Abstract
The data described in this work is related to be the subject of an article in the Forensic Science International, titled: “The harmful chemistry behind “krokodil”: street-like synthesis and product analysis” (http://dx.doi.org/10.1016/j.forsciint.2015.07.042) [1]. The data presented here provides additional description of the chemical profile of “krokodil”. Physicochemical and organoleptic characteristics, TLC profile, UV/Vis, 1H NMR and FTIR spectrum are presented. These data validate the proposed synthetic procedure and pathway and give further information about the contaminants present in “krokodil”.
Keywords: “Krokodil”, Street like synthesis, TLC profile, UV/Vis, 1H NMR, FTIR
Specifications Table
| Subject area | Chemistry |
| More specific subject area | Chemical profile data |
| Type of data | Figures |
| How data was acquired | UV analysis (Varian CARY 100 spectrophotometer from a range of 200 nm to 800 nm. Software: Cary Win UV, v. 3.0), FTIR analysis (Nicolet iS10 from Thermo Scientific. Smart OMNI-Transmisson accessory. Software OMNIC 8.3) and 1H NMR analysis (Bruker DRX-300 spectrometer operating at 300.13 MHz for1H) |
| Data format | Analyzed data |
| Experimental factors | Additional chemical profile data from “krokodil” samples |
| Experimental features | Crude “krokodil” obtained using street-like synthesis and its extract after alkalization and organic extraction using ethyl acetate as solvent were analyzed |
| Data source location | Porto, Portugal |
| Data accessibility | Data is provided in this article |
Value of the data
-
•
Detailed description of organoleptic properties and pH range of “krokodil” as well as the disclosure of UV/Vis and 1H NMR spectra provide additional data to the establishment of the chemical profile of “krokodil”.
-
•
The description of the chemical profile of “krokodil” will eventually aid the competent authorities in dealing with this drug, in terms of identification and characterization.
-
•
Further insight regarding the complex nature of “Krokodil” was revealed by TLC analysis and FTIR spectrum.
1. Data
Data presented here describes the additional chemical analysis of the “krokodil” samples obtained using the street-like synthesis. Physical and organoleptic characters, UV/Vis and 1H NMR spectra were described on a “krokodil” sample freshly prepared (crude “krokodil”). Organic extract of “krokodil” (extracted “krokodil”) was obtained after alkalization of the crude product and extraction using ethyl acetate. This organic extracts were analyzed by TLC, FTIR and 1H NMR techniques.
2. Experimental design, materials and methods
The synthesis was carried out as described previously [1]. “Krokodil extract” samples were obtained by the treatment of 4 mL of crude “krokodil” with NaOH 20% (m/v) until alkalization, followed by extraction with ethyl acetate. The organic phases were gathered, dried over anhydrous sodium sulfate, filtered and concentered until dryness.
All pH measurements were made with a Model pH-meter GLP 22 (Crison, Allela, Spain).
UV/Vis spectra of water-diluted solutions of crude “krokodil” were recorded on a Varian CARY 100 spectrophotometer from a range of 200 nm to 800 nm (software: Cary Win UV, v. 3.0). 1H NMR spectrum was recorded on a Bruker DRX-300 spectrometer (operating at 300 MHz for 1H) using D2O (Deutero GmbH) as solvent.
TLC experiments were carried out on pre-coated plates (silica gel, 60 F254 Merck) with 0.2 mm of thickness. Elution took place at a CAMAG Horizontal Developing chamber and five mobile phases were tested. Chromatograms visualization was conducted under UV light at 254 and 365 nm.
FTIR spectrum was obtained in a FTIR spectrometer Nicolet iS10 from Thermo Scientific, using KBr disks. Spectra analysis was performed with Smart OMNI-Transmission accessory (Software OMNIC 8.3).
Crude “krokodil” appeared as a yellow to light brown solution due to the presence of iodine [2] and with a very characteristic acidic smell. The final product did not reveal any signal of iodine crystals and red phosphorus sediments were successfully removed by filtration. The pH of crude “krokodil” samples was 1.15±0.30. The low pH value is in accordance with the literature [1], [2]. However, this is the first time that pH value was reported with analytical precision.
