Highlights
-
•
We studied patients with refractory temporal lobe epilepsy who underwent surgery.
-
•
Hippocampal internal architecture (HIA) did not correlate with seizure outcome.
-
•
HIA correlated with hippocampal neuronal density, Wyler grades, and hippocampal volume.
-
•
HIA on MRI has a histopathological basis.
-
•
Postoperative seizure outcome cannot be predicted by HIA on MRI.
Keywords: Epilepsy surgery, Hippocampus, Histopathology, Outcome, Prognosis
Abstract
Purpose
Semi-quantitative analysis of hippocampal internal architecture (HIA) on MRI has been shown to be a reliable predictor of the side of seizure onset in patients with temporal lobe epilepsy (TLE). In the present study, we investigated the relationship between postoperative seizure outcome and preoperative semi-quantitative measures of HIA.
Methods
We determined HIA on high in-plane resolution preoperative T2 short tau inversion recovery MR images in 79 patients with presumed unilateral mesial TLE (mTLE) due to hippocampal sclerosis (HS) who underwent amygdalohippocampectomy and postoperative follow up. HIA was investigated with respect to postoperative seizure freedom, neuronal density determined from resected hippocampal specimens, and conventionally acquired hippocampal volume.
Results
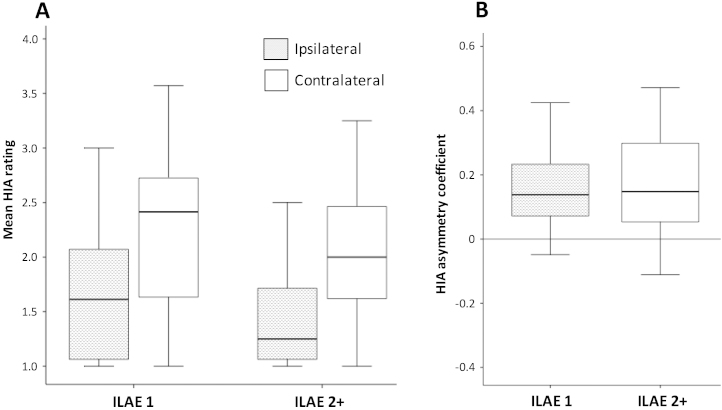
HIA ratings were significantly related to some neuropathological features of the resected hippocampus (e.g. neuronal density of selective CA regions, Wyler grades), and bilaterally with preoperative hippocampal volume. However, there were no significant differences in HIA ratings of the to-be-resected or contralateral hippocampus between patients rendered seizure free (ILAE 1) compared to those continuing to experience seizures (ILAE 2-5).
Conclusions
This work indicates that semi-quantitative assessment of HIA on high-resolution MRI provides a surrogate marker of underlying histopathology, but cannot prospectively distinguish between patients who will continue to experience postoperative seizures and those who will be rendered seizure free. The predictive power of HIA for postoperative seizure outcome in non-lesional patients with TLE should be explored.
1. Introduction
Hippocampal sclerosis (HS) is the primary neuropathological correlate and most common cause of refractory mesial temporal lobe epilepsy (mTLE). The preoperative identification of HS is associated with an improved postoperative seizure outcome relative to patients with suspected TLE and no MRI lesion [1], [2]. However, approximately 40% of patients with unilateral mTLE and HS will continue to experience debilitating postoperative seizures after short (i.e. 1–2 years) follow-up periods [2], [3]. This figure may extend substantially when any kind of postoperative seizure is considered [3] or when long (i.e. >10 years) postoperative follow up periods are used [4]. It is unknown why such a large proportion of seemingly excellent candidates are not successfully rendered seizure free after temporal lobe surgery. Bitemporal epileptiform contributions remain a possible cause of persistent postoperative seizures; our recent findings indicate that groups of patients with persistent postoperative seizures have significant alterations of bilateral thalamohippocampal structural networks relative to patients surgically rendered seizure free [5], [6]. Other work supports the hypothesis that, as a group, patients with residual postoperative seizures have increased contralateral hippocampal structural atrophy relative to patients with an optimal outcome [7], [8]. Whilst these morphometric findings are novel and fit well with known epileptiform networks in mTLE, they do not permit the prospective classification of individual patients according to outcome. An imaging marker that can stratify patients according to outcome and can be easily incorporated into presurgical evaluation would represent an important tool.
