Abstract
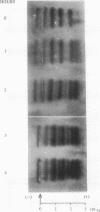
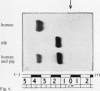
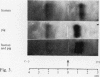
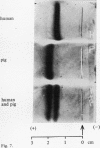
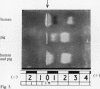
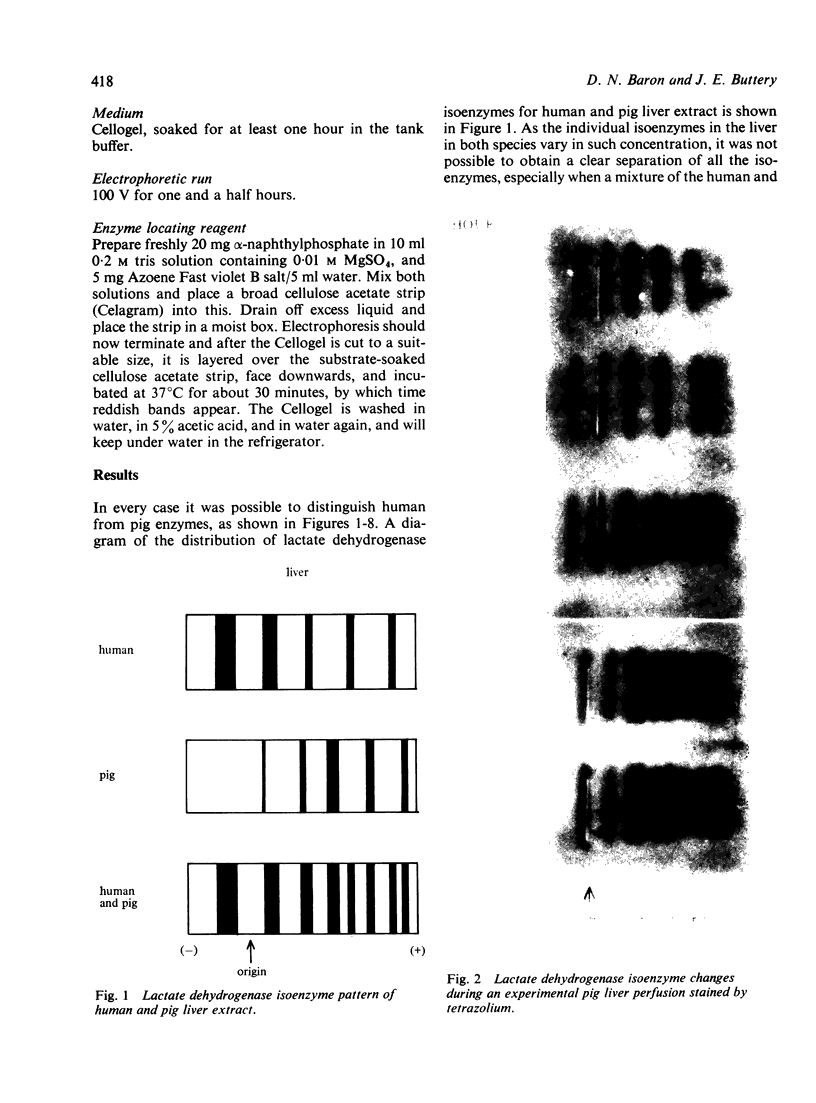
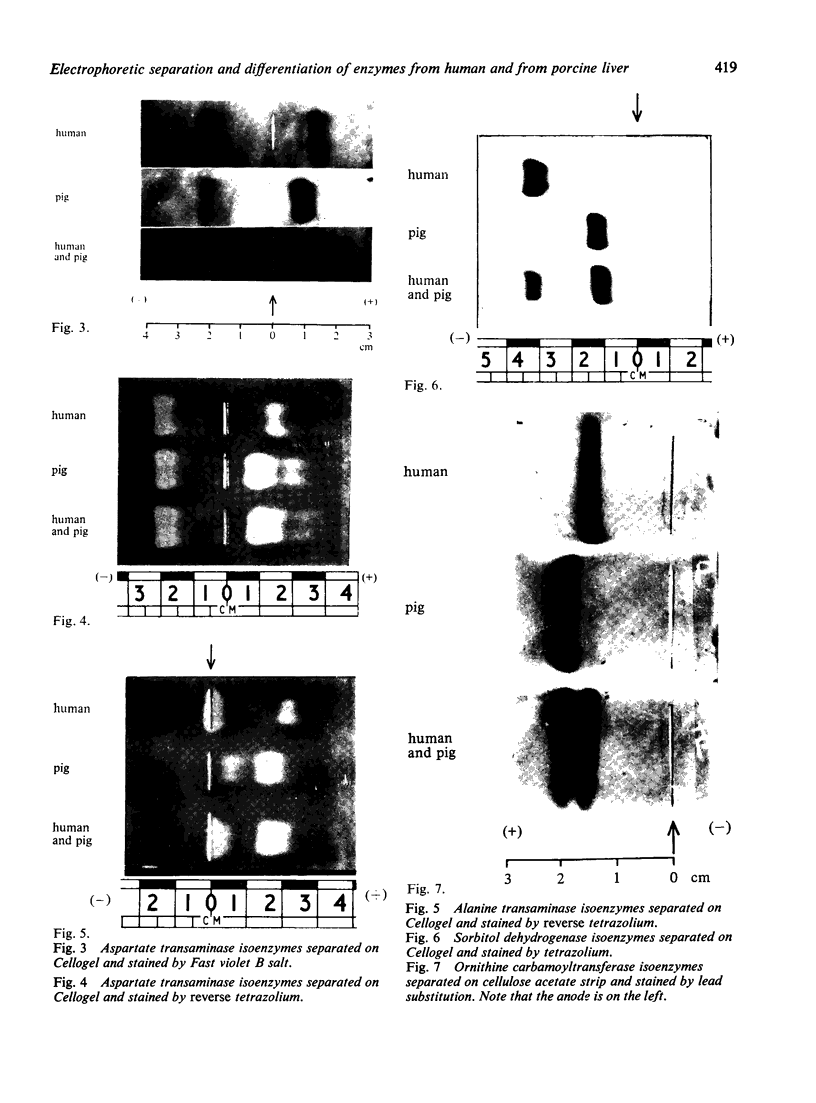
The electrophoretic separations of some human and pig liver enzymes on cellulose acetate and Cellogel were investigated, with reference to their joint occurrence in serum of patients undergoing treatment by extracorporeal pig liver perfusion. In every case it was possible to distinguish between the human and pig enzymes. Pig lactate dehydrogenase isoenzymes occupy a position slightly anodic to the corresponding human bands. The aspartate transaminase band of human is more anodic than that of pig, but their cathodic bands have the same mobility. Alanine transaminase of both human and pig liver extract is shown to exist as two bands each towards the anode. The faster moving human band is more anodic than the corresponding pig band, while the other human band is less anodic. Sorbitol dehydrogenase, alkaline phosphatase, and ornithine carbamoyltransferase all exist as one band each. Human sorbitol dehydrogenase is more cathodic than the pig enzyme, human alkaline phosphatase more anodic than the pig enzyme, while human ornithine carbamoyltransferase is less anodic than the pig enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abouna G. M., Ashcroft T., Hull C., Hodson A., Kirkley J., Walder D. N. The assessment of function of the isolated perfused porcine liver. Br J Surg. 1969 Apr;56(4):289–295. doi: 10.1002/bjs.1800560413. [DOI] [PubMed] [Google Scholar]