UV/Vis spectrum of crude “krokodil” was performed to evaluate the presence and extension of chromophores (Fig. 1). Two main absorption bands were observed in the spectrum, one in the range of 215–250 nm (λmax at 225 nm) and other in the range of 250–300 nm (λmax at 276 nm). Absorptions in the 215–250 nm range are associated with presence of organic substances with unsaturated bonds and few conjugated systems (π→π* transitions). The absorptions in the 250–300 nm range are associated with organic substances with stronger chromophores and also with auxochromes (n→π* transitions) or conjugated systems [3]. It is noteworthy, that the band in the 250–300 nm range is compatible with the λmax of absorption of some morphinans (λmax 284 nm for desomorphine and λmax 285 nm for codeine) [4], [5].
Fig. 1.
UV/Vis spectrum of crude “krokodil”.
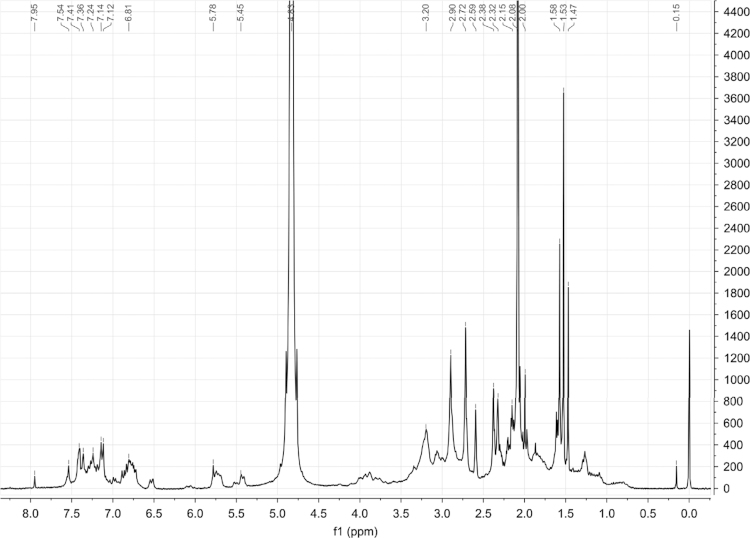
1H NMR spectrum of crude “krokodil” was also recorded (Fig. 2). The spectrum exhibits a lower frequency value signal (δ=0.15 ppm) which may be due to the presence/contamination with some raw materials used in the synthesis, like silicone grease (polydimethylsiloxane) or a similar compound [6]. The presence of several signals in the range of 0.5–2 ppm, was also observed, suggesting the presence of aliphatic protons and several signals in the range of 2–3.5 ppm, suggesting the presence of heteroatoms adjacent to carbons. As “krokodil” is an aqueous solution, D2O was used as solvent for NMR analysis. The residual H2O signal (D2O was not 100% deuterated), a broad signal at 4.83 ppm, can overlap proton signals due to alcohols, phenols, amides or amines [7]. The absorptions in the range of 5–6 ppm are compatible with vinylic protons. A substantial number of different aromatic protons are also present once there are several signals in the range of 6.5–8 ppm.
Fig. 2.
1H NMR spectrum of crude “krokodil”.
In “krokodil” manufacture, organic and inorganic reactants are involved and it is agreed that organic and inorganic compounds are produced. Considering the organic compounds present in “Krokodil”, morphinans are very important, since they are the responsible for the psychoactive effects of this drug. In order to study these organic components, crude “krokodil” was basified with sodium hydroxide and extracted with ethyl acetate yielding an organic extract designated as “krokodil extract”. The treatment of crude “krokodil” with a strong base assured the presence of morphinans as free bases, which were subsequently extracted with ethyl acetate, an organic solvent of intermediate polarity [8]. However, these procedures are not specific for morphinan molecules, since all organic compounds, with the exception of acidic substances, are also extracted.
Taking in account the fact that “krokodil” contains a mixture of morphinans, several mobile phases described in the literature for thin-layer chromatography (TLC) analysis of morphinans were tested [9], [10]. For the TLC analysis a mixture of hexane/ethyl acetate/diethylamine was selected as mobile phase (6:4:0.5). The chromatographic profile of “krokodil extract” with this mobile phase is shown in Fig. 3.
Fig. 3.
Thin-layer chromatographic profile of extracted “krokodil” samples. Mobile phase: hexane/ethyl acetate/diethylamine (6:4:0.5).