Hippocampal volume is not a consistent reliable marker of postoperative outcome in patients with mTLE and HS [9]. It is therefore plausible to consider other imaging features of the hippocampus that may have predictive value. Semi-quantitative assessment of internal hippocampal architecture (HIA) has been shown to be a significant predictor of the laterality of seizure onset in TLE [10], [11]. Changes in HIA have been reported in patients with histopathologically proven HS in whom no MRI-determined hippocampal atrophy was evident [12]. Loss of normal HIA is thought to be due to neuronal cell loss and replacement of the normal anatomical layers with gliotic tissue [13]. One primary advantage of semi-quantitative assessment of HIA is the potential ease of implementation into radiological evaluation for prospective patients being considered for surgery. We sought for the first time to assess the significance of preoperative HIA of the to-be-resected and contralateral hippocampi on short-term (1–3 years) postoperative seizure outcome in patients with mTLE. We additionally investigated the relationship between HIA and histopathological alterations of resected hippocampi and conventionally acquired hippocampal volumes.
2. Methods
2.1. Patients
Detailed demographic, clinical and preoperative workup information for patients studied in this investigation is described in our recent papers [5], [6]. All patients were recruited and investigated at University Hospital Bonn, had presumed unilateral mTLE by virtue of seizure semiology and electrophysiological investigations, had neuroradiologically defined ipsilateral HS, and no evidence of bilateral HS or a secondary lesion that may be epileptogenic in nature. All patients were consecutively recruited on the basis of the above criteria between 2006 and 2011. Of 115 patients with mTLE and HS being enrolled into this study, 87 patients underwent selective amygdalohippocampectomy between 2006 and 2012 and received standardised postoperative follow up and outcome assessment using the International League Against Epilepsy (ILAE) outcome classification system [14].
2.2. Histopathological assessment
We determined ILAE [15] and Wyler [16] semi-quantitative gradings of HS from resected specimens. ILAE ratings included specimens with severe neuronal cell loss and gliosis predominantly in CA1 and CA4 regions (ILAE type 1), predominantly in CA1 (ILAE type 2), predominantly in CA4 (ILAE type 3) or with no HS. Wyler grades provided grades of overall hippocampal cell loss ranging from no pathology to Grade 4, with three incremental grades (mild, moderate, moderate to marked, and marked). Furthermore, quantitative neuronal cell density (number of neurons/μm2) was obtained from each specimen, as recently described [17]. After scanning of Neu-N immunohistochemistry using a Mirax scanner (3DHistech, Hungary), rectangular regions-of-interest (ROIs) within hippocampal subfields CA1-4 were selected. NeuN-positive cells within these ROIs were counted with a quantitative software solution (HistoQuant, 3DHistech, Hungary) after manual control of correct cell identification.
2.3. MRI
All patients underwent MRI at the Life & Brain Center in Bonn on a 3 Tesla scanner (Magnetom Trio, Siemens, Erlangen, Germany) using an 8-channel head coil. For the assessment of HIA, we acquired a T2 short tau inversion recovery (STIR) sequence in the coronal plane angulated perpendicular to the long axis of the hippocampus (40 slices, TR = 5600 ms, TI = 100 ms, TE = 18 ms, resolution 0.45 × 0.45 × 2.0 mm, flip angle 0°). We determined hippocampal volume from 3D T1-weighted MPRAGE images acquired for each patient (160 slices, TR = 1300 ms, TI = 650 ms, TE = 3.97 ms, resolution 1.0 × 1.0 × 1.0 mm, flip angle 10°).
HIA was visually assessed using the HIA rating scale as described previously [10], [11] on consecutive coronal T2-STIR sections in a rostral to caudal direction. All ratings were performed blind to diagnosis. The anterior limit of HIA assessment was taken as the first slice in which the uncus detaches from the hippocampal head and forms the hippocampal body, at which point the uncus is no longer visible. The posterior limit of HIA assessment was taken as the first slice in which the inferior and superior colliculi come clearly into view. Each hippocampal image was graded with a HIA score from ‘1’ indicating no perceptible internal architecture to ‘4’ indicating excellent internal architecture differentiation. Fig. 1 provides examples of coronal sections illustrating HIA scoring in patients investigated in the present study. HIA scores were based on assessment of the laminar appearance of apposing gray and white matter arising from the structure of Ammon's horn, giving rise to a dark hypointense band depicted in coronal sections. Left and right hippocampi were rated independently blind to patient identification and outcome information. An average HIA score was determined for each hippocampus in each patient, and a HIA asymmetry score was then determined by subtracting the left average HIA score from the right [10]. Using this approach, a positive HIA asymmetry score indicated preferential loss of HIA clarity on the ipsilateral (to-be-resected) side and a negative HIA asymmetry score indicated preferential loss on the contralateral side. Intra- (one rater, SE) and inter- (two raters, SE and SSK) rater reliability studies were performed on ten participants from the main cohort. These ten participants were randomly selected with no knowledge of diagnosis, including side of HS. Reliability studies indicated high levels of repeatability (intra-rater intraclass coefficient, two-way mixed model for consistency (ICC): left HIA = 0.97, right HIA = 0.98) and reproducibility (ICC: left HIA = 0.94, right HIA = 0.93). We additionally determined hippocampal volume from T1-weighted MPRAGE images using our previously described and well-validated approach that employed stereology in conjunction with point counting [18], [19].
