scholar]
- 20. Annema JT, van Meerbeeck JP, Rintoul RC et al Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: A randomized trial. JAMA 2010; 304: 2245–2252. [DOI] [PubMed] [Google Scholar]
- 21. Yarmus LB, Akulian J, Lechtzin N et al Comparison of 21‐gauge and 22‐gauge aspiration needle in endobronchial ultrasound‐guided transbronchial needle aspiration: Results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013; 143: 1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeyabalan A, Shelley‐Fraser G, Medford AR. Impact of needle gauge on characterization of endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) histology samples. Respirology 2014; 19: 735–739. [DOI] [PubMed] [Google Scholar]
- 23. Nakajima T, Yasufuku K, Takahashi R et al Comparison of 21‐gauge and 22‐gauge aspiration needle during endobronchial ultrasound‐guided transbronchial needle aspiration. Respirology 2011; 16: 90–94. [DOI] [PubMed] [Google Scholar]
- 24. Hermens FH, van Engelenburg TC, Visser FJ, Thunnissen FB, Termeer R, Janssen JP. Diagnostic yield of transbronchial histology needle aspiration in patients with mediastinal lymph node enlargement. Respiration 2003; 70: 631–635. [DOI] [PubMed] [Google Scholar]