- Bersohn I., Kew M. C., Rolle M., Miency C. J., Myburgh J. A. Porcine lactic dehydrogenase in the serum of patients treated by extracorporeal porcine liver perfusion. Br Med J. 1969 Apr 12;2(5649):84–85. doi: 10.1136/bmj.2.5649.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery J. E., Parbhoo S. P., Baron D. N. Serum enzyme and isoenzyme changes in the assessment of experimental and clinical extracorporeal pig liver perfusion. Clin Sci. 1971 Aug;41(2):4P–4P. doi: 10.1042/cs041004p. [DOI] [PubMed] [Google Scholar]
- Eiseman B. Treatment of hepatic coma by extracorporeal liver perfusion. Ann R Coll Surg Engl. 1966 Jun;38(6):329–348. [PMC free article] [PubMed] [Google Scholar]
- Hof J. O., Wolf U., Krone W. Studies on isozymes of sorbitol dehydrogenase in some vertebrate species. Humangenetik. 1969;8(3):178–182. doi: 10.1007/BF00280570. [DOI] [PubMed] [Google Scholar]
- Myers R. C., Van Remortel H. The use of a reagent gel to locate LDH isoenzymes separated on cellulose acetate membranes. Clin Chem. 1968 Nov;14(11):1131–1134. [PubMed] [Google Scholar]
- Op't Hof J. Isoenzymes and population genetics of sorbit dehydrogenase (EC: 1.1.1.14) in swine (Sus scrofa). Humangenetik. 1969;7(3):258–259. doi: 10.1007/BF00273178. [DOI] [PubMed] [Google Scholar]
- Posen S., Neale F. C., Birkett D. J., Brudenell-Woods J. Intestinal alkaline phosphatase in human serum. Am J Clin Pathol. 1967 Jul;48(1):81–86. doi: 10.1093/ajcp/48.1.81. [DOI] [PubMed] [Google Scholar]
- ROMEL W. C., LAMANCUSA S. J. ELECTROPHORESIS OF GLUTAMIC OXALACETIC TRANSAMINASE IN SERUM, BEEF HEART, AND LIVER HOMOGENATES ON CELLULOSE ACETATE. Clin Chem. 1965 Feb;11:131–136. [PubMed] [Google Scholar]
- SWICK R. W., BARNSTEIN P. L., STANGE J. L. THE METABOLISM OF MITOCHONDRIAL PROTEINS. I. DISTRIBUTION AND CHARACTERIZATION OF THE ISOZYMES OF ALANINE AMINOTRANSFERASE IN RAT LIVER. J Biol Chem. 1965 Aug;240:3334–3340. [PubMed] [Google Scholar]
- Ziegenbein R. 2 different forms of glutamic pyruvic transaminase in rat heart and their intracellular localization. Nature. 1966 Nov 26;212(5065):935–935. [PubMed] [Google Scholar]