Fig. 1.
T2-STIR coronal sections demonstrating HIA ratings of a selection of hippocampi. Top. A randomly selected patient with clear left HS (on right of image). HS is characterised by hippocampal atrophy and loss of HIA. HIA scores on this section were 2 and 4 for the left and right hippocampi, respectively. The magnified box shows the approximate locations of CA1 (1), CA2 (2), CA3 (3), CA4 (4), dentate gyrus (dg) and subiculum (s). Bottom. Left (L) and right (R) hippocampal sections that were graded as I, II, III and IV according to the criteria defined by ver Hoef [10], [11]. All hippocampi are presented in radiological orientation (left = right). All hippocampi presented are from different patients.
2.4. Statistical analysis
We used SPSS (IBM SPSS Statistics, Version 21.0, Armonk, NY) for statistical analysis. To determine differences between patient groups, a univariate ANOVA was used including group (seizure free vs persistent seizures; history of childhood febrile seizures vs no history) and HIA score, neuronal density and hippocampal volume as dependent variables. Seizure freedom was defined as an International League Against Epilepsy score of I (ILAE 1), and persistent postoperative seizures defined as ILAE 2–6 [14]. Chi-square tests were used to determine whether histopathological gradings and a history of childhood febrile convulsions were related to HIA and seizure outcome. Pearson's Correlation Coefficients were used to investigate relationships between imaging and clinical variables. Paired samples t-tests were used to determine neuronal density differences between CA regions across all patients.
3. Results
Preoperative T2-STIR MRI data was available for 86 patients with a full detailed clinical history. Two patients were excluded due to image motion artefact. HIA ratings were performed for 84 patients who had complete clinical information, including age at seizure onset, duration of seizure disorder, seizure frequency and history of febrile seizures. Of the 84 patients, all underwent amygdalohippocampectomy and 79 had postoperative outcome ratings on average at two years after surgery, and at a minimum of one year. Five patients were lost to postoperative follow-up. Quantitative histopathological data was available for 46 patients.
3.1. HIA and outcome
Across all patients, mean HIA scores ranged from 1.00 to 3.00 (mean 1.53, SD 0.54) and 1.00 to 3.57 (mean 2.11, SD 0.67) for the ipsilateral and contralateral hippocampi, respectively (t = 9.01, p < 0.0001). Of the 79 patients who underwent surgery and had outcome data available, 44 (55.7%) achieved an ILAE outcome of ILAE 1, and 35 (44.3%) achieved an outcome of ILAE 2–5. No patient had a postoperative outcome of ILAE 6. There was no significant difference in mean ipsilateral HIA, contralateral HIA and HIA asymmetry between patient outcome groups (Table 1, Fig. 2). Sixty-nine (82.1%) patients were found to have a positive HIA asymmetry, ten (11.9%) patients were found to have a negative asymmetry, and five (6.0%) had symmetry. Five of the patients with a positive HIA asymmetry did not have postoperative outcome assessment. There was no significant difference between outcome groups in the distribution of patients with positive HIA asymmetry, negative asymmetry and asymmetry (Table 1).
Table 1.
Top: Mean (and SD) of ipsilateral HIA, contralateral HIA and HIA asymmetry according to outcome group. Bottom: Number (and percentage) of patients with a positive HIA asymmetry (+ve HIA Asym), negative HIA asymmetry (−ve HIA Asym) and HIA symmetry (Sym) according to outcome group. F = one-way ANOVA results); χ2 = chi-square results.
| Ipsilateral HIA | Contralateral HIA | HIA Asymmetry | |
|---|---|---|---|
| ILAE 1 | 1.64 (0.56) | 2.20 (0.70) | 0.14 (0.15) |
| ILAE 2-5 | 1.44 (0.51) | 2.01 (0.62) | 0.16 (0.15) |
| F = 2.20, p = 0.12 | F = 0.92, p = 0.40 | F = 0.46, p = 0.64 |
| +ve HIA Asym | −ve HIA Asym | HIA Sym | |
|---|---|---|---|
| ILAE 1 | 36 (81.8%) | 5 (11.4%) | 3 (6.8%) |
| ILAE 2-5 | 28 (80.0%) | 5 (14.3%) | 2 (5.7%) |
| χ2 = 0.04, p = 0.84 | χ2 = 0.002, p = 0.96 | χ2 = 0.04, p = 0.84 |
Fig. 2.