The FTIR spectrum of “krokodil” showed an absorption compatible with a phenolic hydroxyl (3382 cm−1, m, PhO–H stretch), a group present in acetaminophen, desomorphine and morphinan-4,5-epoxy-3-ol (Fig. 4). The presence of vinylic carbons, which was already recognized in 1H NMR spectrum, is also suggested (3028 cm−1, m, =C–H stretch). In accordance with the 1H NMR spectrum, FTIR spectrum also shows several bands compatible with the aromatic C=C stretching (1559 cm−1, m; 1542 cm−1, m; 1509 cm−1, m; 1456 cm−1, m).
Fig. 4.
– FTIR Spectrum of “krokodil” in KBr microplate.
Conflict of interest statement
Authors declare no conflict of interest.
Acknowledgments
Emanuele Alves and Annibal D. Pereira Netto acknowledge Brazilian agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process 245844/2012-0) for research grants and scholarship. José Soares acknowledges Fundação para a Ciência e a Tecnologia (FCT) for PhD (SFRH/BD/98105/2013). Ricardo Dinis-Oliveira acknowledges Fundação para a Ciência e a Tecnologia (FCT) for his Investigator Grant (IF/01147/2013). Sara Cravo and Carlos Afonso acknowledges FCT through the strategic project CEQUIMED-UP (Pest-OE/SAU/UI4040/2014). Artur Silva thanks FCT/MEC for the financial support of the QOPNA research Unit (FCT UID/QUI/00062/2013), through national founds and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement, and to the Portuguese NMR Network.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2015.11.046.
Contributor Information
Emanuele Amorim Alves, Email: manuhpa@hotmail.com.
Ricardo Jorge Dinis-Oliveira, Email: ricardinis@med.up.pt, ricardinis@sapo.pt.
Carlos Manuel Afonso, Email: cafonso@ff.up.pt.
Appendix A. Supplementary material
Supplementary material
References
- 1.Alves E.A., Soares J.X., Afonso C.M., Grund J.C., Agonia A.S., Cravo S.M. The harmful chemistry behind "krokodil": street-like synthesis and product analysis. Forensic Sci. Int. 2015;257:76–82. doi: 10.1016/j.forsciint.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 2.Alves E.A., Grund J.P., Afonso C.M., Netto A.D., Carvalho F., Dinis-Oliveira R.J. The harmful chemistry behind krokodil (desomorphine) synthesis and mechanisms of toxicity. Forensic Sci. Int. 2015;249C:207–213. doi: 10.1016/j.forsciint.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Scott A.I. Pergamon Press; Oxford: 1964. Interpretation of Ultraviolet Spectra of Natural Products. [Google Scholar]
- 4.Chemicals C. Safety Data Sheet Desomorphine. Cayman Chemicals; 2014.
- 5.Sigma-Aldrich. Codeine Data Sheet. Sigma-Aldrich; 2014.
- 6.Gottlieb H.E., Kotlyar V., Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 7.Gerothanassis I.P., Troganis A., Exarchou V., Barbarossou K. Nuclear Magnetic Resonance (NMR) spectrocospy: basici principles and phenomena, and their applications to chemistry, biology and medicine. Chem. Educ. Res. Pract. 2002;3:229–252. [Google Scholar]
- 8.Riffault L., Colas C., Destandau E., Pasquier L., Andre P., Elfakir C. Non-targeted molecular characterisation of a rose flower ethyl acetate extract using ultra-HPLC with atmospheric pressure photoionisation and quadrupole time-of-flight MS/MS. Phytochem. Anal. 2015;26:189–201. doi: 10.1002/pca.2552. [DOI] [PubMed] [Google Scholar]
- 9.Ahadi A., Partoazar A., Abedi-Khorasgani M.H., Shetab-Boushehri S.V. Comparison of liquid-liquid extraction-thin layer chromatography with solid-phase extraction-high-performance thin layer chromatography in detection of urinary morphine. J. Biomed. Res. 2011;25:362–367. doi: 10.1016/S1674-8301(11)60048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pothier J., Galand N. Automated multiple development thin-layer chromatography for separation of opiate alkaloids and derivatives. J. Chromatogr. A. 2005;1080:186–191. doi: 10.1016/j.chroma.2005.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material