HIA ratings and postoperative outcome. (A) Boxplots of mean HIA ratings of the ipsilateral and contralateral hippocampus in patients with excellent (ILAE 1) and suboptimal (ILAE 2) postoperative outcomes. (B) HIA rating asymmetries and outcome. No significant differences between outcome groups were identified for ipsilateral and contralateral ratings, or rating asymmetries.
3.2. Relationships with histopathology and volume
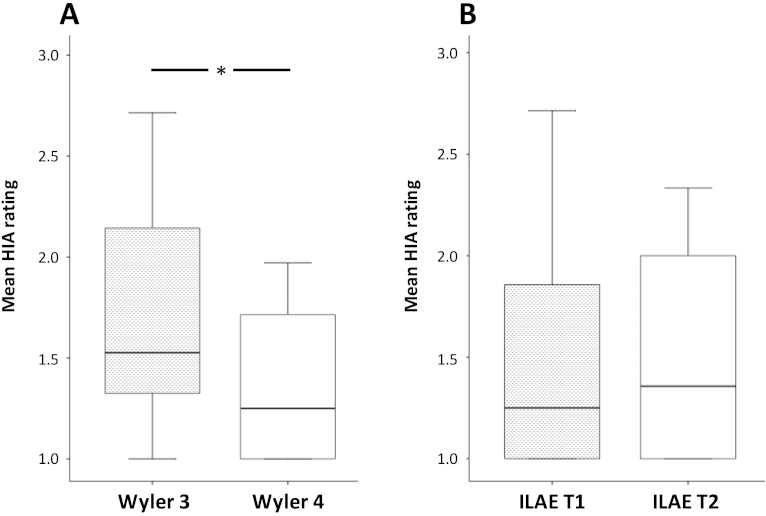
Of the 46 resections available for analysis, hippocampal damage was determined to be Wyler Grade II in three (6.5%), Grade III in 22 (47.8%) and Grade IV in 21 (45.7%) cases. Resections classified as Wyler Grade IV (most significant hippocampal damage defined histopathologically) had significantly lower ipsilateral HIA ratings relative to resections classified as Grade III (mean 1.35 (SD 0.35) and 1.72 (SD 0.53), respectively; F = 4.3, p = 0.02; Fig. 3A). The patterns of HS were determined to be ILAE type 1 in 38 (82.6%) and ILAE type 2 in eight (17.4%) cases. No patient showed evidence of ILAE type 3. There were no statistically significant differences in outcome (χ2 = 0.19, p = 0.67), but this was likely due to the small number of patients in the ILAE type 2 group. There was an increased percentage of patients attaining excellent seizure control in those with typical patterns of HS relative to those with atypical patterns (Table 2). Ipsilateral HIA ratings did not significantly differ between patients with ILAE type 1 (mean 1.51, SD 0.55) and 2 (mean 1.51, SD 0.57) patterns of HS (F = 0.001, p = 0.98; Fig. 3B).
Fig. 3.
HIA ratings and histopathological gradings. (A) Boxplots of ipsilateral mean HIA ratings in patients with moderate to marked (Wyler 3) and marked (Wyler 4) hippocampal sclerosis. (B) Ipsilateral mean HIA ratings in patients with typical (ILAE T1) and atypical (ILAE T2) patterns of hippocampal sclerosis.
*Significantly different (p = 0.02).
Table 2.
Pattern of hippocampal sclerosis shown with respect to postoperative seizure outcome.
| Outcome | HS: ILAE type 1 | HS: ILAE type 2 |
|---|---|---|
| ILAE 1 | 21 (58.3%) | 4 (50%) |
| ILAE 2 | 2 (5.6%) | 0 |
| ILAE 3 | 7 (19.4%) | 1 (12.5%) |
| ILAE 4 | 5 (13.9%) | 1 (12.5%) |
| ILAE 5 | 1 (2.8%) | 2 (25%) |
| Total | 36 (100%) | 8 (100%) |
Ipsilateral HIA was significantly correlated with neuronal density in CA3 (r = .31, p = 0.04) and CA4 (r = .45, p = 0.002), but not with neuronal density of CA1 (r = .15, p = 0.33) or CA2 (r = .15, p = 0.35) (Fig. 4). There was a non-significant trend for patients who were rendered seizure free to have a lower neuronal density in CA1 relative to patients who continued to experience postoperative seizures (0.34, SD 0.04; 0.59. SD 0.15; F = 3.26, p = 0.08). There were no significant differences between outcome groups in neuronal density of CA2 (F = 0.07, p = 0.80), CA3 (F = 0.09, p = 0.77) or CA4 (F = 0.39, p = 0.54) (Fig. 5). Across all patients, neuronal density was significantly lower in (i) CA1 (mean = 0.39 [×104/μm2], SD = 0.37) relative to CA2 (mean = 1.66, SD = 0.78; t = 11.67, p < 0.001), CA3 (mean = 0.97, SD = 0.63; t = 7.00, p < 0.001) and CA4 (mean = 0.70, SD = 0.60; t = 3.54, p = 0.001), (ii) CA4 relative to CA2 (t = 9.43, p < 0.001) and CA3 (t = 4.77, p < 0.001), and (iii) CA3 relative to CA2 (t = 6.37, p < 0.001).
Fig. 4.
Relationship between ipsilateral mean HIA ratings and quantitative neuronal density (ND) of CA1 (top left), CA2 (top right), CA3 (bottom left) and CA4 (bottom right).
*Significant correlation (p = 0.04).
Fig. 5.
Neuronal densities in CA1-4 in patients with excellent (ILAE 1) and suboptimal (ILAE 2-5) postoperative outcome. No significant differences are observed.
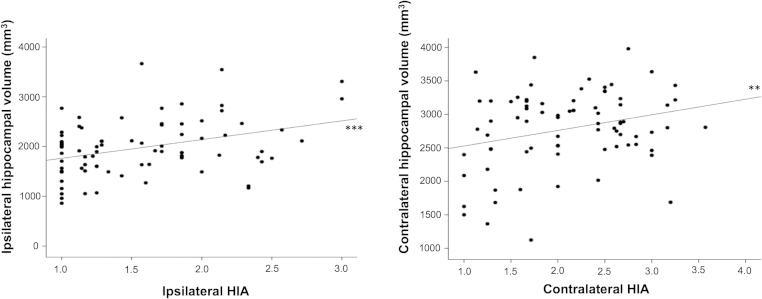
Ipsilateral (r = .36, p = 0.001) and contralateral (r = .27, p = 0.02) HIA was significantly correlated with ipsilateral and contralateral hippocampal volume, respectively (Fig. 6). There were no significant differences between patient outcome groups in the volume of the ipsilateral (F = 0.19, p = 0.67) or contralateral (F = 0.10, p = 0.32) hippocampus. There were no significant relationships between HIA ratings of the ipsilateral or contralateral hippocampus (and HIA asymmetry) and age of onset of mTLE, preoperative duration of mTLE, estimated seizure frequency, duration of postoperative follow up, or a history of childhood febrile convulsions (all p > 0.05).
Fig. 6.
Significant relationships between ipsilateral HIA and hippocampal volume (left) and contralateral HIA and hippocampal volume (right).
**p = 0.02.
***p = 0.001.
4. Discussion
Using a previously published and validated method to assess HIA on high in-plane resolution MR images, we have demonstrated that assessment of HIA does not have significance for the prediction of postoperative seizure outcome, at least in patients with mTLE and HS. The advantage of the HIA approach we adopted was with respect to the ease of application, its potential incorporation into presurgical evaluation programmes, and reliability for determining the side of seizure onset in patients with TLE [10], [11]. We have furthermore established that assessment of HIA is a sensitive marker of the underlying hippocampal structural pathology in TLE through correlation with histopathological analysis. Unlike the majority of time consuming manual volumetric assessment of the hippocampus, and automated analyses of hippocampal morphology that are dependent on sophisticated image processing pipelines, assessment of HIA can be easily incorporated into routine radiological evaluation, potentially at the time of scanning. There is therefore a real incentive to determine the clinical applicability of this approach.
We have shown that the ratings of HIA on high in-plane resolution images are significantly correlated with the underlying histopathology in mTLE and hippocampal volume, indicating that this method of assessment is a sensitive marker of hippocampal pathology. These findings are in keeping with earlier suggestions indicating that although disruption of HIA may be a separate hallmark of HS, it tends to be closely correlated with other features of HS, including hippocampal atrophy [13]. Our findings also reinforce the notion that global hippocampal atrophy is not a predictor of postoperative seizure outcome in patients with mTLE and radiological evidence of HS [5], [6], [20], [21]. Increasing hippocampal atrophy may be related to improved postoperative outcome, however, when patients with all forms of refractory mesial TLE (i.e. including patients with and without neuroradiological evidence of HS) are considered together [9], [22]. Furthermore, we have recently shown that the extent of surgical resection in this group of patients did not significantly influence postoperative seizure outcome [5], although this is a contentious issue in the wider literature [23], [24], [25], [26], [27].
Ten patients had a negative HIA asymmetry score, indicating that the hippocampus contralateral to radiologically defined atrophy and intended resection had more internal structural disruption. Five of these patients had an excellent postoperative outcome, and five had a suboptimal outcome (Table 1). We were unable to identify any clinical, histological, extrahippocampal MRI or surgical differences between these ten patients and the rest of the sample. These findings indicate that whilst lateralised atrophy and HIA disruption are not mutually exclusive, it is likely that contralateral HIA disruption in patients with diagnosed HS is not a reliable marker of persistent postoperative seizures due to bilateral hippocampal seizure onset zones.
There are methodological issues that merit discussion. Firstly, although evaluation of HIA is a subjective measure, the approach we have used has a sensitivity of 85% and specificity of 100% for correctly predicting the laterality of seizure onset in patients with mTLE [10]. It is important to note that the T2-STIR sequence we used is different from the T2-weighted TSE sequence used by Ver Hoef et al. [10], [11]. It is unknown how these technical differences may have affected HIA gradings and reliability studies. Hippocampal features on preoperative T2-STIR images have been shown to correlate well with histologically determined anatomical boundaries on hippocampal resections in patients with TLE and HS [28]. Furthermore, we have shown this method to have high levels of intra- and inter-rater reliability based on repeat measurements of ten subjects. There was natural variance between raters in gradings on individual hippocampal sections (e.g. Grade II or Grade III). However, given that the HIA score was calculated by taking an average from all slices evaluated (with most cases having between six to nine sections rated per hippocampus), it is likely that such borderline cases will have received an average score representative of the overall architecture of the hippocampus. Secondly, although we observed significant relationships between loss of HIA clarity and (i) Wyler gradings of overall hippocampal damage and (ii) CA3 and CA4 neuronal density, we did not observe a significant relationship between HIA ratings and neuronal density within CA1. Given that classical HS is characterised by preferential loss of CA1 neurons, and to a lesser extent CA3 and CA4 neurons, with relative preservation of CA2 neurons [17], [29], this finding is unexpected and difficult to explain. One possible explanation is that CA1 neuronal density was significantly lower with a much smaller variance relative to all other CA regions. Given that most of our patients had severe HS (Wyler Grades III and IV constituted 93.5% of our sample), with the primary neuropathological feature being loss of CA1 neurons, it may be that there was not enough variance in CA1 neuronal density to observe a significant relationship with HIA. The distribution of neuronal densities shown in Fig. 4 supports this idea. Finally, there is convincing evidence indicating that patients with atypical patterns of HS (e.g. circumscribed loss of CA1 neurons) have a significantly worse postoperative seizure outcome compared to patients with classical (neuronal loss of CA1, CA3 and CA4) or total (CA1-CA4) HS [29], [30]. We were unfortunately not able to test this hypothesis using quantitative measures of neuronal density of hippocampal subfields given the absence of non-epilepsy control hippocampal neuronal densities to quantify patterns of HS in patients. However, our semi-quantitative ILAE gradings of HS indicated no significant differences in outcome between patients with type 1 (classical HS) and type 2 (predominant CA1 cells loss). This is likely due to a low number of patients who had ILAE type 2 HS in our study; patients with ILAE type 1 did have an increased incidence of excellent outcomes relative to patients with ILAE type 2.
The confident prediction of postoperative seizure outcome by virtue of non-invasive imaging methods represents an important and so far unfulfilled clinical endeavour. Semi-quantitative assessment of HIA on high in-plane resolution MRI can be easily incorporated into routine presurgical evaluation, has lateralising value for patients with TLE, and is sensitive to underlying hippocampal histopathology. However, confident prediction of short-term postoperative seizure outcome using HIA assessment is not possible. There remains the possibility of bihemispheric mesial temporal pathology in patients with TLE and postoperative seizures, and the development of sensitive imaging measures that can detect mesial temporal pathological alterations contralateral to the side of intended resection would represent important scientific and clinical progress.
Conflict of interest
None.
Acknowledgements
This work was supported by a UK Medical Research Council grant awarded to SSK (Grant Number MR/K023152/1). MPR is supported by a UK Medical Research Council Programme Grant (Grant Number MR/K013998/1) and the National Institute for Health Research (NIHR) Biomedical Research Centre at the South London and Maudsey NHS Foundation Trust. BW was supported by a Heisenberg-Grant of the Deutsche Forschungsgemeinschaft (WE 4427/3-1).
References
- 1.McIntosh A.M., Kalnins R.M., Mitchell L.A., Fabinyi G.C., Briellmann R.S., Berkovic S.F. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127:2018–2030. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 2.Berkovic S.F., McIntosh A.M., Kalnins R.M., Jackson G.D., Fabinyi G.C., Brazenor G.A., Bladin P.F., Hopper J.L. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology. 1995;45:1358–1363. doi: 10.1212/wnl.45.7.1358. [DOI] [PubMed] [Google Scholar]
- 3.Wiebe S., Blume W.T., Girvin J.P., Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study, G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 4.de Tisi J., Bell G.S., Peacock J.L., McEvoy A.W., Harkness W.F., Sander J.W., Duncan J.S. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378:1388–1395. doi: 10.1016/S0140-6736(11)60890-8. [DOI] [PubMed] [Google Scholar]
- 5.Keller S.S., Richardson M.P., Schoene-Bake J.C., O’Muircheartaigh J., Elkommos S., Kreilkamp B., Goh Y.Y., Marson A.G., Elger C., Weber B. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol. 2015;77:760–774. doi: 10.1002/ana.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller S.S., Richardson M.P., O’Muircheartaigh J., Schoene-Bake J.C., Elger C., Weber B. Morphometric MRI alterations and postoperative seizure control in refractory temporal lobe epilepsy. Hum Brain Mapp. 2015;36:1637–1647. doi: 10.1002/hbm.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J.J., Salamon N., Dutton R.A., Lee A.D., Geaga J.A., Hayashi K.M., Toga A.W., Engel J., Jr., Thompson P.M. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology. 2005;65:1094–1097. doi: 10.1212/01.wnl.0000179003.95838.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller S.S., Cresswell P., Denby C., Wieshmann U., Eldridge P., Baker G., Roberts N. Persistent seizures following left temporal lobe surgery are associated with posterior and bilateral structural and functional brain abnormalities. Epilepsy Res. 2007;74:131–139. doi: 10.1016/j.eplepsyres.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Bonilha L., Keller S.S. Quantitative MRI in refractory temporal lobe epilepsy: relationship with surgical outcomes. Quant Imaging Med Surg. 2015;5:204–224. doi: 10.3978/j.issn.2223-4292.2015.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ver Hoef L.W., Williams F.B., Kennedy R.E., Szaflarski J.P., Knowlton R.C. Predictive value of hippocampal internal architecture asymmetry in temporal lobe epilepsy. Epilepsy Res. 2013;106:155–163. doi: 10.1016/j.eplepsyres.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ver Hoef L.W., Paige A.L., Riley K.O., Cure J., Soltani M., Williams F.B., Kennedy R.E., Szaflarski J.P., Knowlton R.C. Evaluating hippocampal internal architecture on MRI: inter-rater reliability of a proposed scoring system. Epilepsy Res. 2013;106:146–154. doi: 10.1016/j.eplepsyres.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson G.D., Kuzniecky R.I., Cascino G.D. Hippocampal sclerosis without detectable hippocampal atrophy. Neurology. 1994;44:42–46. doi: 10.1212/wnl.44.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Jackson G.D., Berkovic S.F., Duncan J.S., Connelly A. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am J Neuroradiol. 1993;14:753–762. [PMC free article] [PubMed] [Google Scholar]
- 14.Wieser H.G., Blume W.T., Fish D., Goldensohn E., Hufnagel A., King D., Sperling M.R., Luders H., Pedley T.A., Commission on Neurosurgery of the International League Against E. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- 15.Blumcke I., Thom M., Aronica E., Armstrong D.D., Bartolomei F., Bernasconi A., Bernasconi N., Bien C.G., Cendes F., Coras R., Cross J.H., Jacques T.S., Kahane P., Mathern G.W., Miyata H., Moshe S.L., Oz B., Ozkara C., Perucca E., Sisodiya S., Wiebe S., Spreafico R. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013;54:1315–1329. doi: 10.1111/epi.12220. [DOI] [PubMed] [Google Scholar]
- 16.Wyler A.R., Dohan F.C., Jr., Schweitzer J.B., Berry A.D. A grading system for mesial temporal pathology (Hippocampal Sclerosis) from anterior temporal lobectomy. J Epilepsy. 1992;5:220–225. [Google Scholar]
- 17.Schoene-Bake J.C., Keller S.S., Niehusmann P., Volmering E., Elger C., Deppe M., Weber B. In vivo mapping of hippocampal subfields in mesial temporal lobe epilepsy: relation to histopathology. Hum Brain Mapp. 2014;35:4718–4728. doi: 10.1002/hbm.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller S.S., Mackay C.E., Barrick T.R., Wieshmann U.C., Howard M.A., Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage. 2002;16:23–31. doi: 10.1006/nimg.2001.1072. [DOI] [PubMed] [Google Scholar]
- 19.Keller S.S., Wieshmann U.C., Mackay C.E., Denby C.E., Webb J., Roberts N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002;73:648–655. doi: 10.1136/jnnp.73.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quigg M., Bertram E.H., Jackson T., Laws E. Volumetric magnetic resonance imaging evidence of bilateral hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsia. 1997;38:588–594. doi: 10.1111/j.1528-1157.1997.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 21.Mueller C.A., Scorzin J., von Lehe M., Fimmers R., Helmstaedter C., Zentner J., Lehmann T.N., Meencke H.J., Schulze-Bonhage A., Schramm J. Seizure outcome 1 year after temporal lobe epilepsy: an analysis of MR volumetric and clinical parameters. Acta Neurochir (Wien) 2012;154:1327–1336. doi: 10.1007/s00701-012-1407-0. [DOI] [PubMed] [Google Scholar]
- 22.Jack C.R., Jr., Sharbrough F.W., Cascino G.D., Hirschorn K.A., O’Brien P.C., Marsh W.R. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol. 1992;31:138–146. doi: 10.1002/ana.410310204. [DOI] [PubMed] [Google Scholar]
- 23.Joo E.Y., Han H.J., Lee E.K., Choi S., Jin J.H., Kim J.H., Tae W.S., Seo D.W., Hong S.C., Lee M., Hong S.B. Resection extent versus postoperative outcomes of seizure and memory in mesial temporal lobe epilepsy. Seizure. 2005;14:541–551. doi: 10.1016/j.seizure.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Bonilha L., Kobayashi E., Mattos J.P., Honorato D.C., Li L.M., Cendes F. Value of extent of hippocampal resection in the surgical treatment of temporal lobe epilepsy. Arq Neuropsiquiatr. 2004;62:15–20. doi: 10.1590/s0004-282x2004000100003. [DOI] [PubMed] [Google Scholar]
- 25.Hardy S.G., Miller J.W., Holmes M.D., Born D.E., Ojemann G.A., Dodrill C.B., Hallam D.K. Factors predicting outcome of surgery for intractable epilepsy with pathologically verified mesial temporal sclerosis. Epilepsia. 2003;44:565–568. doi: 10.1046/j.1528-1157.2003.39202.x. [DOI] [PubMed] [Google Scholar]
- 26.Kanner A.M., Kaydanova Y., deToledo-Morrell L., Morrell F., Smith M.C., Bergen D., Pierre-Louis S.J., Ristanovic R. Tailored anterior temporal lobectomy, Relation between extent of resection of mesial structures and postsurgical seizure outcome. Arch Neurol. 1995;52:173–178. doi: 10.1001/archneur.1995.00540260079020. [DOI] [PubMed] [Google Scholar]
- 27.Jack C.R., Jr., Sharbrough F.W., Marsh W.R. Use of MR imaging for quantitative evaluation of resection for temporal lobe epilepsy. Radiology. 1988;169:463–468. doi: 10.1148/radiology.169.2.3174994. [DOI] [PubMed] [Google Scholar]
- 28.Howe K.L., Dimitri D., Heyn C., Kiehl T.R., Mikulis D., Valiante T. Histologically confirmed hippocampal structural features revealed by 3 T MR imaging: potential to increase diagnostic specificity of mesial temporal sclerosis. AJNR Am J Neuroradiol. 2010;31:1682–1689. doi: 10.3174/ajnr.A2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumcke I., Pauli E., Clusmann H., Schramm J., Becker A., Elger C., Merschhemke M., Meencke H.J., Lehmann T., von Deimling A., Scheiwe C., Zentner J., Volk B., Romstock J., Stefan H., Hildebrandt M. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol. 2007;113:235–244. doi: 10.1007/s00401-006-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thom M., Liagkouras I., Elliot K.J., Martinian L., Harkness W., McEvoy A., Caboclo L.O., Sisodiya S.M. Reliability of patterns of hippocampal sclerosis as predictors of postsurgical outcome. Epilepsia. 2010;51:1801–1808. doi: 10.1111/j.1528-1167.2010.02681.x. [DOI] [PubMed] [Google Scholar]